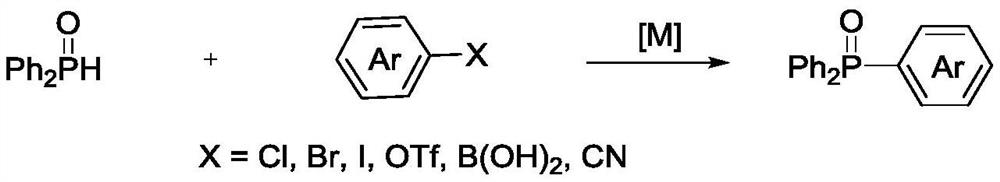Patents
Literature
40results about How to "Reaction conditions are green and environmentally friendly" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of nitrogen-doped fluorescent carbon dots
ActiveCN105586036AEvenly dispersedReaction conditions are green and environmentally friendlyNanoopticsLuminescent compositionsSonicationOrganic solvent
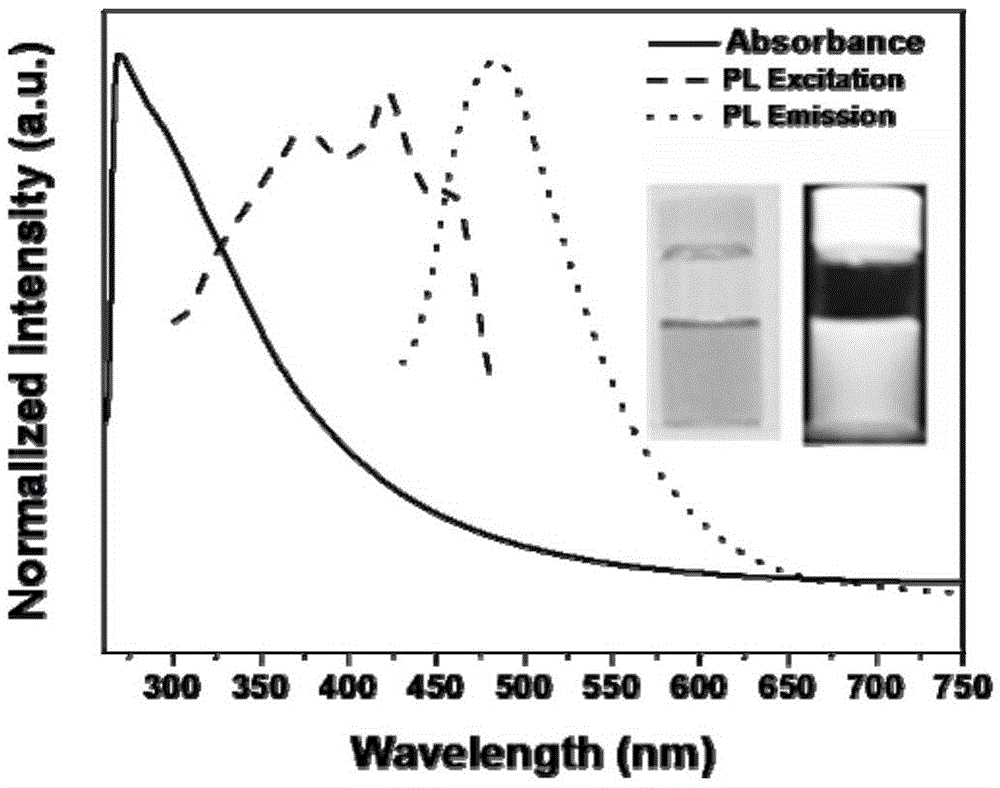
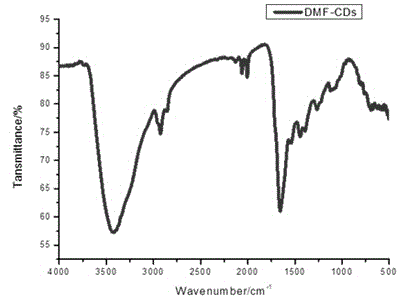
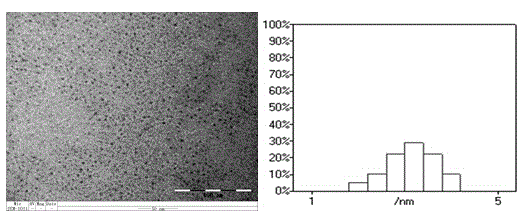
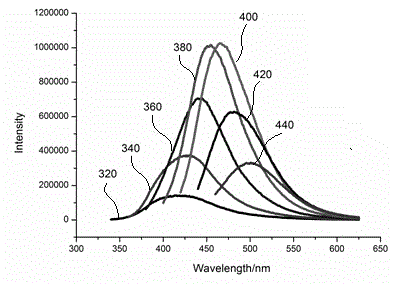
The invention relates to the technical field of preparation of carbon nanomaterials and provides a preparation method of nitrogen-doped fluorescent carbon dots. The preparation method comprises the following steps: 1, a carbon source is broken, ground, sieved by a 200-mesh sieve and dried in an oven; 2, the carbon source dried by the oven is weighed and placed into a 50-ml beaker, then an organic solvent is added, and a mixture is stirred sufficiently and subjected to ultrasonic processing for 0.8-1.2 h; 3, the mixture prepared in the step 2 is placed in a 50-ml hydrothermal kettle to have a hydrothermal reaction for 2-12 h, and the temperature is controlled at 60-180 DEG C; 4, after step 3, a suspension is taken out and placed into a high-speed desk centrifuge, supernatant liquor is taken out after 10-20 min of centrifugation, and hydrosol of the fluorescent carbon dots is obtained; 5, the hydrosol, obtained in the step 4, of the fluorescent carbon dots is placed in rotary evaporation equipment, the organic solvent is removed, and the target product fluorescent carbon dots are prepared. The preparation method of the nitrogen-doped fluorescent carbon dots has the characteristics of green and environment-friendly reaction conditions, easiness in control and easiness in mass production and preparation and is expected to be widely applied to the fields of photoelectric devices, bio-imaging, detection, sensing and the like.
Owner:DALIAN UNIV OF TECH
Preparation method for organic phase carbon dots
ActiveCN104531149AHigh yieldGood dispersionNanoopticsLuminescent compositionsDispersitySolvothermal reaction
The invention discloses a preparation method for organic phase carbon dots. The preparation method comprises the steps that raw material are fetched and added into a reaction kettle, and the raw materials are subjected to a solvothermal reaction for 0.5-72 hours under the condition of 150-300 DEG C, so that an organic phase carbon dot solution is obtained; liquid is concentrated, an obtained solvent returns to the reaction kettle for cycle use, and solid is dried to obtain organic phase carbon quantum dots, wherein the raw materials are one of or the composition of several of N,N-dimethyl formamide, N,N-dimethylacetamide, dimethyl sulfoxide, normal hexane, cyclohexane, methylbenzene and xylene. The technological method is simple and rapid, reaction conditions are simple and friendly to environment, yield is high, and reaction time is reduced. The adopted raw materials are cheap and easy to obtain, catalyst does not need to be added, and the solvent can continue to be recycled. The prepared organic phase carbon dots have good dispersity in an organic solvent, and have wide application in the fields of organic coating, substance detection, catalysis, fluorescent ink, novel nanocomposite, novel electrochemical electrodes, novel optical materials and the like.
Owner:NANJING UNIV
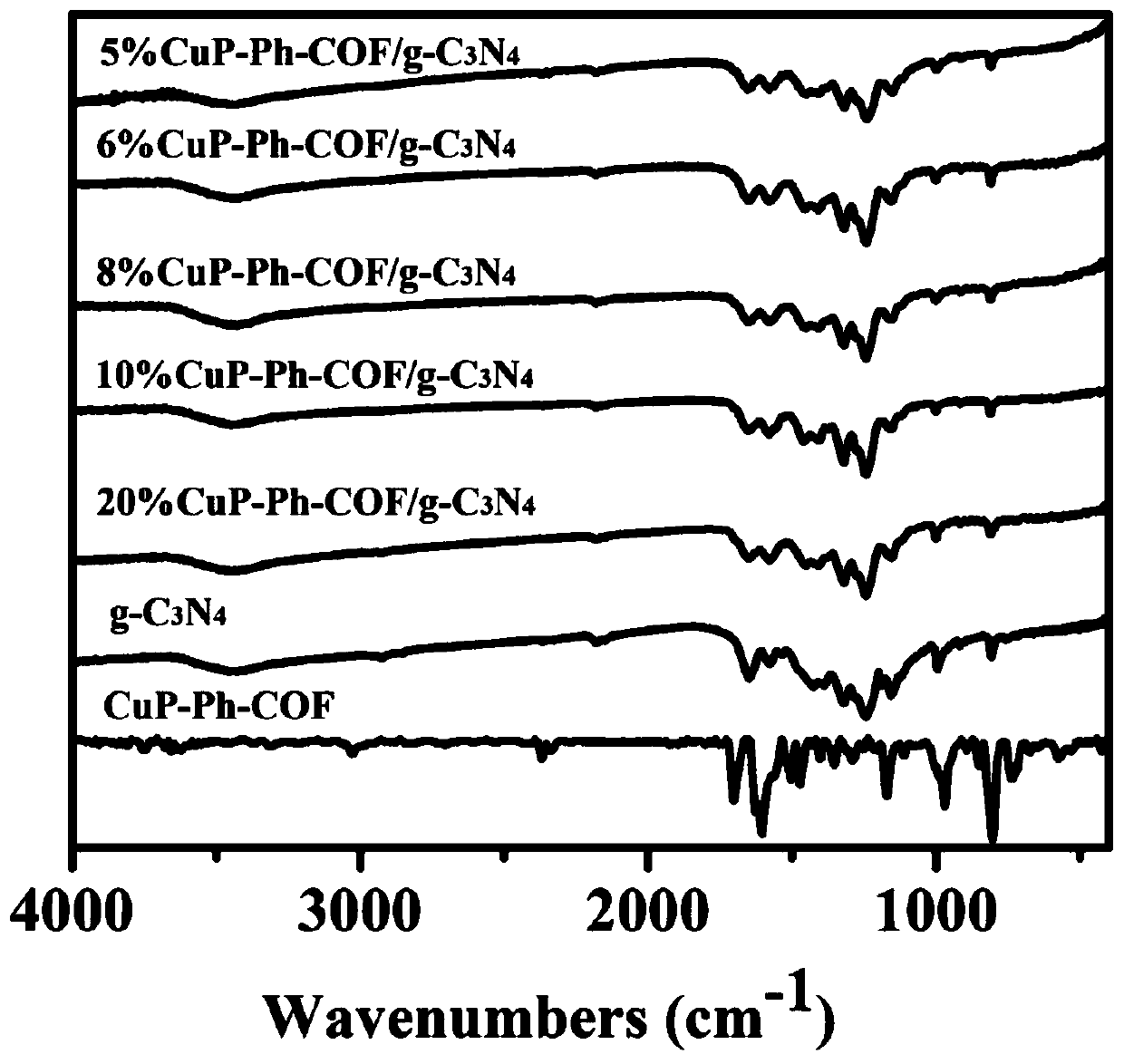
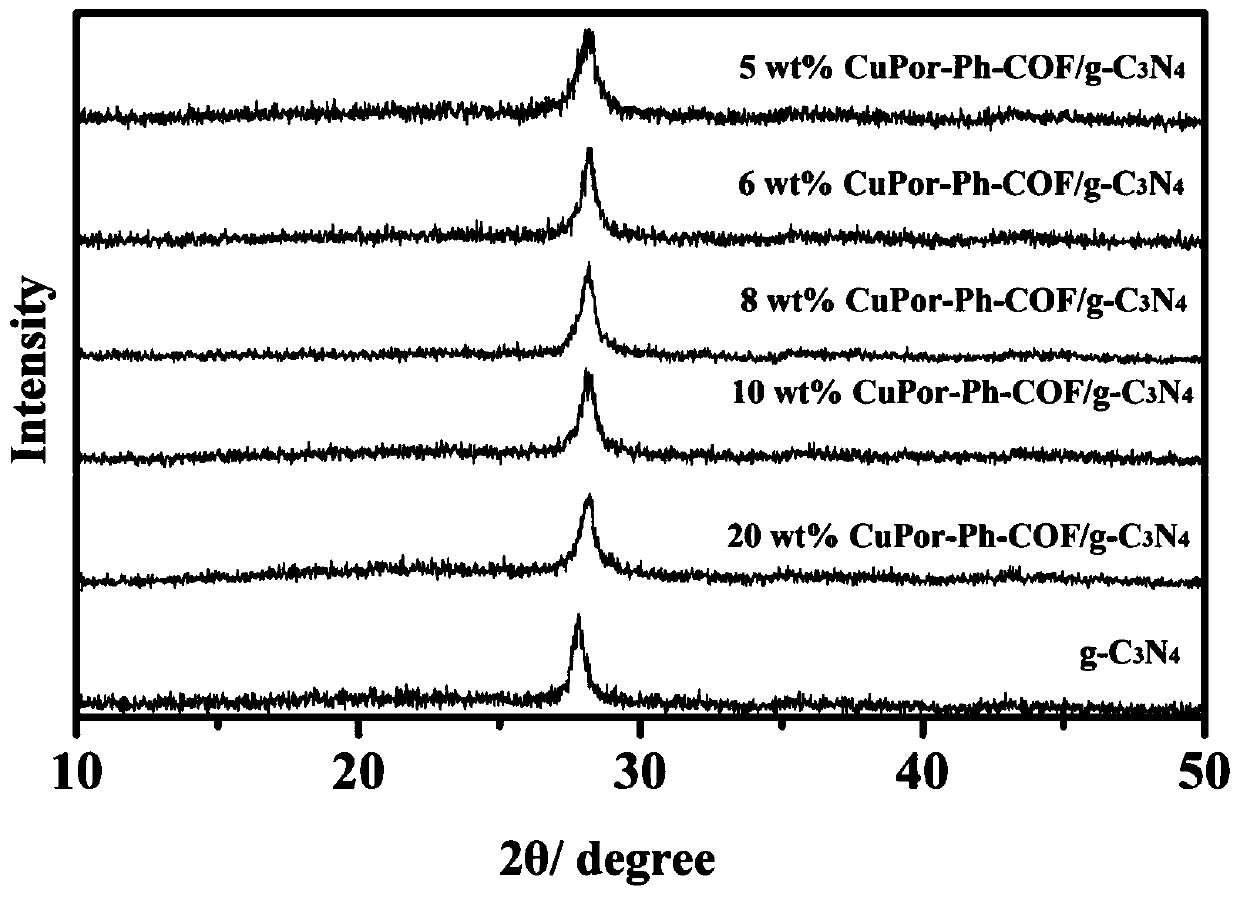
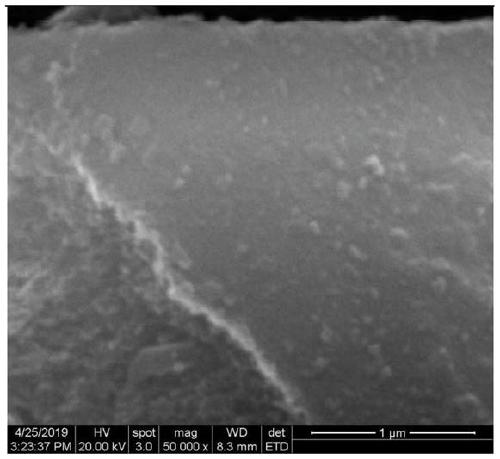
Preparation method of porphyrin COF and carbon nitride composite material and application in photocatalytic degradation of organic dyes
InactiveCN110227550AMild reaction conditionsNew synthetic methodWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsPhotocatalytic degradationCarbon composites
Belonging to the technical field of inorganic chemistry, the invention discloses a preparation method of a porphyrin COF and carbon nitride composite material and application in photocatalytic degradation of organic dyes. Copper tetraminophenyl porphyrin and terephthalaldehyde are compounded on the surface of g-C3N4 through amine-aldehyde condensation reaction by grinding. The method has the advantages of simple operation, mild reaction conditions, high yield, low cost, easy large-scale production and the like. The material CuP-Ph-COF / g-C3N4 is used as a photocatalyst to degrade the dye rhodamine B, shows excellent ability of photocatalytic degradation of organic pollutants, and is expected to be practically applied in dye wastewater treatment.
Owner:HENAN INST OF SCI & TECH
Preparation method of natural polyelectrolyte gel adsorbent for wastewater treatment
InactiveCN103623797ALarge swellingEasy to separateOther chemical processesWater/sewage treatment by sorptionMethacrylateChemical reaction
The invention belongs to the technical field of environmental protection, and relates to a preparation method of a natural polyelectrolyte gel adsorbent for treating wastewater containing heavy metal ions and dyes. The method comprises the following steps: dissolving natural polyelectrolyte in water to prepare a polyelectrolyte water solution of which the mass concentration is 1-5%, adding 15-30 parts by weight of bis-acrylate or bis-methacrylate terminated polyethyleneglycol high-polymer monomer into 100 parts by weight of polyelectrolyte water solution, dissolving by stirring, adding 0.1-0.5 part of photoinitiator, uniformly mixing, carrying out ultrasonic treatment for 5-15 minutes, pouring into a mold, and irradiating under low-intensity ultraviolet light with the wavelength of 365nm to obtain the natural polyelectrolyte gel adsorbent. The preparation process does not relate to any organic solvent, toxic crosslinking agent or related chemical reaction; and thus, the preparation method is simple and quick to use, and can be used for treating wastewater containing heavy metal ions, dyes and the like.
Owner:WUHAN TEXTILE UNIV
Method for degradation of composite material by directional bond breaking and recovery of fiber from composite material
ActiveCN110527137ASimple componentsEasy to separate, purify and reusePlastic recyclingSolvent effectsFiber strength
The invention provides a method for degradation of a composite material by directional bond breaking and recovery of fibers from the composite material. The method comprises the following steps that (1) the composite material is cut into composite material blocks with the target size for standby application; (2) the composite material blocks, solvents, metal salts, ligands, pH regulators and oxidants are mixed evenly, and then heated, the composite material blocks are naturally cooled to the room temperature after being completely degraded, and then high-speed centrifugal separation is conducted to obtain crude fiber products; and (3) the crude fiber products are washed and dried, and finally fiber fine products are obtained. According to the method, through the hot solvent effect, unsaturated complexation and weak complexation, the solvents, the metal salts, the ligands, the pH regulators, the oxidants and the composite material are mixed, and then heated, directional bond breaking degradation of resin is achieved, finally the fibers with little surface resin residue, basically no defect and the fiber strength retention rate up to 97.1% are obtained, and the degradation rate of the resin is as high as 100%.
Owner:WUHAN UNIV OF TECH
Method of synthesizing quinazolinone-structured compound
InactiveCN105732520AReaction conditions are green and environmentally friendlyNo pollution in the processOrganic chemistrySolventOxide
A method of synthesizing a quinazolinone-structured compound includes the steps of adding an alcohol and amine as substrates in a liquid solvent with a peroxide as an oxidizing agent, and performing a reaction under the effects of a catalyst at 0-150 DEG C for 10-20 h to synthesize the quinazolinone-structured compound, wherein the catalyst includes metal oxides and / or metal oxides having a defect locus, and the metal oxides include one or more than two of [alpha]-MnO2, [beta]-MnO2, [delta]-MnO2, Mn2O3, Mn3O4, Fe2O3, V2O5, Co3O4 or MoO3, and the metal oxides having the defect locus is MnO2 treated in the presence of argon gas. The method, under a mild condition, is used for synthesizing the quinazolinone-structured compound at high efficiency and selectivity. The method has mild operation conditions and reaches 96% in yield of the quinazolinone-structured compound.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
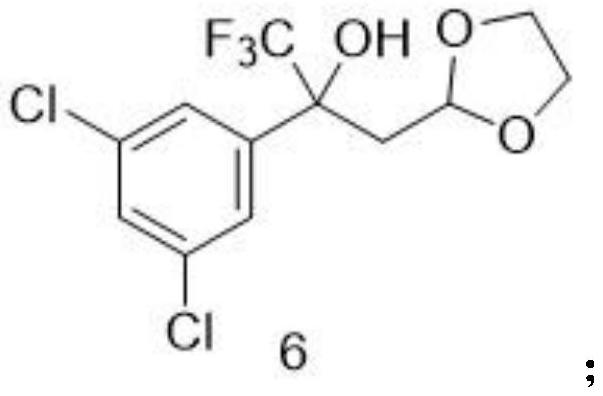
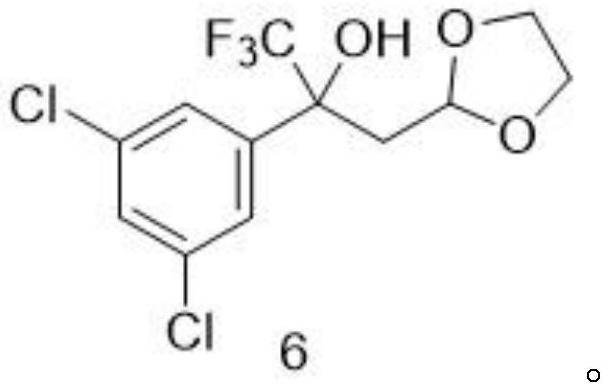
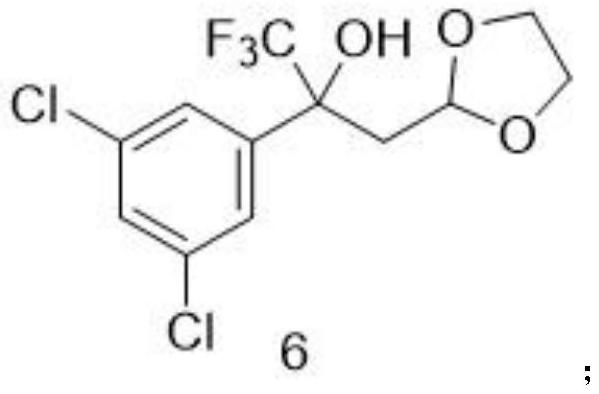
Method for preparing fluralana intermediate, prepared intermediate and application of fluralana intermediate
ActiveCN113461653ALow costLow priceOrganic chemistryAgainst vector-borne diseasesPtru catalystGrignard reagent
A method for preparing a fluralana intermediate, provided by the invention, comprises the following steps: carrying out nucleophilic addition reaction on 1-(3, 5-dichlorophenyl)-2, 2, 2-trifluoroethanone, carrying out acidolysis, reacting with a Grignard reagent, carrying out oxidation, carrying out oxidation cyclization reaction, reacting with hydroxyl(toluenesulfonyloxy)benzene iodide, and reacting with a trimethoxyphosphorus solution to finally generate the fluralan. In the preparation process, a catalyst and dimethoxy zinc are not needed, ultralow-temperature reaction conditions of -78 DEG C are not needed, operation is easy and convenient, meanwhile, reagents such as ozone and dimethyl sulfide which are harmful to the environment are replaced, and the method is more environmentally friendly. The invention also provides the intermediate for preparing the fluralan.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
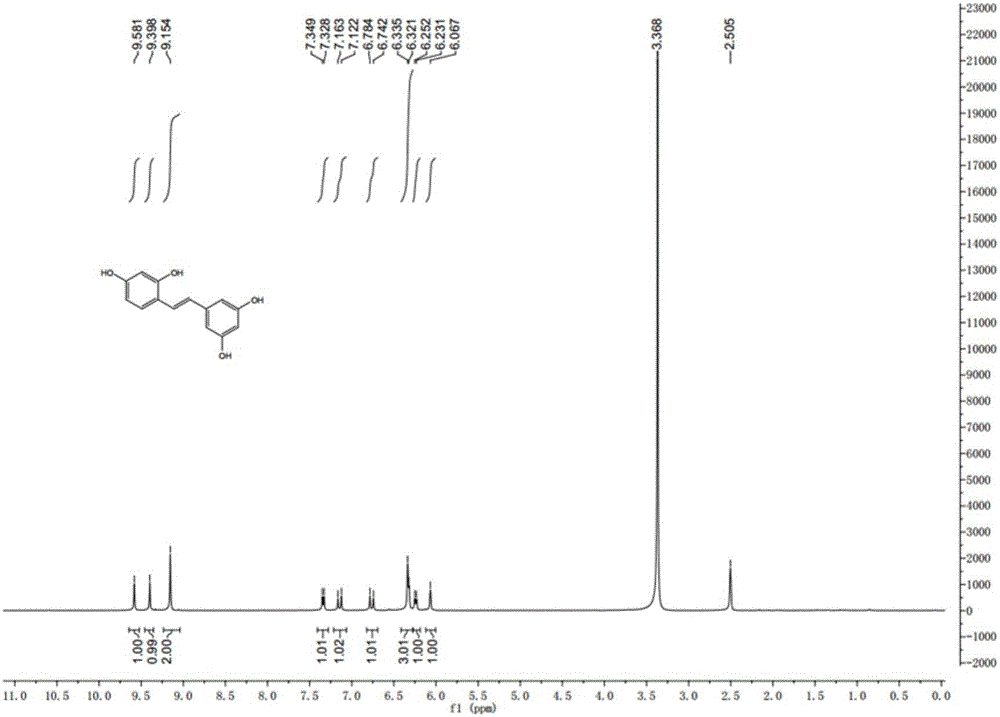
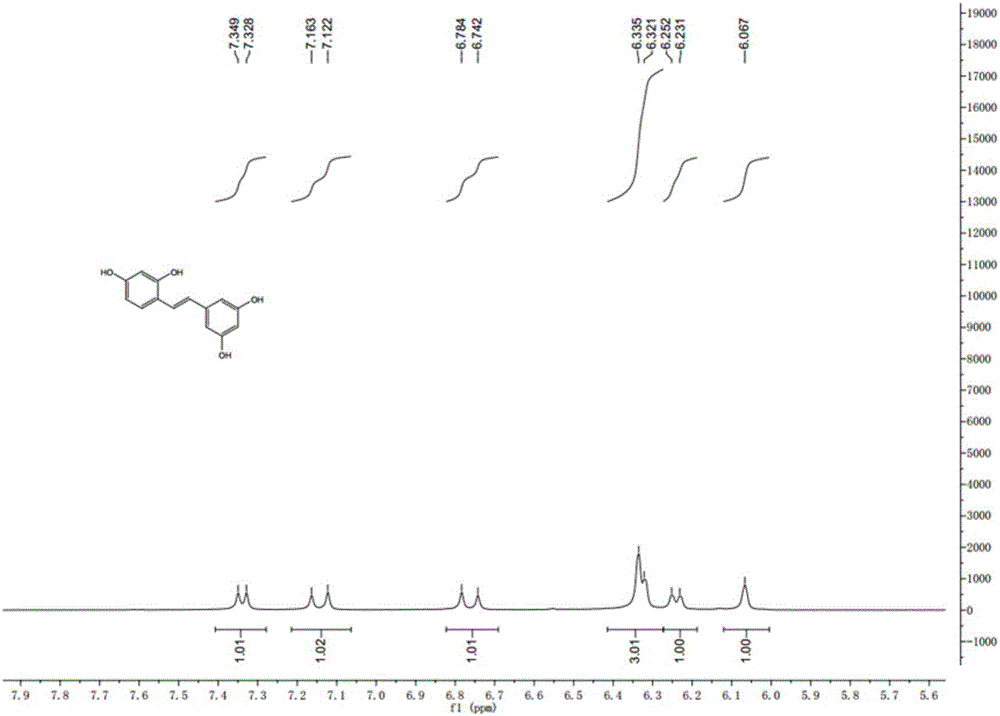
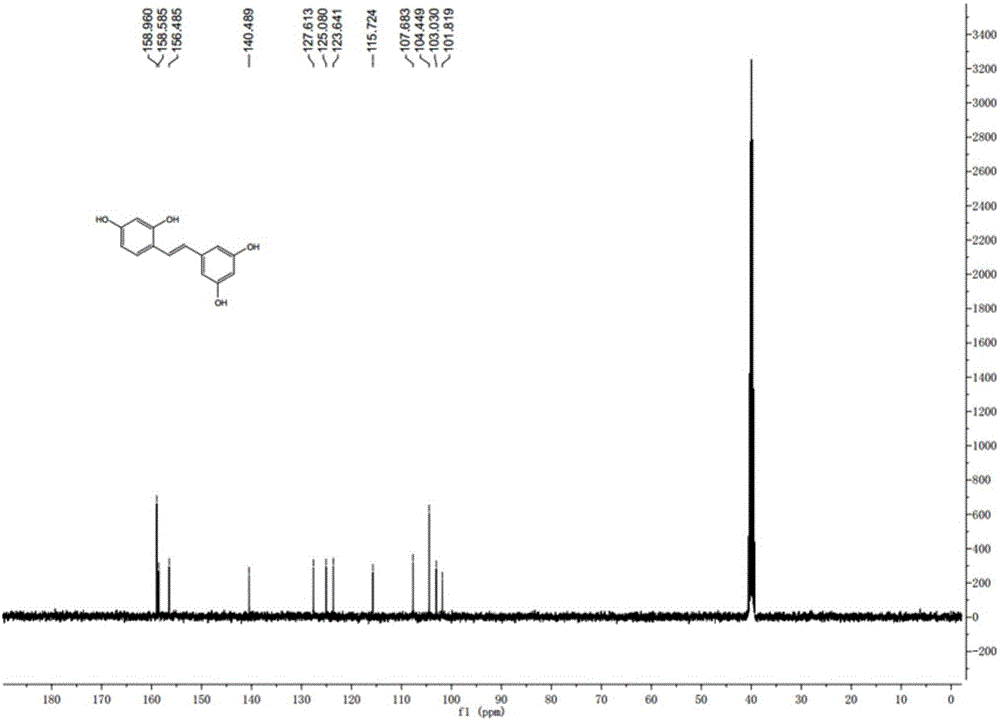
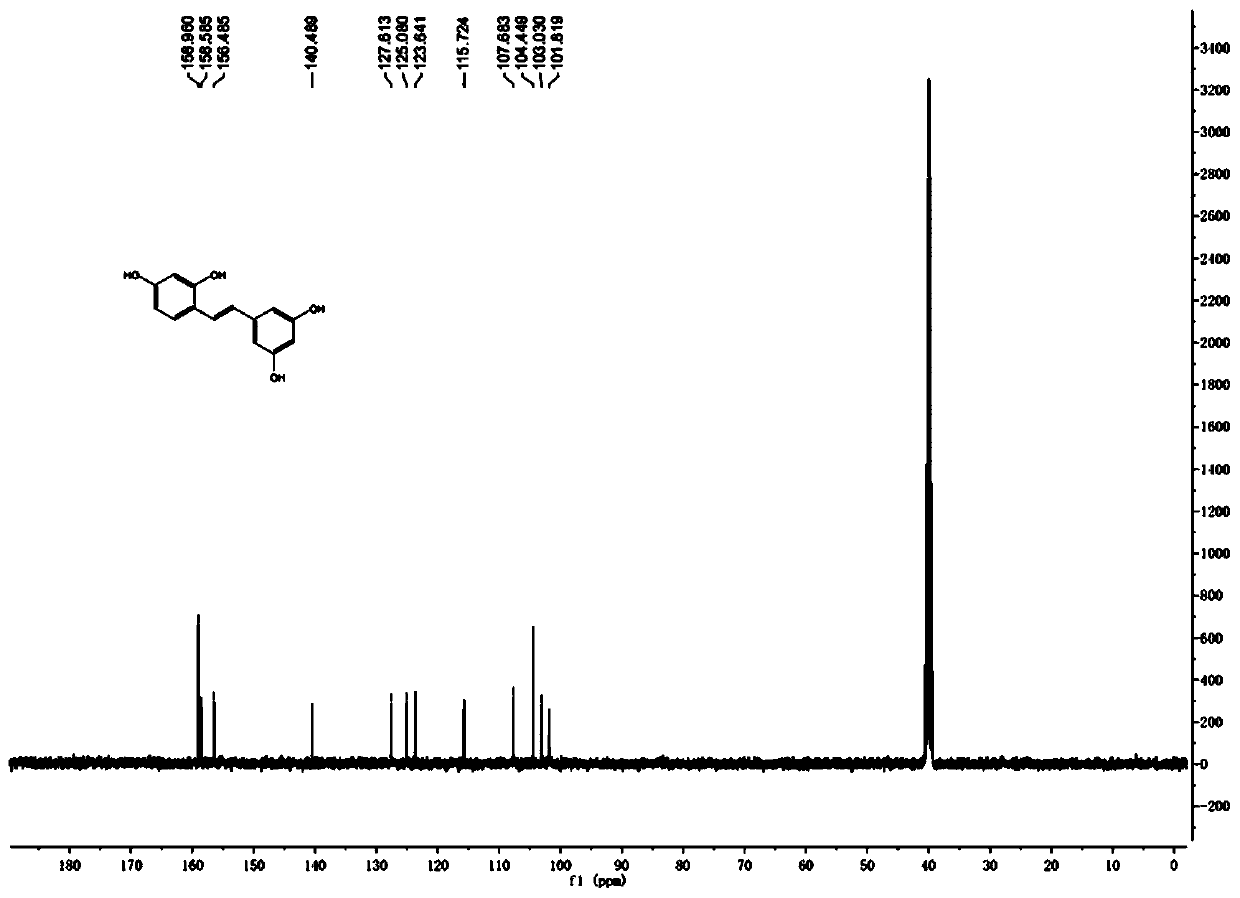
Synthesis method of natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene
ActiveCN106748662ASimple stepsNo protectiveOxygen-containing compound preparationOrganic compound preparationSynthesis methodsBenzaldehyde
The invention discloses a synthesis method of a natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene. According to the method, 1,3-acetone dicarboxylic acid dimethyl ester is used as a starting raw material; condensation and aromatization reactions are performed to obtain 3,5-dihydroxy-2,4-dicarboxylate methyl phenyl acetate; then, hydrolysis and decarboxylation are performed to obtain 3,5-dyhydroxy phenylacetic acid; the 3,5-dyhydroxy phenylacetic acid and 2,4-dihydroxy benzaldehyde take condensation reaction under the existence of alkali to obtain 3-(3,5-dihydroxy phenyl)-7-hydroxy coumarin; next, open loop decarboxylation reaction is performed under the alkaline condition; the natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene is obtained. The method has the advantages that the raw materials are easily obtained; the reaction route is simple and fast; the operation is convenient; the yield is higher.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
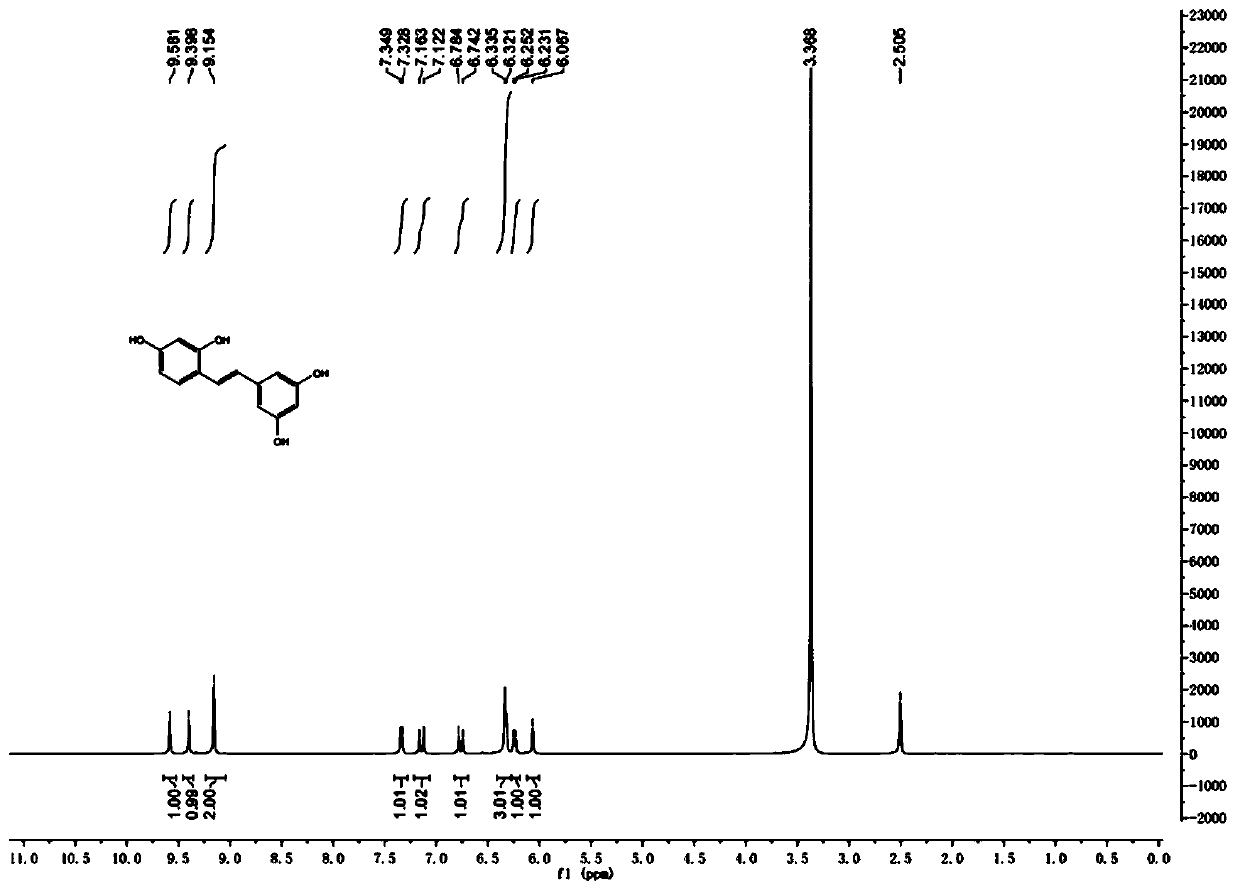
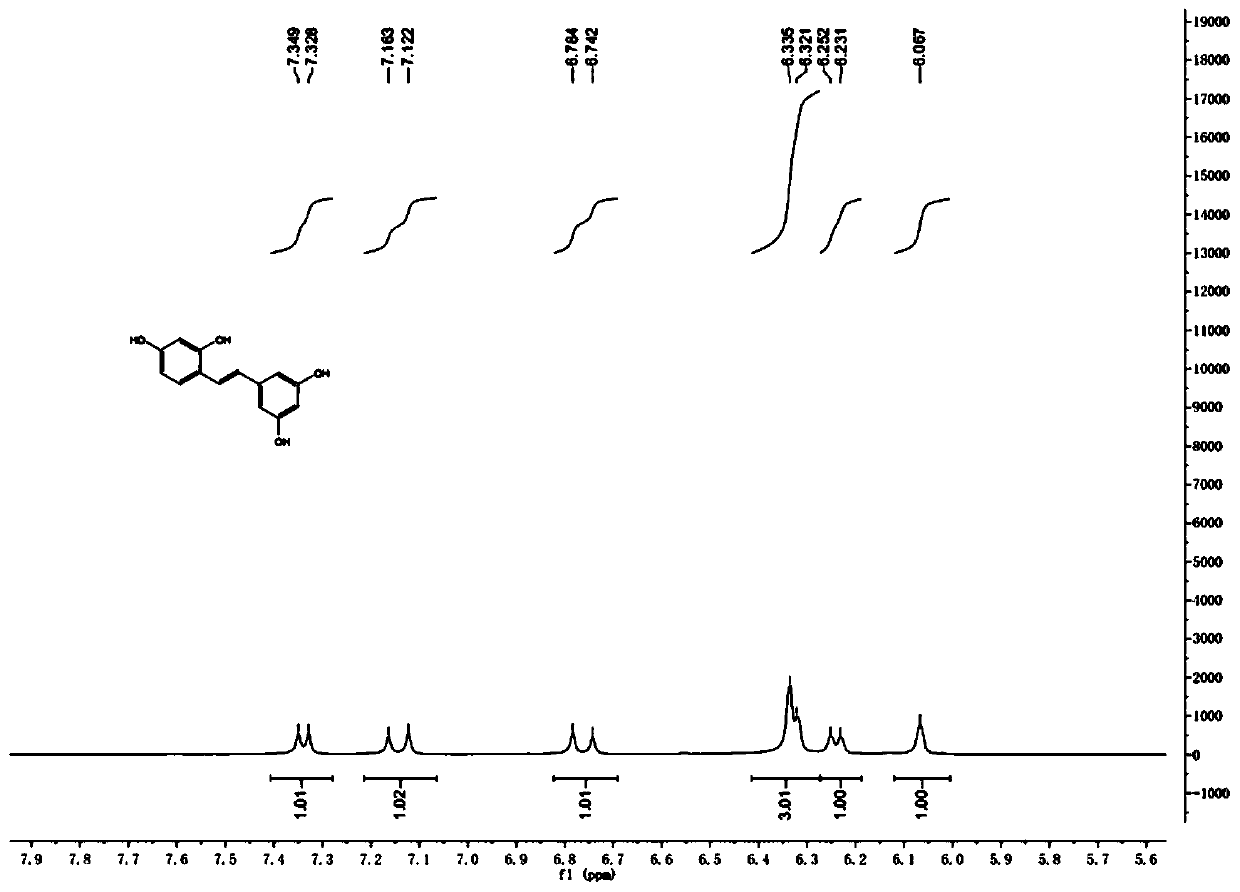
A kind of synthetic method of natural product e-2,3',4,5'-tetrahydroxystilbene
ActiveCN106748662BSimple stepsGood atom economyOxygen-containing compound preparationOrganic compound preparationSynthesis methodsPhenylacetic acid
The invention discloses a synthesis method of a natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene. According to the method, 1,3-acetone dicarboxylic acid dimethyl ester is used as a starting raw material; condensation and aromatization reactions are performed to obtain 3,5-dihydroxy-2,4-dicarboxylate methyl phenyl acetate; then, hydrolysis and decarboxylation are performed to obtain 3,5-dyhydroxy phenylacetic acid; the 3,5-dyhydroxy phenylacetic acid and 2,4-dihydroxy benzaldehyde take condensation reaction under the existence of alkali to obtain 3-(3,5-dihydroxy phenyl)-7-hydroxy coumarin; next, open loop decarboxylation reaction is performed under the alkaline condition; the natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene is obtained. The method has the advantages that the raw materials are easily obtained; the reaction route is simple and fast; the operation is convenient; the yield is higher.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
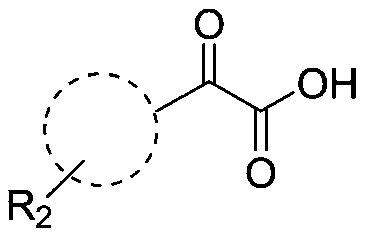
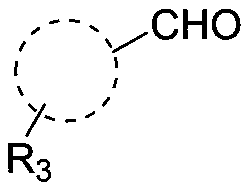
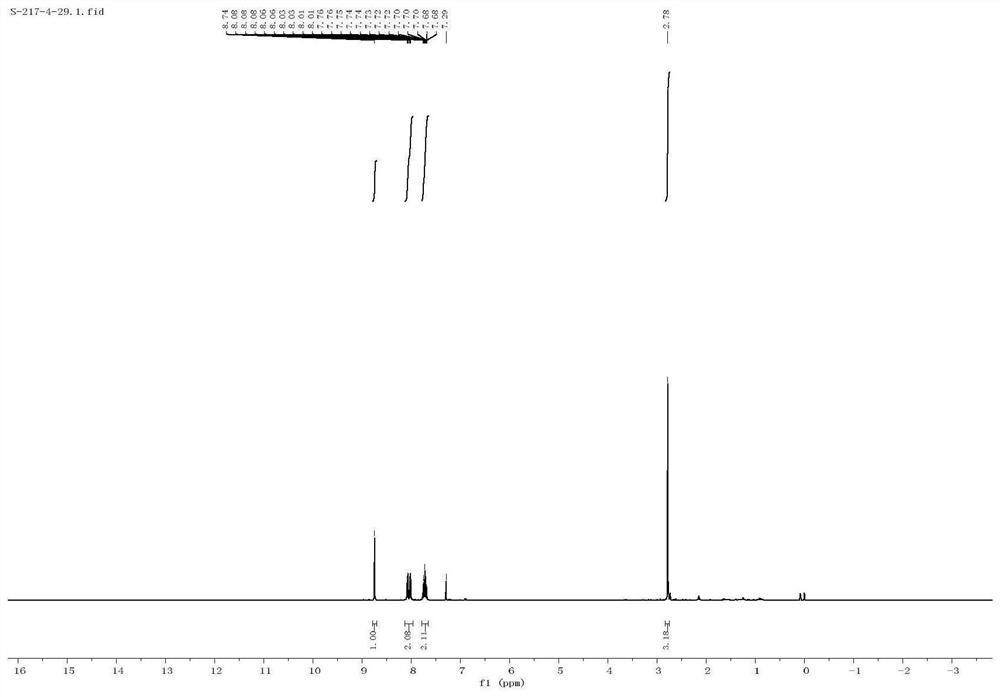
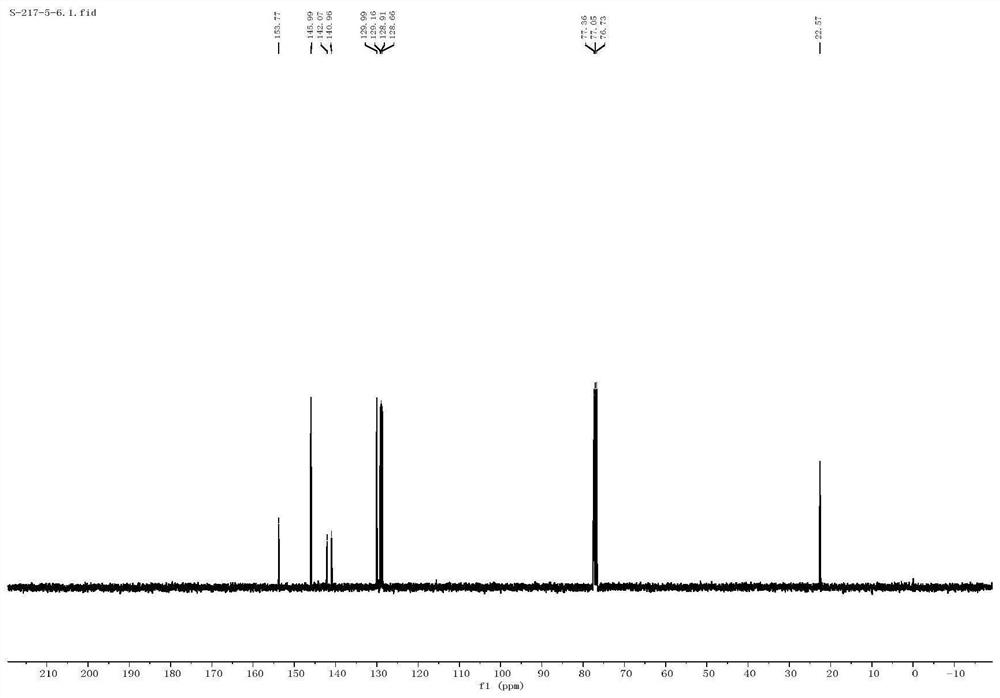
Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition
InactiveCN104610267AReaction conditions are green and environmentally friendlyLow costOrganic chemistryNitrostyrolNitrobenzene
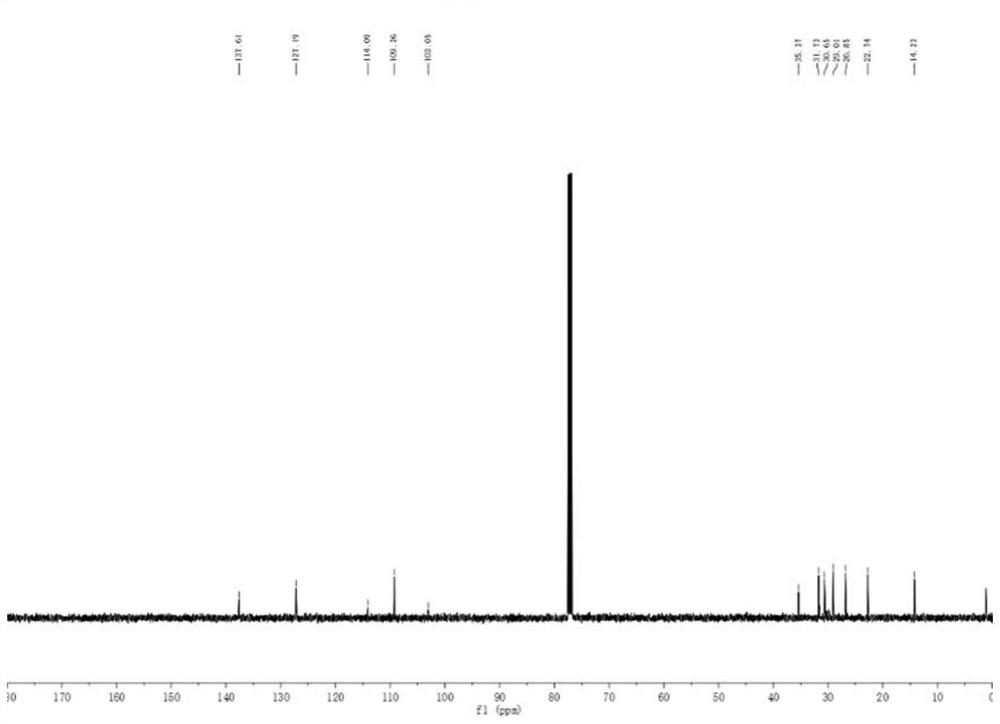
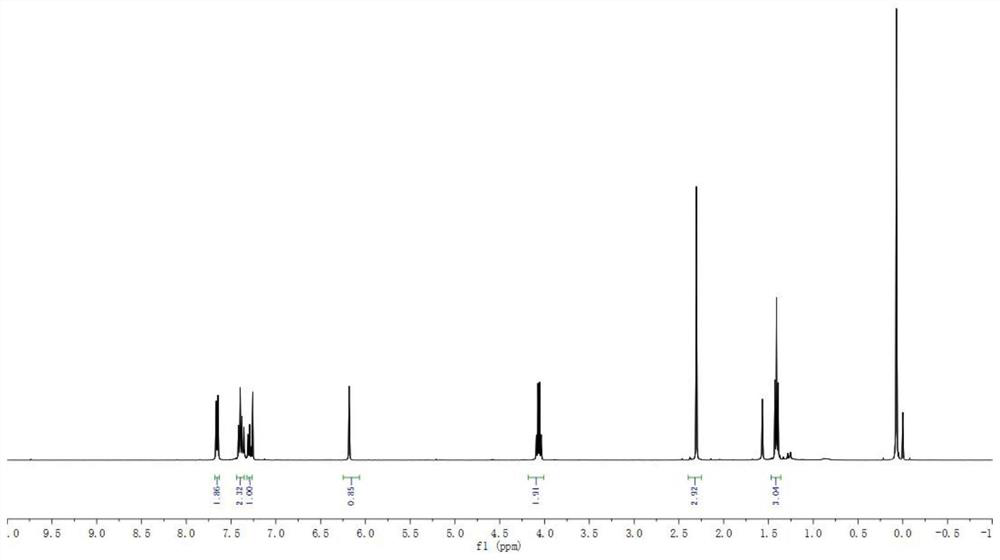
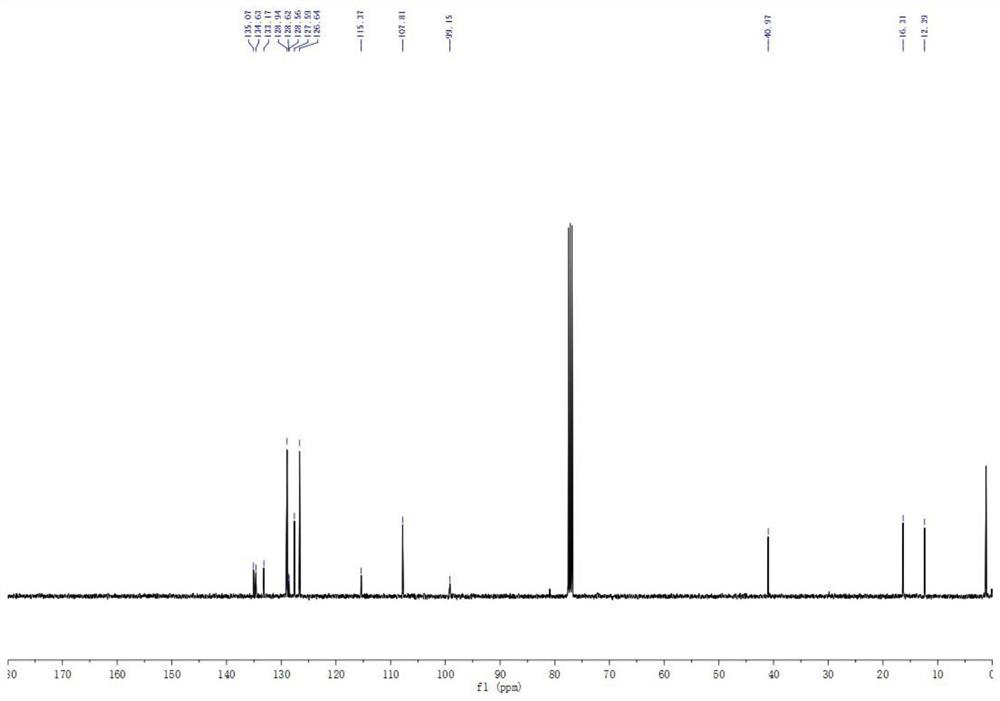
The invention belongs to the technical field of organic chemistry, and particularly relates to a method for efficiently synthetizing pyrazol[1,5-c]-quinazoline skeleton compounds under no catalytic condition. The structure of the compounds is represented and confirmed with 1H NMR, 13 C NMR, MS and other methods. The method disclosed by the invention comprises the following steps: reacting 1,3-dipolar quinazoline dipoles obtained from o-nitrobenzaldehyde and a series of compounds of triethyl orthoformate with beta-nitrostyrolene under the condition of no any catalysts based on DMSO as a solvent at the temperature of 110 DEG C to generate a series of pyrazol[1,5-c]-quinazoline derivatives. By adopting the method, the pyrazol[1,5-c]-quinazoline compounds can be efficiently prepared. The method disclosed by the invention is mild in reaction conditions and simple to oeprate, the cost is greatly lowered with comparison to the cost of previous reaction between the 1,3-dipolar quinazoline dipoles and terminal alkyne, the reaction conditions are environmentally friendly, the production purity is high, separation and purification are convenient, the method is suitable for large-scale preparation, and the pyrazol[1,5-c]-quinazoline skeleton compounds have excellent application prospect in new drug research and development since the structure of quinazoline compounds has broad-spectrum biological activity.
Owner:JIANGXI NORMAL UNIV
Bismuth-containing catalyst for preparation of glyceric acid through selective oxidation of glycerin and preparation method of catalyst
ActiveCN107321360AEasy to prepareEasy to operateOrganic compound preparationHeterogenous catalyst chemical elementsFiltrationActive component
The invention discloses a bismuth-containing catalyst for preparation of glyceric acid through selective oxidation of glycerin and a preparation method of the catalyst. The bismuth-containing catalyst is characterized in that the catalyst takes bismuth as an active component and layered hydroxide or layered metal composite oxide as a carrier. The preparation method comprises steps as follows: a bismuth salt solution is prepared; a soluble metallic salt solution is prepared, the two solutions are slowly added to a certain quantity of soluble carbonate solutions for pH value adjustment, stirred at the constant temperature to be aged, cooled, washed to be neutral, subjected to suction filtration under reduced pressure and vacuum drying and then calcined, and the catalyst is obtained. The prepared catalyst is used for preparation of glyceric acid through selective oxidation of glycerin and has the characteristics of high glycerin conversion rate, good selectivity of the product, namely, glyceric acid, simple preparation method, good repeated use performance and low cost.
Owner:NANJING INST OF TECH
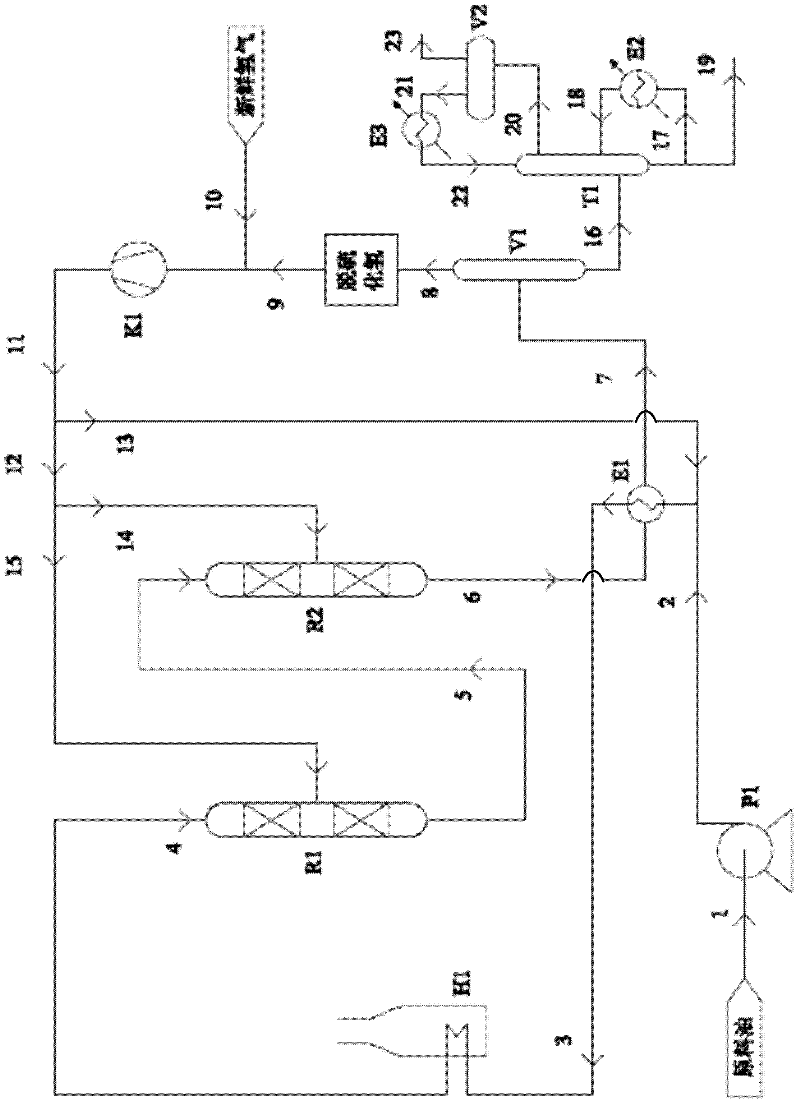
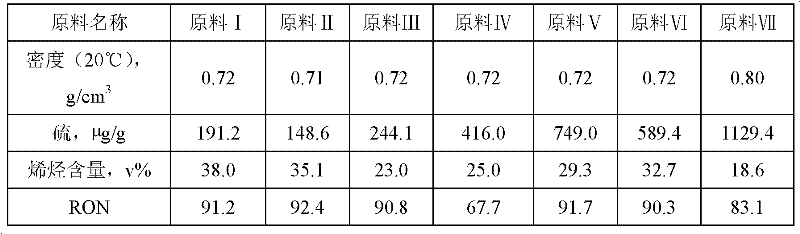
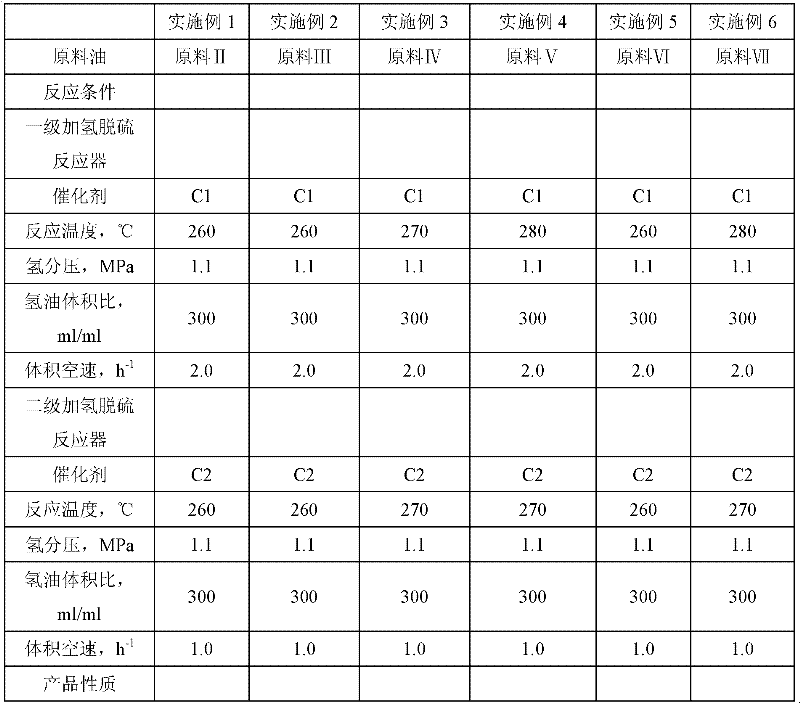
Method for reducing sulfur content in sulfur-containing light oil
InactiveCN102399588AReduce lossesControl reductionTreatment with hydrotreatment processesHydrodesulfurizationFixed bed
The invention relates to a method used for reducing the sulfur content in sulfur-containing light oil. The method comprises steps that: a light oil raw material is delivered into a primary hydrodesulfurization processing reactor, and is subject to contact reaction with a hydrodesulfurization catalyst C1; an effluent is delivered into a secondary hydrodesulfurization processing reactor, and is subject to contact reaction with a hydrodesulfurization catalyst C2; an effluent is processed through gas-liquid separation, such that a light oil product with low sulfur content is obtained. According to the invention, a fixed bed reactor structure is adopted in both the primary hydrodesulfurization processing reactor and the secondary hydrodesulfurization processing reactor. The two stages of hydrodesulfurization processing reactors are connected in series. The sulfur-containing light oil is processed through two times of continuous hydrodesulfurization reactions, such that sulfur content is reduced. Hydrodesulfurization is ensured, an alkene reducing phenomenon can be effectively controlled, loss of octane number can be controlled, and the reaction conditions are mild. The hydrogenation-modified light oil product provided by the invention is not required to be processed through alkali washing, the octane number loss of the product is low, and a yield of the product is higher than 99%.
Owner:DALIAN UNIV OF TECH
Preparation method of high-entropy oxide ceramic fiber material
PendingCN113754432AUniform shapeGood dispersionInorganic material artificial filamentsOxide ceramicMetal nitrate
The invention belongs to the technical field of ceramic fiber materials, and discloses a preparation method of a high-entropy oxide ceramic fiber material. The preparation method comprises the steps: preparing high-entropy oxide ceramic fibers through a template impregnation method, taking viscose as a template, and subjecting the viscose to vacuum impregnation in a metal nitrate mixed solution, wherein metal nitrate in the metal nitrate mixed solution is formed by mixing at least four of cerous nitrate, zirconium nitrate, yttrium nitrate, hafnium nitrate, titanium nitrate and lanthanum nitrate in equal molar mass; and after the impregnation,performing washing, centrifuging, drying and heat treatment to obtain the high-entropy oxide ceramic fiber. According to the preparation method, liquid-phase burdening is adopted, the method can guarantee that multiple metal elements are mixed uniformly, the prepared high-entropy oxide ceramic fiber material is uniform in morphology, the phase structure is of a fluorite type, and the proportion of elements contained in the fiber material is close to equal moles.
Owner:SINOSTEEL LUOYANG INST OF REFRACTORIES RES
Preparation method of 3-aryl-2H-benzo [beta] [1, 4] benzoxazine-2-ketone compound
ActiveCN111100085ASolve the problem of environmental protectionSynthetic method is cheapOrganic chemistryM-aminophenolKetonic acids
The invention discloses a preparation method of a 3-aryl-2H-benzo [beta] [1, 4] benzoxazine-2-ketone compound, which comprises the following steps: carrying out magnetic stirring on a reaction substrate 2-aminophenol compound and a ketonic acid compound in an oil bath pot by using a deep eutectic solvent as a reaction solvent, and carrying out after-treatment after the reaction is finished, thereby obtaining the 3-aryl-2H-benzo [beta] [1, 4] benzoxazine-2-ketone compound. The deep eutectic solvent is formed by uniformly mixing choline chloride and urea according to a molar ratio of 1: 2, and then heating the mixed solution to a uniform and transparent liquid at 80 DEG C. The method is simple in operation process, mild in reaction condition, low in cost and environmentally friendly, and hasapplication and popularization value.
Owner:EAST CHINA UNIV OF TECH
Method for synthesizing quinoxaline compound under visible light induced iron catalysis condition
ActiveCN113087674AAchieve synthesisHigh reaction conditionsOrganic chemistryQuinoxalinePhotosensitizer
The invention belongs to the technical field of compound synthesis, and particularly relates to a method for synthesizing quinoxaline compounds under visible light induced iron catalysis conditions. The method comprises the following steps: by taking non-activated aliphatic amine and o-phenylenediamine as raw materials, under the action of a photosensitizer, under the illumination of visible light, reacting in a solvent at room temperature and under oxygen conditions to generate the quinoxaline compound. The method has better substrate universality and relatively mild reaction conditions, not only realizes synthesis of the quinoxaline compound for the first time, but also widens the field of organic synthesis.
Owner:HENAN UNIVERSITY
Synthesis method of 2-amino-4, 6-dihydroxypyrimidine
InactiveCN106167469AReduce the difficulty of separationHigh purityOrganic chemistrySynthesis methodsReaction temperature
The invention relates to a synthesis method of 2-amino-4, 6-dihydroxypyrimidine. Guanidine nitrate and diethyl malonate are employed as the raw materials for one-step cyclization reaction. The steps include: employing a new-made sodium ethoxide solution, diethyl malonate and self-made guanidine nitrate to carry out stirring reflux reaction for 0.5-1.5h, distilling off ethanol, then dissolving the obtained white solid in water, then using a 4-6% acetic acid solution to conduct acidification to a pH value of 4-6, and carrying out filtering, washing and drying, thus obtaining a white solid 2-amino-4, 6-dihydroxypyrimidine. The method provided by the invention has the advantages of simple reaction steps, low reaction temperature, safety and environmental protection, is suitable for industrial production, and has wide application prospect.
Owner:NANTONG TENDENCI CHEM
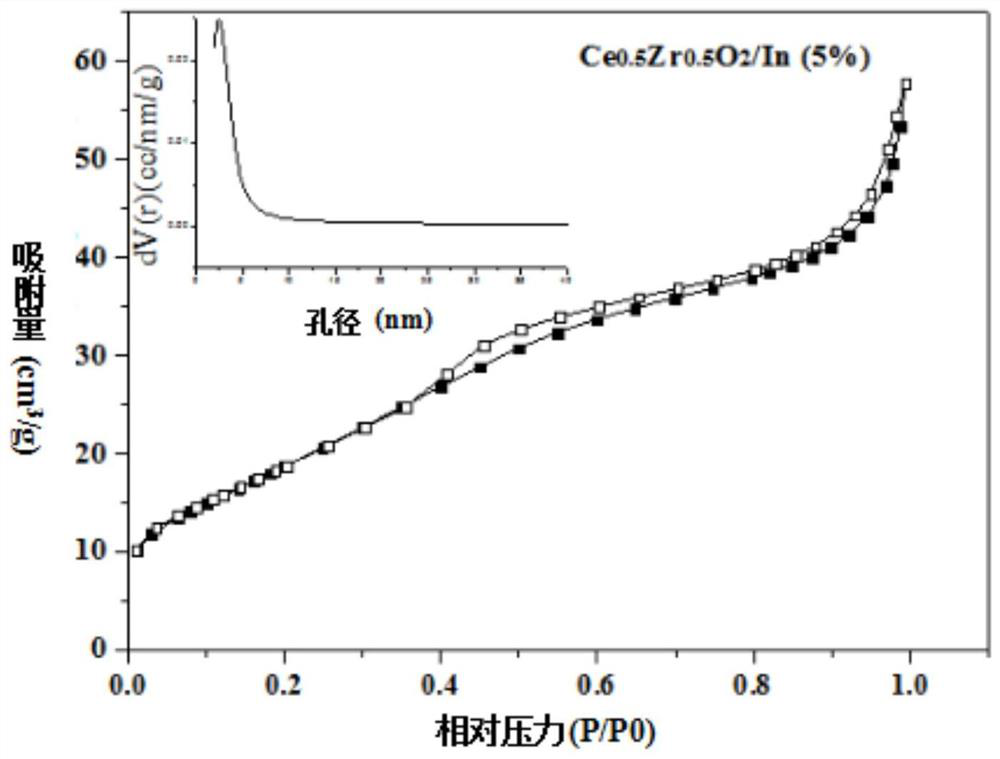
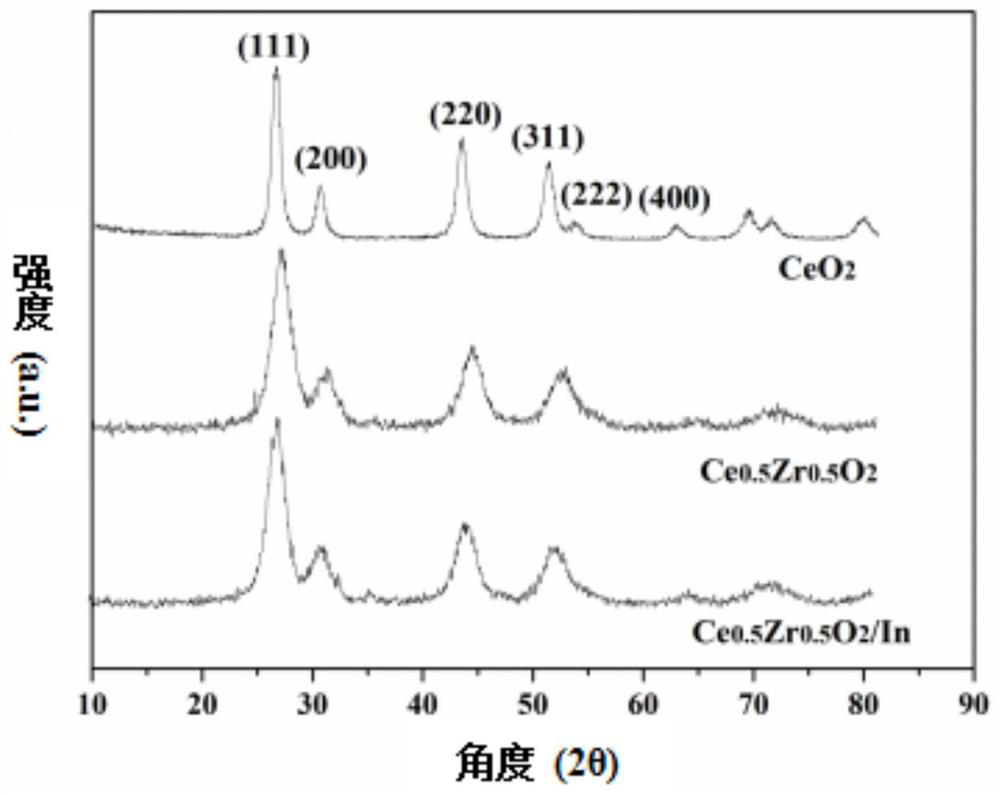
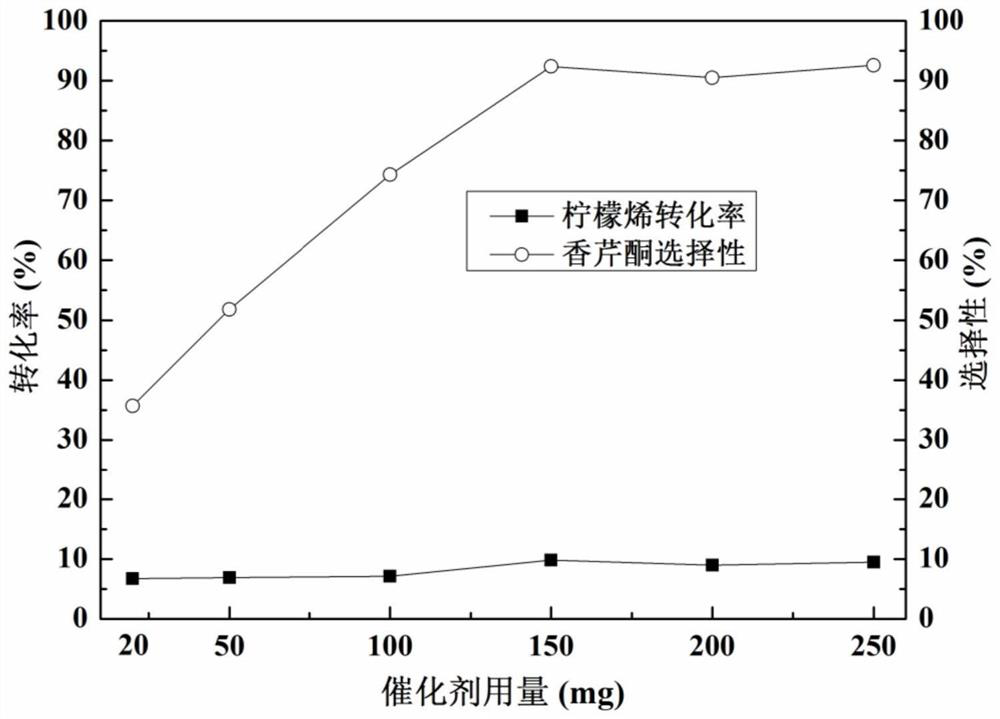
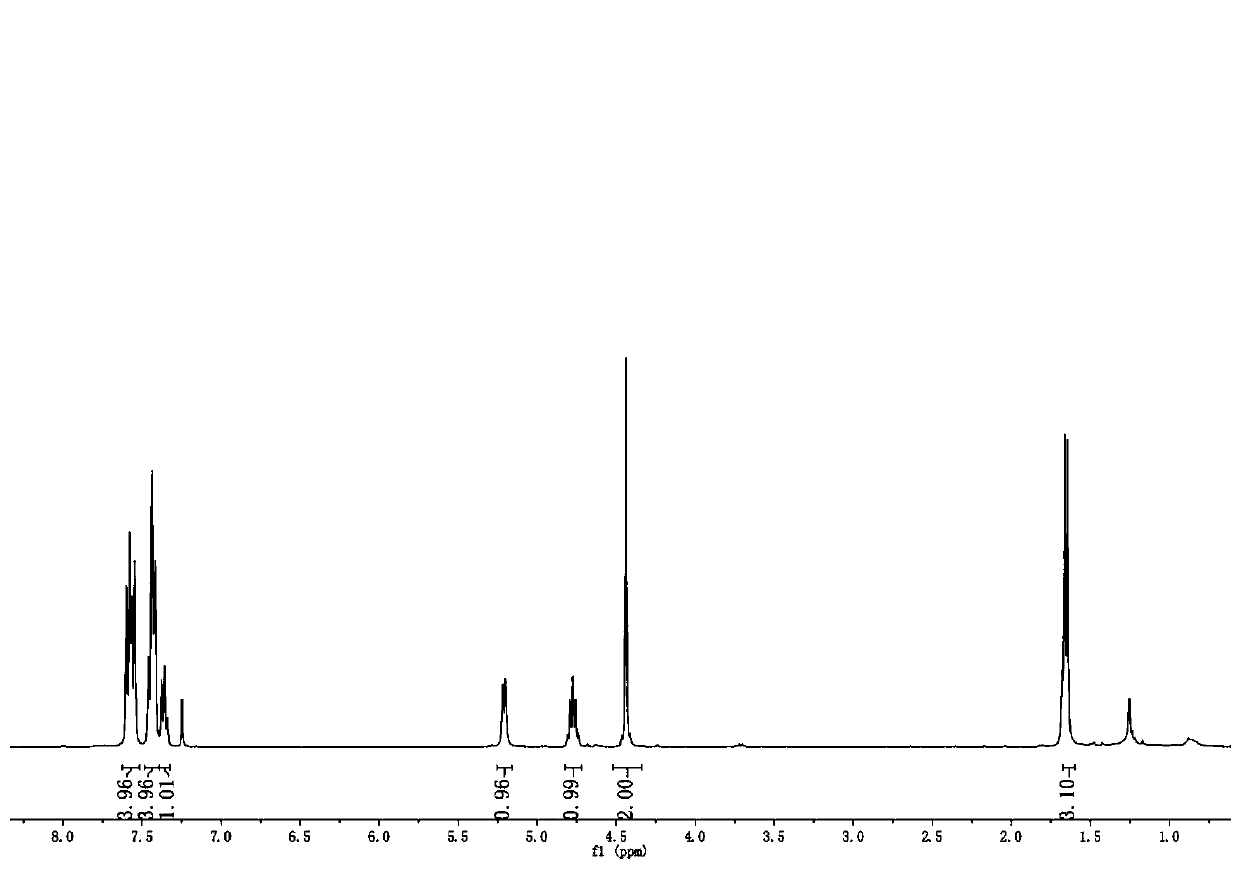
Metal-loaded cerium-zirconium solid solution material, preparation method thereof and application of metal-loaded cerium-zirconium solid solution material in catalytic synthesis of carvone
ActiveCN111729669AIdeal theoretical yieldReaction conditions are green and environmentally friendlyOrganic compound preparationHeterogenous catalyst chemical elementsPtru catalystPhysical chemistry
The invention discloses a metal-loaded cerium-zirconium solid solution material and a preparation method thereof, and application of the metal-loaded cerium-zirconium solid solution material in catalytic synthesis of carvone. The metal-loaded cerium-zirconium solid solution material is used as a catalyst for selective catalytic synthesis of carvone, and the optimal condition for catalytic synthesis of carvone is found; and a new way is provided for green synthesis of carvone.
Owner:CHINA TOBACCO YUNNAN IND
A kind of preparation method of straw-based resin fertilizer
InactiveCN104262001BMild reaction conditionsReaction conditions save energyOrganic fertilisersUrea compound fertilisersWater bathsFiltration
The invention relates to a method for preparing a straw-based resin fertilizer. The method comprises the following steps: step 1, grinding crop straws, screening, thereby obtaining straw powder; step 2, sequentially adding an acrylic acid solution, acrylamide, polyethylene glycol biacrylate, urea, K2S2O8 and the straw powder into the reactor under the cold bath and stirring conditions, adding water to the needed volume, thereby obtaining a monomer phase; step 3, adding cyclohexane and Span-60 into the reactor, slowly adding the monomer phase prepared by the step 2, adding Na2SO3, performing suction filtration after the reaction is ended, thereby obtaining bead gel, performing vacuum drying at the temperature of 42-48 DEG C for 4 hours, thereby obtaining the powdered straw-based salt-resistant resin controlled release fertilizer. According to the method disclosed by the invention, the straw-based super absorbent resin fertilizer is prepared at normal temperature, the heating is not needed during the reaction, the reaction is carried out at room temperature, and the method is mild in reaction conditions, energy-saving and environmentally friendly. The prepared fertilizer is high in water absorption and water-retaining property and good in controlled-release effect, and the preparation process is simple and low in price.
Owner:徐州禾动力肥料有限公司
A kind of preparation method of fatty amine promoted by mechanical force
The invention discloses an aliphatic amine preparation method. The aliphatic amine preparation method comprises the following steps: taking alkyl, aryl and the like as a substrate 1 and taking a nitrogen source as a substrate 2; adding an additive, an oxidizing agent and a noble metal catalyst which are suitable; and reacting under the effect of mechanical force to obtain aliphatic amine. The provided method is different from traditional organic reaction, addition of any solvents in reaction can be avoided completely, only raw materials are mixed, a target product can be obtained by simple ball-milling through mechanical force, and therefore, the aliphatic amine preparation method has the characteristics of environmental protection, high yield, gentle condition, simplicity and conveniencein operation and the like.
Owner:HUAZHONG UNIV OF SCI & TECH
A bismuth-containing catalyst for selectively oxidizing glycerin to prepare glyceric acid and its preparation method
ActiveCN107321360BEasy to prepareEasy to operateOrganic compound preparationHeterogenous catalyst chemical elementsFiltrationActive component
The invention discloses a bismuth-containing catalyst for preparation of glyceric acid through selective oxidation of glycerin and a preparation method of the catalyst. The bismuth-containing catalyst is characterized in that the catalyst takes bismuth as an active component and layered hydroxide or layered metal composite oxide as a carrier. The preparation method comprises steps as follows: a bismuth salt solution is prepared; a soluble metallic salt solution is prepared, the two solutions are slowly added to a certain quantity of soluble carbonate solutions for pH value adjustment, stirred at the constant temperature to be aged, cooled, washed to be neutral, subjected to suction filtration under reduced pressure and vacuum drying and then calcined, and the catalyst is obtained. The prepared catalyst is used for preparation of glyceric acid through selective oxidation of glycerin and has the characteristics of high glycerin conversion rate, good selectivity of the product, namely, glyceric acid, simple preparation method, good repeated use performance and low cost.
Owner:NANJING INST OF TECH
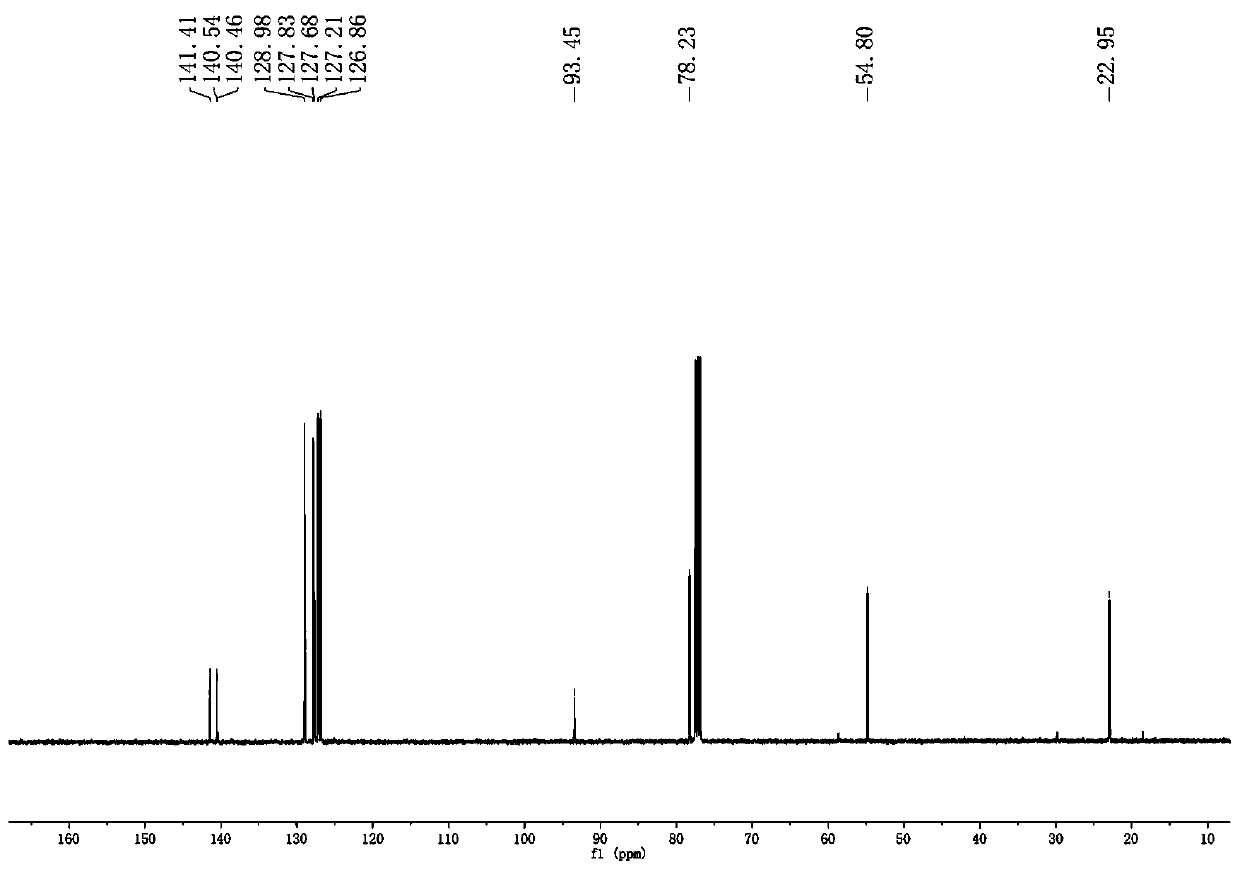
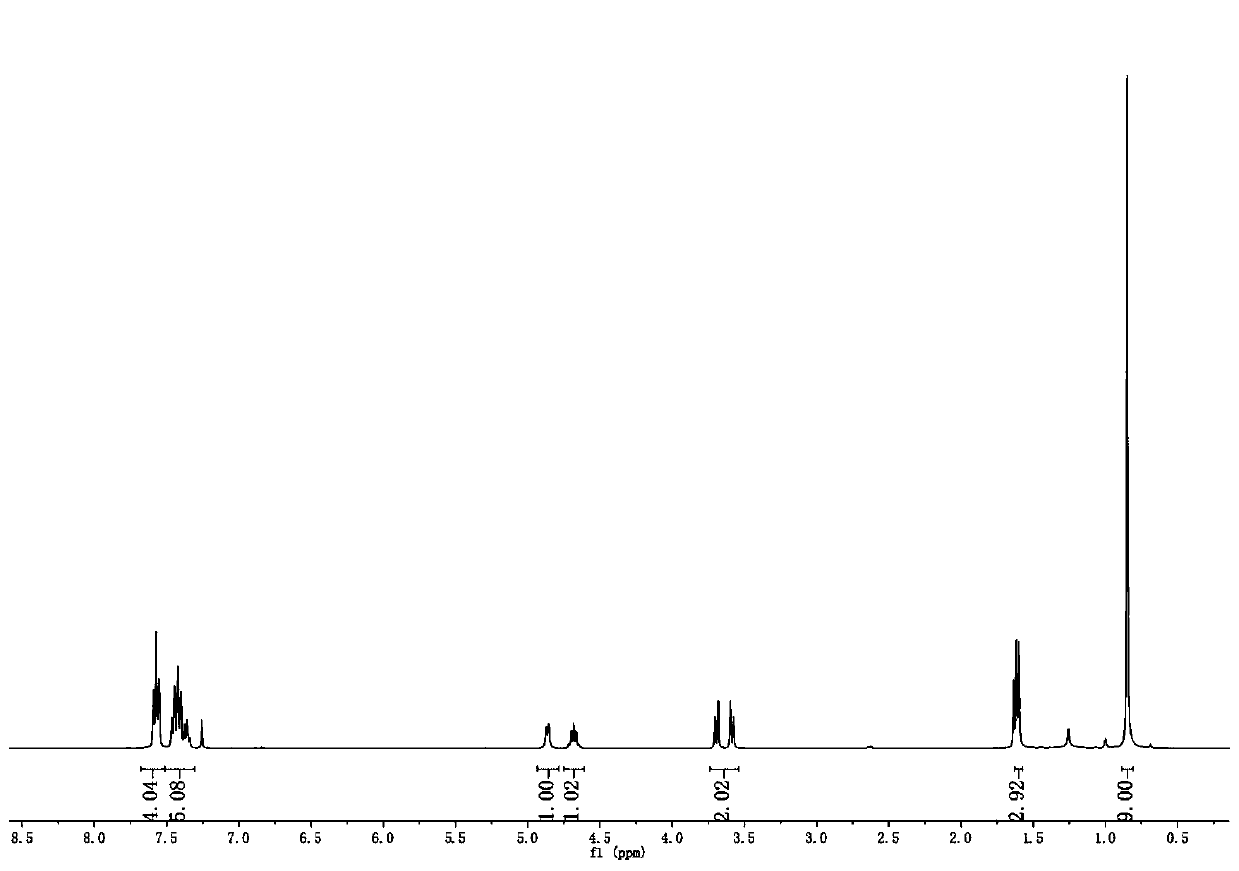
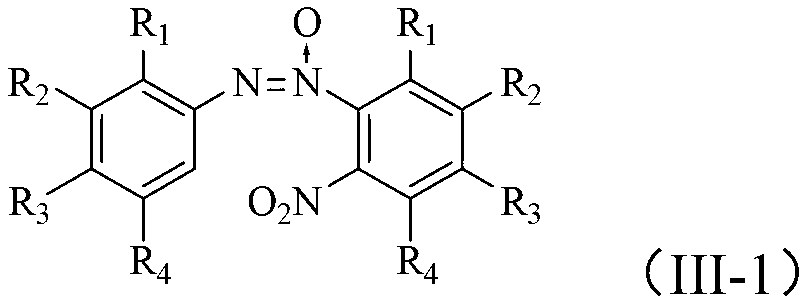
Method for synthesizing benzotriazole compounds
ActiveCN102285926BMild reaction conditionsReaction conditions are green and environmentally friendlyOrganic chemistryEnvironmental resistanceSolvent
The invention discloses a method for synthesizing benzotriazole compounds, which comprises the following steps: subjecting o-nitroazoxy compounds of formula (III) to reduction reaction I under an alkaline condition by using water or an ethanol aqueous solution as a reaction solvent and glucose as a reducer; after full reaction, adding zinc chloride into the reaction system as a catalyst, and adding aluminum powder under an alkaline condition to perform a reduction ring-closure reaction II; and after the reaction is accomplished completely, obtaining the benzotriazole compounds of the formula (IV). The method provided by the method solves the problems of waste acid, lots of waste water and the like from a diazotization reaction, adopts mild reaction condition, is environment-friendly and low in cost and is an economic and practical environment-friendly technical route.
Owner:徐州博创建设发展集团有限公司
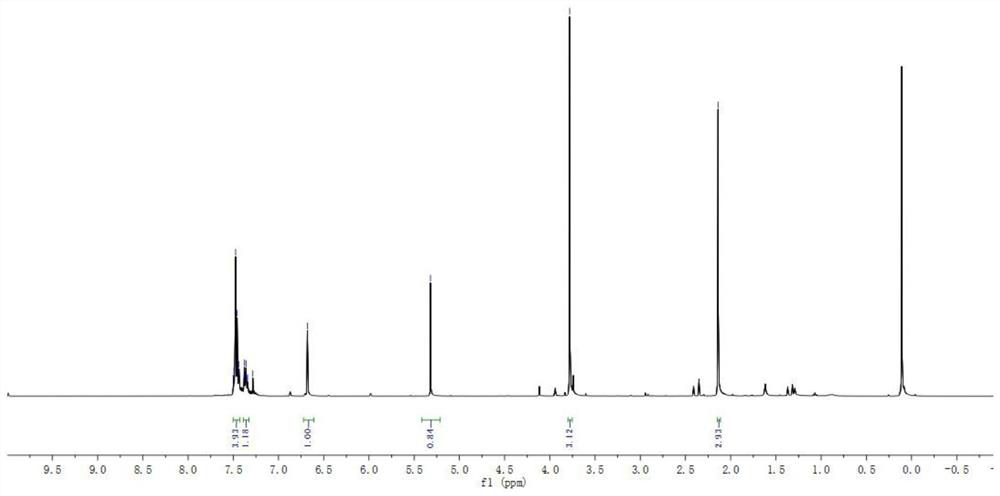
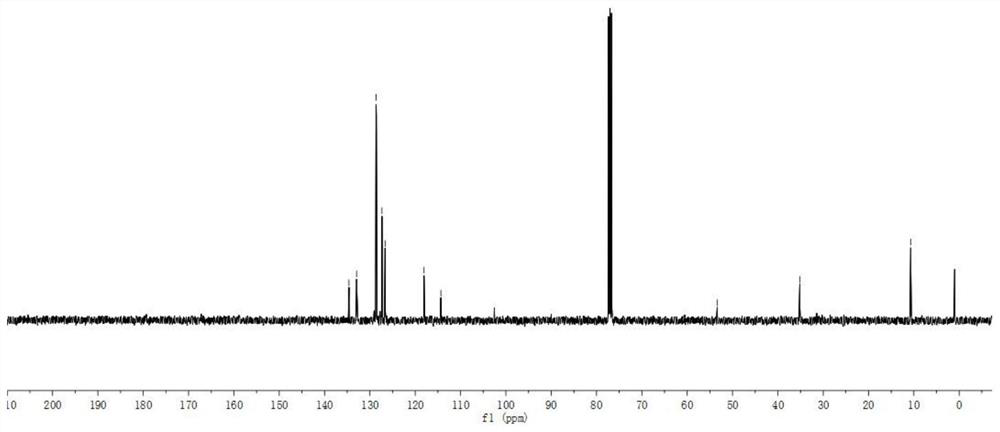
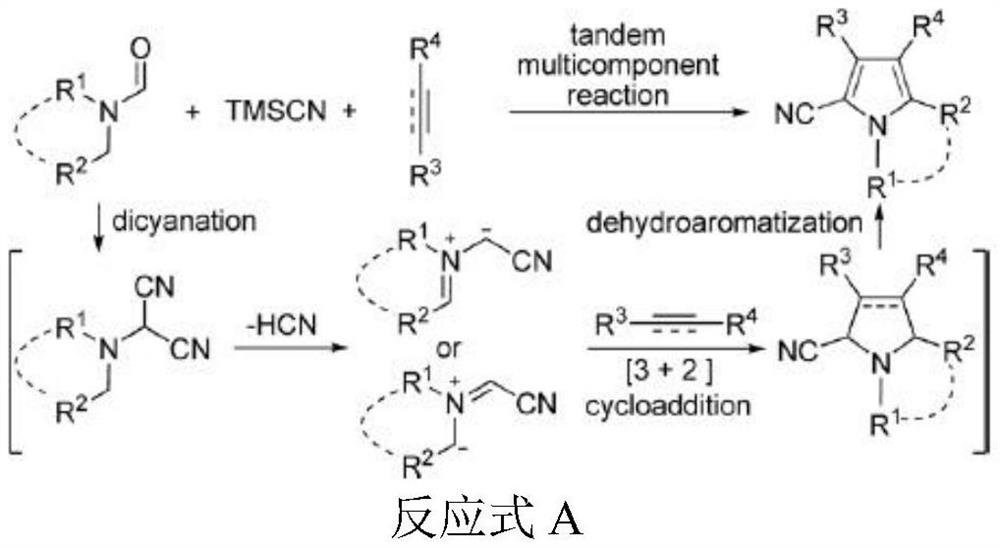
A microwave radiation-assisted synthesis method of n-methyl-2-cyano-3,4-disubstituted pyrrole compound
ActiveCN110713451BHigh yieldReduce the difficulty of catalysisOrganic chemistryPtru catalystSodium iodide
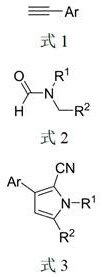
The invention discloses a microwave radiation-assisted synthesis method of N-methyl-2-cyano-3,4-disubstituted pyrrole compound, in which alkyne, trimethylcyanosilane, N,N ‑Dimethylformamide, catalyzed by sodium iodide and assisted by microwaves to carry out a one-pot reaction to generate N‑methyl‑2‑cyano‑3,4‑disubstituted pyrrole compounds. The method has good product selectivity, high yield, simple separation process, low catalyst cost, environmental friendliness, and is beneficial to industrial production and application.
Owner:HUNAN UNIV OF SCI & ENG
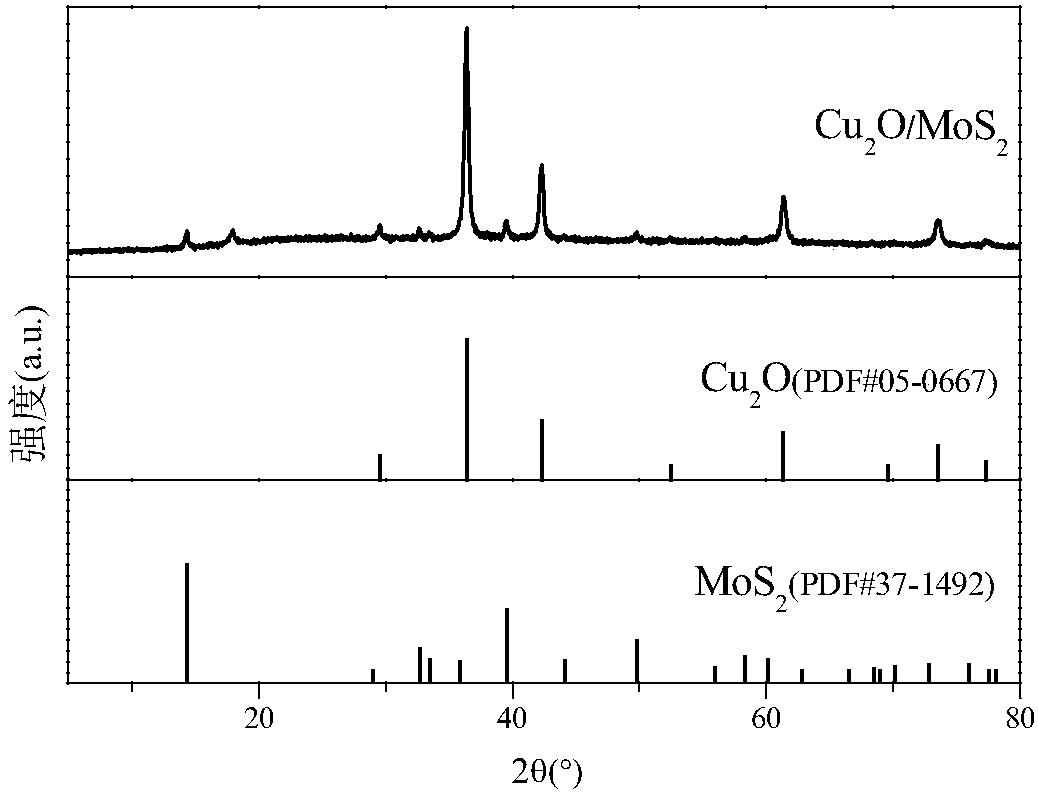
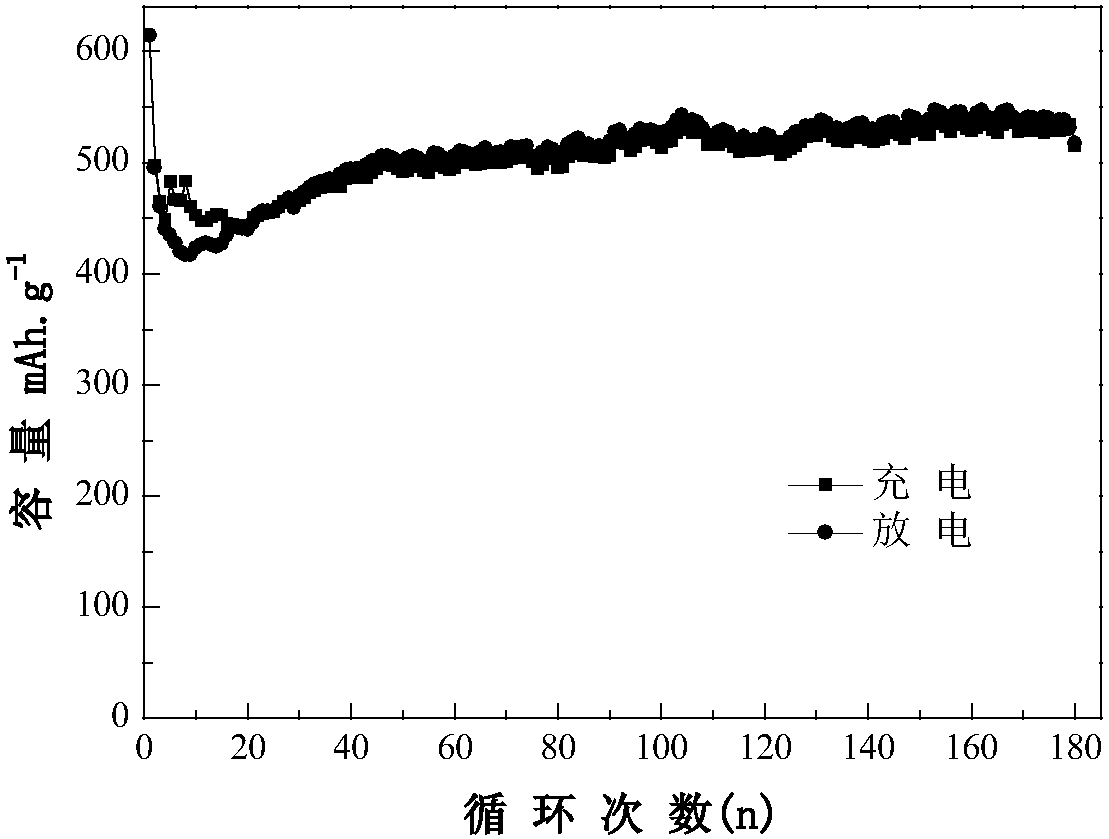
Metal oxide-sulfide composite negative electrode material and preparation method thereof
InactiveCN107611370AReduce manufacturing costEasy to operateMaterial nanotechnologyCell electrodesSulfideRetention ratio
The invention discloses a metal oxide-sulfide composite negative electrode material and a preparation method thereof. Cu2O nanoparticles are uniformly loaded on the surface of a relatively thin MoS2 nanosheet. According to the novel metal oxide-sulfide composite negative electrode material, the Cu2O nanoparticles are relatively uniform in size and regular in shape and form, and can be uniformly loaded on the MoS2 nanosheet through van der waals force. The composite material is used as an active substance of a negative electrode material for a lithium-ion battery, the electrochemical properties(current density: 200mA g<-1> and voltage: 0.01-3V) are tested, and the result shows that the specific capacity and the cycling stability of the composite material are far better than those of a Cu2Omonomer and the capacity retention ratio of the material is extremely high. The preparation method is green, environment-friendly, mild in condition, relatively short in reaction time, easy to operate and suitable for industrial production, and has a broad application prospect.
Owner:SOUTHEAST UNIV
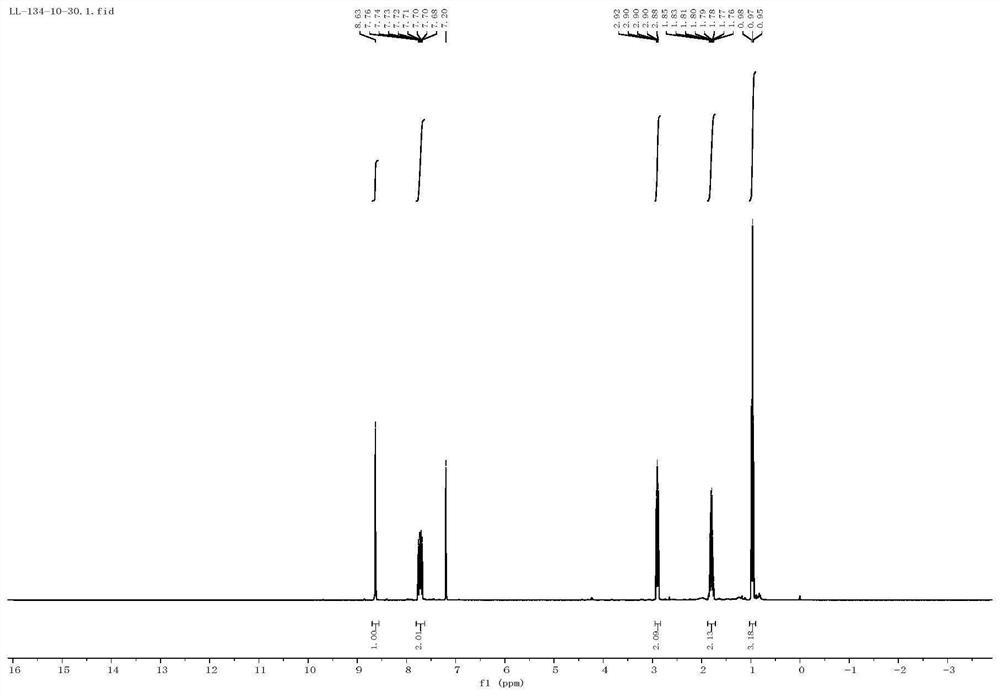
Method for efficiently synthesizing 6-alkylpyrazolo[1,5-c]quinazoline skeleton compounds under non-catalytic conditions
InactiveCN104610267BReaction conditions are green and environmentally friendlyLow costOrganic chemistryNitrostyrolNitrobenzene
Owner:JIANGXI NORMAL UNIV
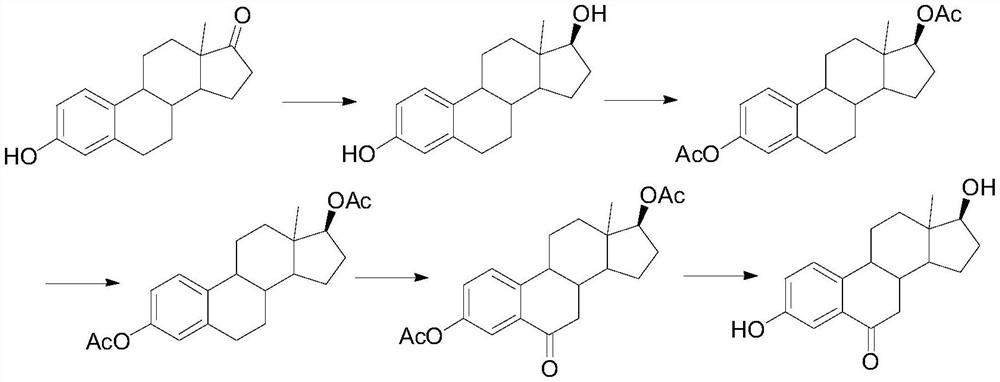
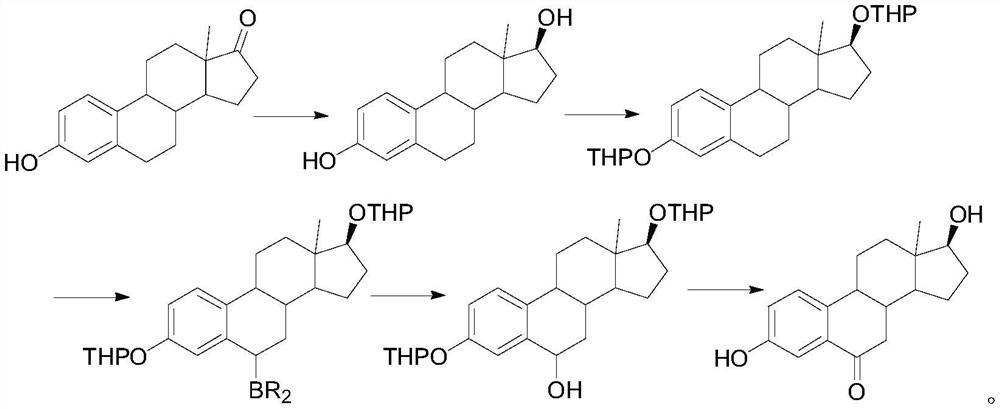
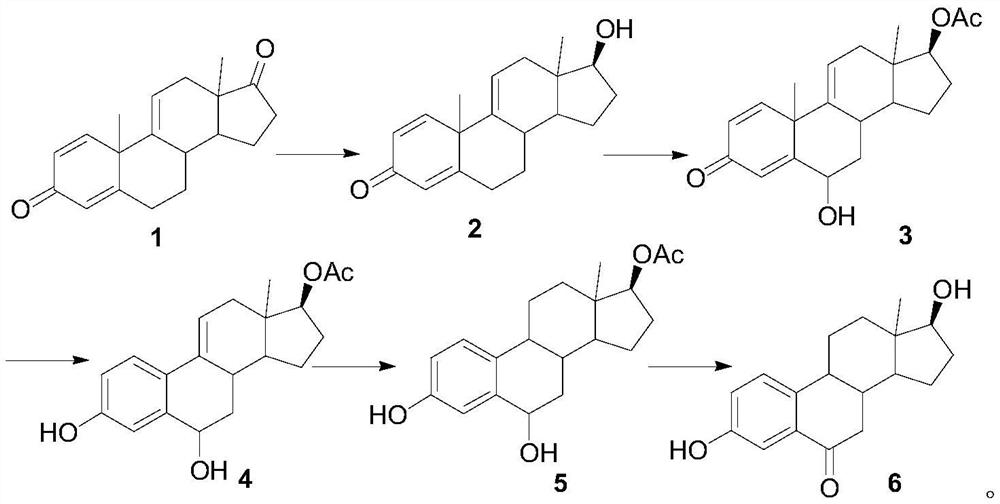
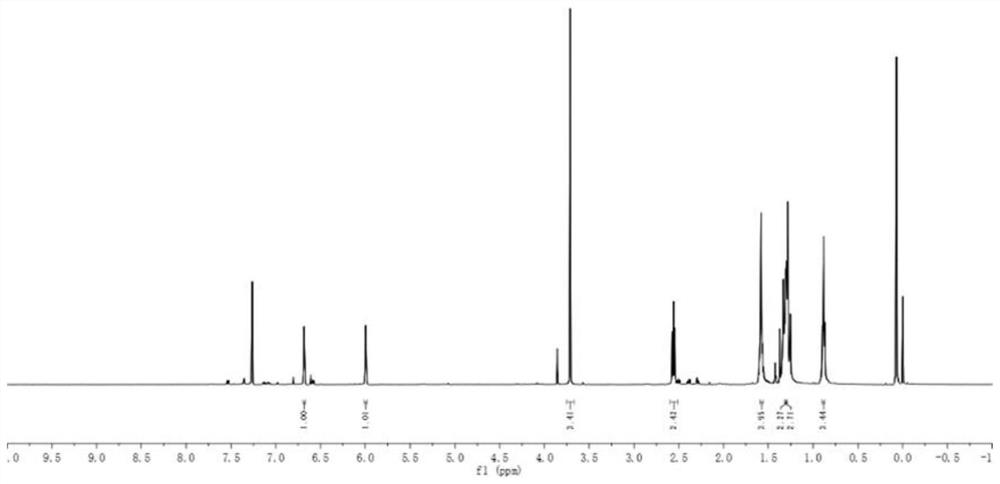
A kind of synthetic method of 6-ketoestradiol
The invention belongs to the technical field of preparation of steroid hormone intermediates, and in particular relates to a synthesis method of 6-ketoestradiol, which uses compound 1 as a raw material, and obtains 6-ketoestradiol through the following reaction route, The reaction route is as follows: the raw material of the present invention is different from estrone, has low price, wide sources and low cost. In the present invention, by optimizing the reaction route, when the 6-position carbonyl is introduced, the 6-position is the only active site, greatly increasing the selectivity of oxidation, and the reaction conditions are also green and environment-friendly. The invention provides a brand-new synthesis route of 6-ketoestradiol, which has mild process conditions, is environmentally friendly, and is suitable for industrial production.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
A kind of synthetic method of 1-methyl-2-cyano-3-aliphatic substituted azacene compound
ActiveCN110746337BHigh yieldReduce the difficulty of catalysisOrganic chemistrySulfite saltAliphatic hydrocarbon
The invention discloses a 1-methyl-2-cyano-3-aliphatic substituted pyrrole compound synthesis method, which comprises: carrying out a three-component addition reaction on terminal aliphatic hydrocarbon, trimethylcyano silane and N,N-dimethylformamide according to an equal chemical molar weight ratio under the catalytic action of ammonium iodide to generate a 1-methyl-2-cyano-3-aliphatic substituted pyrrole compound; and after the reaction is finished, washing the reaction system with saturated sodium sulfite, and extracting the water phase with ethyl acetate to obtain the high-purity 1-methyl-2-cyano-3-aliphatic substituted pyrrole compound. According to the invention, the method is good in product selectivity, high in yield, simple in separation process, low in catalyst cost, environmentally friendly and beneficial to industrial production and application.
Owner:HUNAN BIOLOGICAL & ELECTROMECHANICAL POLYTECHNIC
A microwave synthesis method of 1,2,3,5-tetrasubstituted azocene compounds
ActiveCN110759845BImprove overall utilizationImprove efficiencyOrganic chemistryCombinatorial chemistryAlkyne
The invention discloses a microwave synthesis method for 1,2,3,5-tetrasubstituted azocene compounds, which comprises equimolar amounts of aromatic terminal alkynes, trimethylcyanosilane, formamide compounds, and catalysts Catalyzed reaction assisted by microwave to obtain 1,2,3,5-tetrasubstituted azocene compounds in one pot and one step. The method has good product selectivity, high yield, simple separation process, low catalyst cost, environmental friendliness, and is beneficial to industrial production and application.
Owner:HUNAN UNIV OF SCI & ENG
Catalyst for Knoevenagel condensation reaction of aldehyde and malononitrile and preparation method thereof
ActiveCN114177942AHigh activityReaction conditions are green and environmentally friendlyCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystCarboxylic acid
The invention discloses a catalyst for a Knoevenagel condensation reaction of aldehyde and malononitrile as well as a preparation method and application of the catalyst. The structural formula of the catalyst for the condensation reaction of aldehyde and malononitrile Knoevenagel is as shown in a formula 1, and the catalyst is synthesized by the following steps: placing cobaltous chloride, 3, 2 ', 4'-biphenyltricarboxylic acid, 4, 4 '-dipyridyl and 0.6-1.8 mmol of sodium hydroxide in water, fully stirring, transferring into a reaction kettle with a polytetrafluoroethylene lining, sealing, heating at 130-150 DEG C for 2-3 days, and cooling to room temperature to obtain the catalyst for the condensation reaction of aldehyde and malononitrile Knoevenagel. And turning off the power supply, cooling to room temperature, taking out the mixture in the kettle, washing with water, filtering, drying and separating to obtain the pink blocky crystal catalyst. The synthesis method is simple and environment-friendly, can efficiently catalyze the condensation reaction of aldehyde and malononitrile Knoevenagel in a heterogeneous manner, and has the characteristics of high activity, mild reaction conditions, low catalyst dosage, stable structure, recyclability, wide substrate application range and the like.
Owner:LANZHOU UNIVERSITY
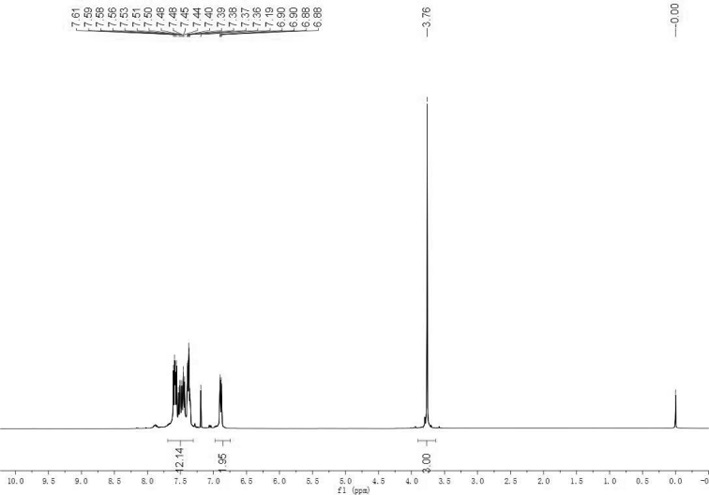
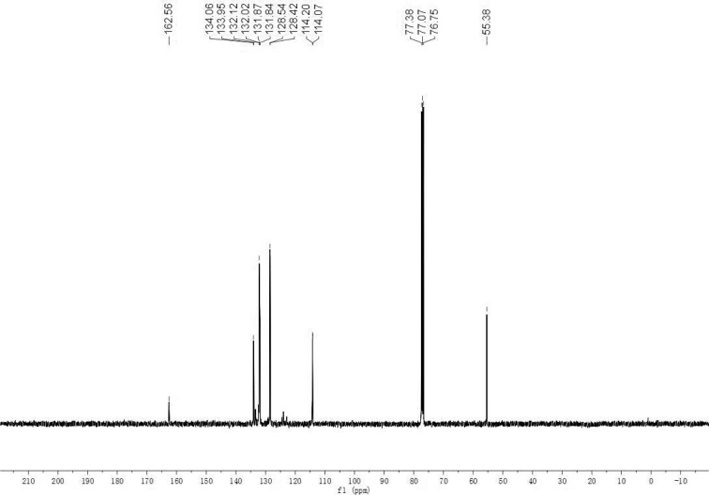
Method for synthesizing trisubstituted arylphosphine oxide species by using diphenyl(tert-butyl)phosphine as substrate
PendingCN113173949ASimple structureHigh reactivityGroup 5/15 element organic compoundsChemical recyclingArylPtru catalyst
The invention relates to a method for synthesizing a trisubstituted arylphosphine oxide species by taking diphenyl(tert-butyl)phosphine as a substrate, belonging to the technical field of organic phosphine synthesis. The method comprises the following steps: with a transition metal palladium salt as a catalyst and a hydrazine compound and diphenyl(tert-butyl)phosphine as reaction substrates, adding the reaction substrates and an alkali compound into a reaction solvent, carrying out stirring and heating, conducting reacting for a certain period of time to obtain a reaction solution, and separating and purifying the reaction solution to obtain a trisubstituted arylphosphine oxide compound, wherein the hydrazine compound is any one selected from aromatic hydrazine and aromatic heterocyclic hydrazine; and the transition metal palladium salt catalyst is selected from any one of Pd(OAc)2, Pd(dba)2 and PdCl2. Compared with a traditional synthesis method, the method has the advantages that the use of diarylphosphine oxide hydrogen is avoided, raw materials are easy to obtain, reaction conditions are mild, yield is high, and reaction selectivity is good. In general, the method is friendly to environment and simple in synthesis process, and has certain industrial application prospects.
Owner:XINXIANG RUNYU NEW MATERIAL TECH CO LTD
Preparation method of 2-aryl benzothiazole compound
PendingCN111072594ASynthetic method is cheapLow costOrganic chemistryAgainst vector-borne diseasesSide productSolvent
The invention discloses a preparation method of a 2-arylbenzothiazole compound, which comprises the following steps: adding a reaction substrate 2-aminothiophenol and ketonic acid(or aldehyde) into adeep eutectic solvent, magnetically stirring in an oil bath pot at 60-90 DEG C, and carrying out after-treatment after the reaction is finished to obtain the 2-arylbenzothiazole compound, wherein a deep eutectic solvent is a reaction solvent, and other catalysts are not needed. The method is simple to operate, mild in reaction condition, efficient in reaction, high in atom economy, few in byproducts and green and environment-friendly in reaction process, and has popularization and application values.
Owner:EAST CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition](https://images-eureka.patsnap.com/patent_img/ef8c07ac-51ca-42e8-a02f-a605cd8029cc/2015100642629100002DEST_PATH_IMAGE002.PNG)
![Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition](https://images-eureka.patsnap.com/patent_img/ef8c07ac-51ca-42e8-a02f-a605cd8029cc/DEST_PATH_IMAGE002.PNG)
![Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition](https://images-eureka.patsnap.com/patent_img/ef8c07ac-51ca-42e8-a02f-a605cd8029cc/DEST_PATH_IMAGE004.PNG)
![Preparation method of 3-aryl-2H-benzo [beta] [1, 4] benzoxazine-2-ketone compound Preparation method of 3-aryl-2H-benzo [beta] [1, 4] benzoxazine-2-ketone compound](https://images-eureka.patsnap.com/patent_img/15fd3ecf-e920-4a57-956c-9d95d30d7066/FDA0002350751770000011.png)
![Preparation method of 3-aryl-2H-benzo [beta] [1, 4] benzoxazine-2-ketone compound Preparation method of 3-aryl-2H-benzo [beta] [1, 4] benzoxazine-2-ketone compound](https://images-eureka.patsnap.com/patent_img/15fd3ecf-e920-4a57-956c-9d95d30d7066/FDA0002350751770000012.png)
![Preparation method of 3-aryl-2H-benzo [beta] [1, 4] benzoxazine-2-ketone compound Preparation method of 3-aryl-2H-benzo [beta] [1, 4] benzoxazine-2-ketone compound](https://images-eureka.patsnap.com/patent_img/15fd3ecf-e920-4a57-956c-9d95d30d7066/BDA0002350751780000021.png)
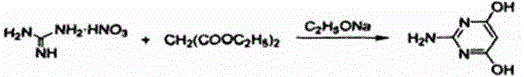
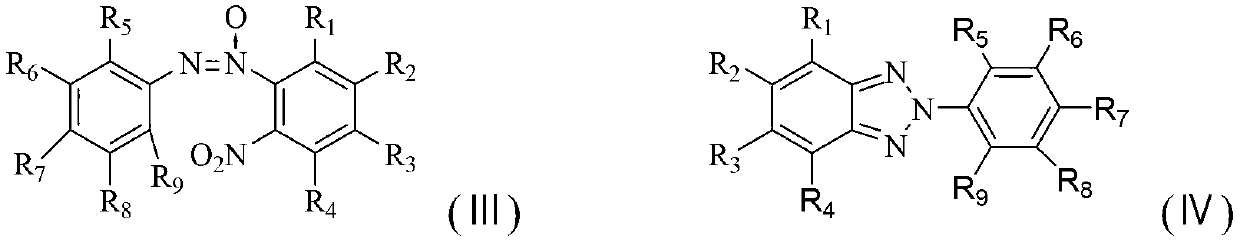
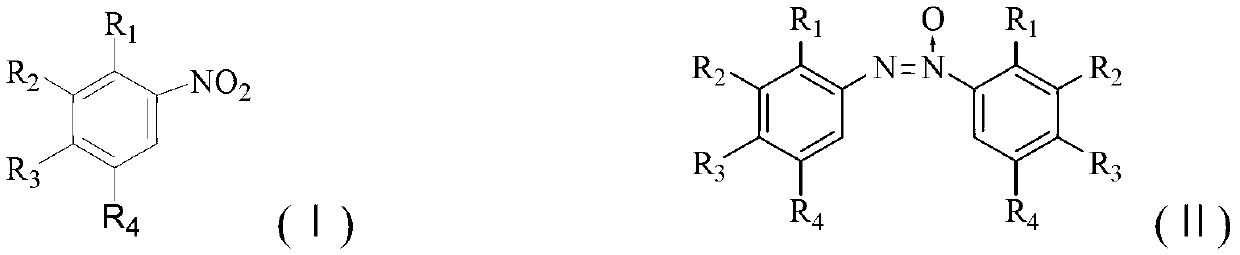
![Method for efficiently synthesizing 6-alkylpyrazolo[1,5-c]quinazoline skeleton compounds under non-catalytic conditions Method for efficiently synthesizing 6-alkylpyrazolo[1,5-c]quinazoline skeleton compounds under non-catalytic conditions](https://images-eureka.patsnap.com/patent_img/4f0ec4c0-9a49-4f62-8d14-c6d2cc9528f5/DEST_PATH_IMAGE002.png)
![Method for efficiently synthesizing 6-alkylpyrazolo[1,5-c]quinazoline skeleton compounds under non-catalytic conditions Method for efficiently synthesizing 6-alkylpyrazolo[1,5-c]quinazoline skeleton compounds under non-catalytic conditions](https://images-eureka.patsnap.com/patent_img/4f0ec4c0-9a49-4f62-8d14-c6d2cc9528f5/DEST_PATH_IMAGE004.png)
![Method for efficiently synthesizing 6-alkylpyrazolo[1,5-c]quinazoline skeleton compounds under non-catalytic conditions Method for efficiently synthesizing 6-alkylpyrazolo[1,5-c]quinazoline skeleton compounds under non-catalytic conditions](https://images-eureka.patsnap.com/patent_img/4f0ec4c0-9a49-4f62-8d14-c6d2cc9528f5/DEST_PATH_IMAGE006.png)