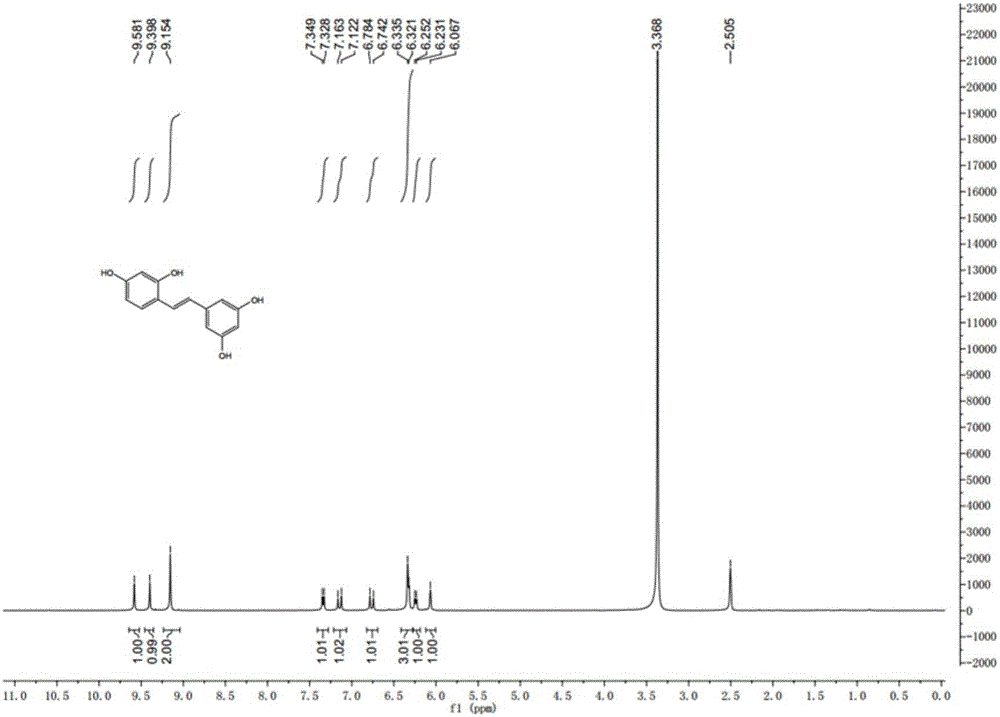
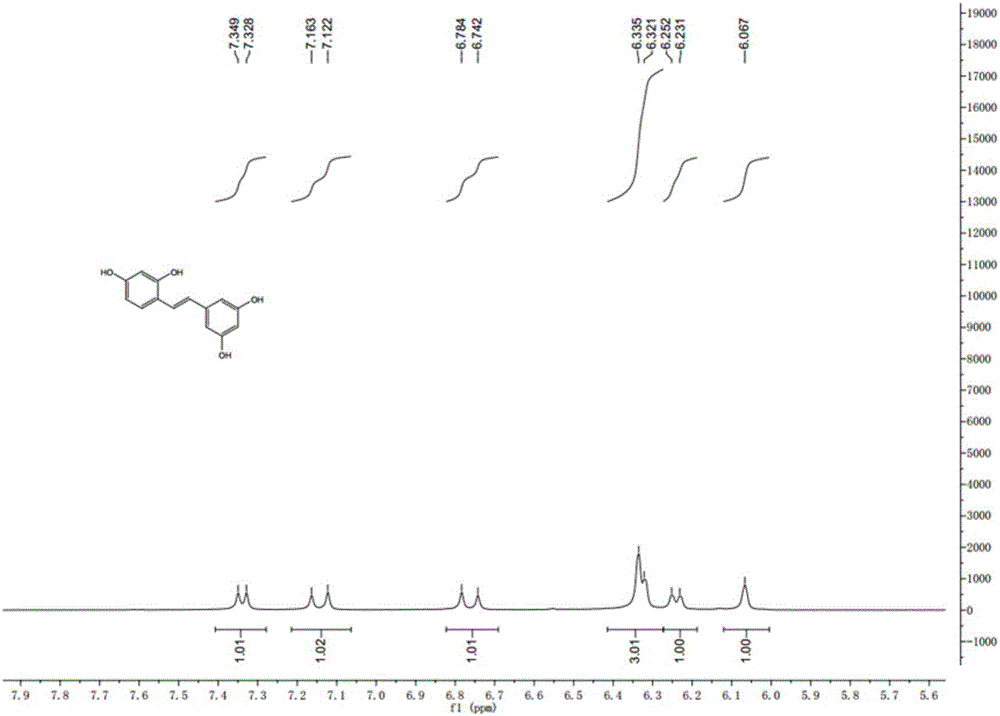
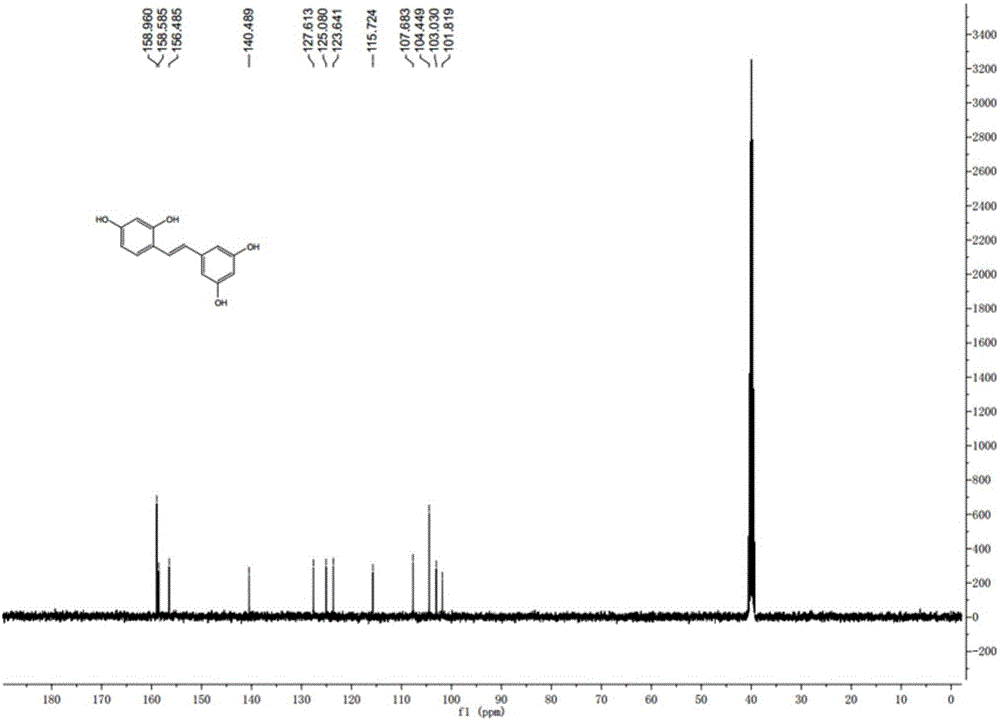
Synthesis method of natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene
A technology of tetrahydroxystilbene and natural products, applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of difficult acquisition of raw materials, difficult large-scale preparation, poor atom economy, etc., and achieve reaction Environmentally friendly conditions, high yield, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of 3,5-dihydroxy-2,4-dimethoxycarbonylphenylacetic acid methyl ester
[0034] Put 1,3-acetonedicarboxylate dimethyl (24.36g, 140mmol) into a single-necked round bottom flask, and then throw in metallic sodium (0.28g, 12mmol), and stir overnight at room temperature. Heated to 140°C, distilled, and reacted for 3h. After the reaction is completed, wash with water and filter with suction to obtain a yellow powder solid, dry it, and recrystallize it with acetone to obtain a light yellow solid, which is methyl 3,5-dihydroxy-2,4-dimethoxycarbonylphenylacetate, weighing 19.74g , yield 95%.
[0035] (2) Preparation of 3,5-dihydroxyphenylacetic acid
[0036] Add 3,5-dihydroxy-2,4-dimethoxycarbonylphenylacetic acid methyl ester (7.15g, 24mmol) into the round bottom flask, then pour 12% sodium hydroxide aqueous solution (64ml, 192mmol), and heat in an oil bath Distill at 140°C for 3h, after the reaction, cool to 80°C, add concentrated sulfuric acid (33.6ml, 240mm...
Embodiment 2
[0042] (1) Preparation of 3,5-dihydroxy-2,4-dimethoxycarbonylphenylacetic acid methyl ester
[0043] Put 1,3-acetonedicarboxylate dimethyl (24.36g, 140mmol) into a single-necked round bottom flask, and then throw in metallic sodium (0.28g, 12mmol), and stir overnight at room temperature. Heated to 140°C, distilled, and reacted for 3h. After the reaction is completed, wash with water and filter with suction to obtain a yellow powder solid, dry it, and recrystallize it with acetone to obtain a light yellow solid, which is methyl 3,5-dihydroxy-2,4-dimethoxycarbonylphenylacetate, weight 19.02g , yield 91%.
[0044] (2) Preparation of 3,5-dihydroxyphenylacetic acid
[0045] Add 3,5-dihydroxy-2,4-dimethoxycarbonylphenylacetic acid methyl ester (7.15g, 24mmol) into the round bottom flask, then pour 12% potassium hydroxide aqueous solution (56ml, 120mmol), and heat in an oil bath Distill at 140°C for 3h. After the reaction, cool to 80°C, add concentrated sulfuric acid (26.9ml, 192m...
Embodiment 3
[0051] (1) Preparation of 3,5-dihydroxy-2,4-dimethoxycarbonylphenylacetic acid methyl ester
[0052] Add 60ml of methanol, sodium metal (0.28g, 12mmol) and dimethyl 1,3-acetonedicarboxylate (24.36g, 140mmol) into a single-necked round bottom flask, and stir overnight at room temperature. Heated to 140°C, distilled, and reacted for 3h. After the reaction is completed, wash with water and filter with suction to obtain a yellow powder solid, dry it, and recrystallize it with acetone to obtain a light yellow solid, which is methyl 3,5-dihydroxy-2,4-dimethoxycarbonylphenylacetate, weight 10.90g , yield 52%.
[0053] (2) Preparation of 3,5-dihydroxyphenylacetic acid
[0054] Drop into 3,5-dihydroxy-2,4-dimethoxycarbonyl phenylacetic acid methyl ester (7.15g, 24mmol) in the round bottom flask, then pour cesium hydroxide (30g, 0.2mol), water (70ml), Heat in an oil bath at 140°C for distillation for 3 hours. After the reaction is complete, cool to 80°C, add concentrated sulfuric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com