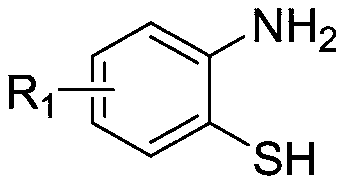
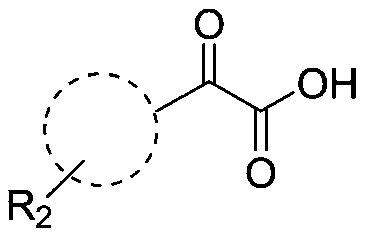
Preparation method of 2-aryl benzothiazole compound
A technology for benzothiazole and compounds, which is applied in the field of preparation of 2-arylbenzothiazole compounds, can solve problems such as insufficient environmental protection of catalysts and limited range of substrates, and achieve cheap synthesis methods, low cost, and few by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of preparation method of 2-aryl benzothiazole compound, its synthetic route is as follows:
[0037]
[0038] Specifically include the following steps:
[0039] (1) Take a 10mL Erlenmeyer flask or test tube, add 0.2mmol 2-aminothiophenol, 0.4mmol benzoylformic acid and 2mL deep eutectic solvent (the molar ratio of choline chloride to tartaric acid is 2:1);
[0040] (2) Put the Erlenmeyer flask or test tube in step (1) into a 60°C oil bath and stir magnetically for 30min to obtain the crude product;
[0041] (3) The crude product obtained in step (2) is separated by column chromatography, the eluent is a mixture of ethyl acetate and petroleum ether, the volume ratio is 1:6, and dried at 30°C for 3 hours to obtain 2-aryl Benzothiazole compounds.
[0042] Structural characterization of the obtained target product, the data are as follows:
[0043] White solid; m, p, 111.9-112.3°C; 1 H NMR (500MHz, CDCl 3 )δ8.12–8.08(m,3H),7.90(d,J=8.0Hz,1H),7.52–7.48(m,4H),7....
Embodiment 2
[0045]
[0046] A kind of preparation method of 2-arylbenzothiazole compound specifically comprises the following steps:
[0047] (1) Take a 10mL Erlenmeyer flask or test tube, add 0.2mmol 2-amino-4 chlorothiophenol, 0.4mmol benzoylformic acid and 2mL deep eutectic solvent (the molar ratio of choline chloride to tartaric acid is 2:1 );
[0048] (2) Put the Erlenmeyer flask or test tube in the step (1) into a 60° C. oil bath and stir magnetically for 30 minutes to obtain the crude product;
[0049] (3) The crude product obtained in step (2) is separated by column chromatography, the eluent is a mixture of ethyl acetate and petroleum ether, the volume ratio is 1:6, and dried at 30°C for 3 hours to obtain 2-aryl Benzothiazole compounds.
[0050] Structural characterization of the obtained target product, the data are as follows:
[0051] White solid; m.p.137-138℃; 1 H NMR (500MHz, CDCl 3 )δ8.09–8.05(m,3H),7.80(d,J=8.5Hz,1H),7.54–7.47(m,3H),7.36(d,J=8.5Hz,1H). 13 C NMR (1...
Embodiment 3
[0053]
[0054] A preparation method of 2-arylbenzothiazole compound, specifically comprising the following steps:
[0055] (1) Take a 10mL Erlenmeyer flask or test tube, add 0.2mmol 2-aminothiophenol, 0.4mmol p-toluoylformic acid and 2mL deep eutectic solvent (the molar ratio of choline chloride to tartaric acid is 2:1 );
[0056] (2) Put the Erlenmeyer flask or test tube in the step (1) into a 60°C oil bath and stir magnetically for 30min to obtain the crude product;;
[0057] (3) The crude product obtained in step (2) is separated by column chromatography, the eluent is a mixture of ethyl acetate and petroleum ether, the volume ratio is 1:6, and dried at 30°C for 3 hours to obtain 2-aryl Benzothiazole compounds.
[0058] Structural characterization of the obtained target product, the data are as follows:
[0059] Light yellow solid; m.p.81.5-81.9℃; 1 H NMR (500MHz, CDCl 3 )δ8.07(d, J=8.1Hz, 1H), 7.99(d, J=8.2Hz, 2H), 7.89(d, J=7.6Hz, 1H), 7.48(t, J=8.3Hz, 1H) ,7.37(t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com