Ternary complex nano-medicine and its preparation method and its application in the preparation of light-controllable release nano-delivery system
A ternary compound and nano-drug technology, which is applied in the fields of polymer material technology and pharmacy, can solve the problems of poor controllability and achieve the effects of cheap raw materials, promoting endocytosis, and improving specific endocytosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
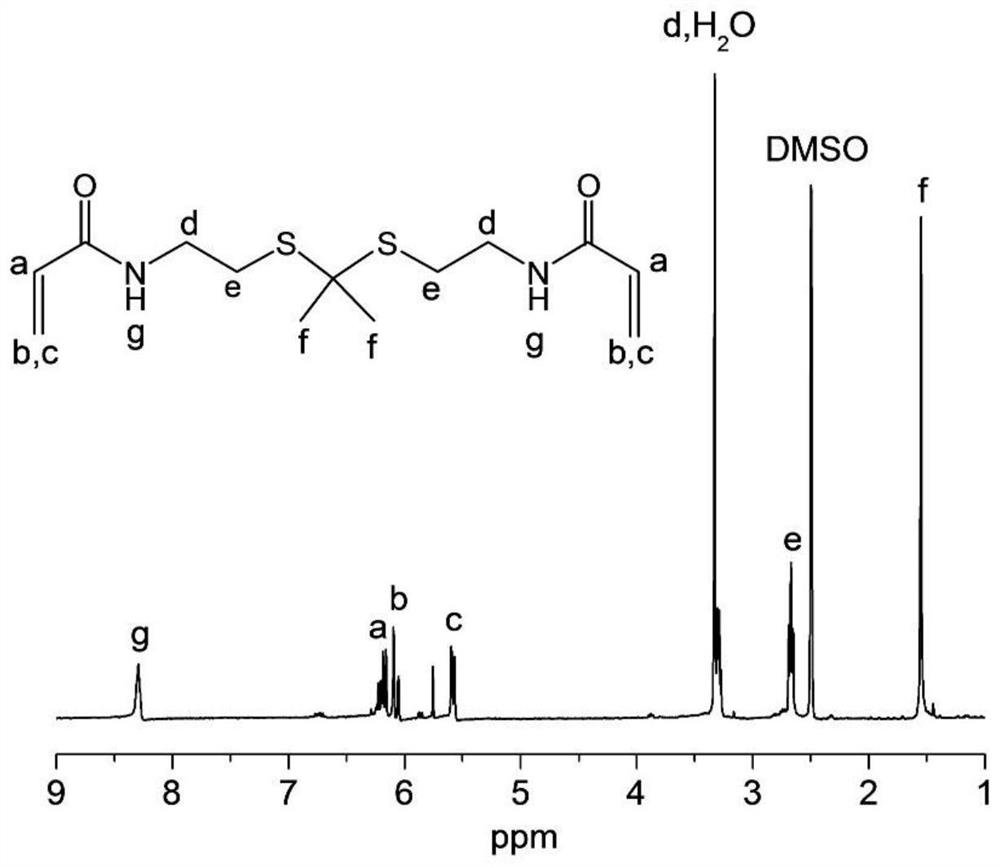
[0068] (1) Cysteamine (20.0 g, 176.0 mmol, 1 equiv) was dissolved in methanol (400 mL) containing triethylamine (35.8 g, 353.9 mmol, 2 equiv). Ethyl trifluoroacetate (26.2 g, 184.8 mmol, 1.05 equiv) was added, and the reaction solution was stirred at room temperature overnight. Acetic acid was then added and the pH was adjusted to 6. The solution was extracted with ethyl acetate (3×100 mL), the combined organic layers were washed with anhydrous MgSO 4 dry. The solution was filtered and rotary evaporated, and the residue was further purified by silica gel column chromatography using n-hexane / ethyl acetate (8 / 1) as the eluent to obtain compound 1 with the following structural formula:
[0069]
[0070] (2) Compound 1 (14.2 g, 82.0 mmol, 2.5 eq) and PTSA (0.2 g, 1.1 mmol, 0.03 eq) were dissolved in benzene (250 mL), which was stirred at room temperature for 10 minutes. Molecular sieves (5 Å, 100.0 g) were added, and the mixture was stirred for an additional 10 min. Then 2-...
Embodiment 2
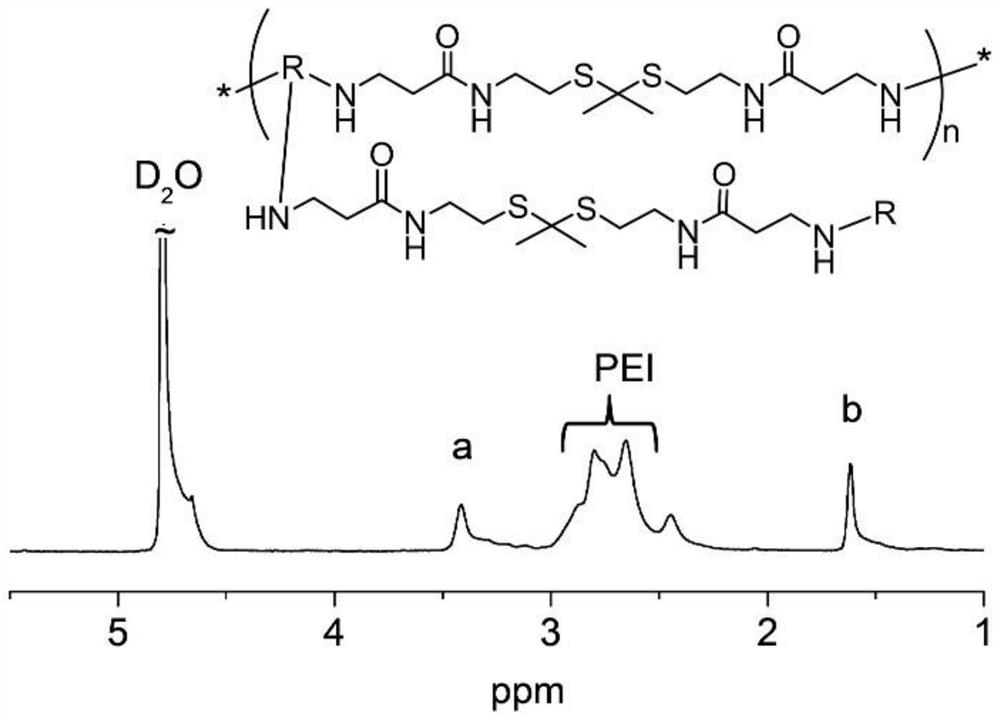
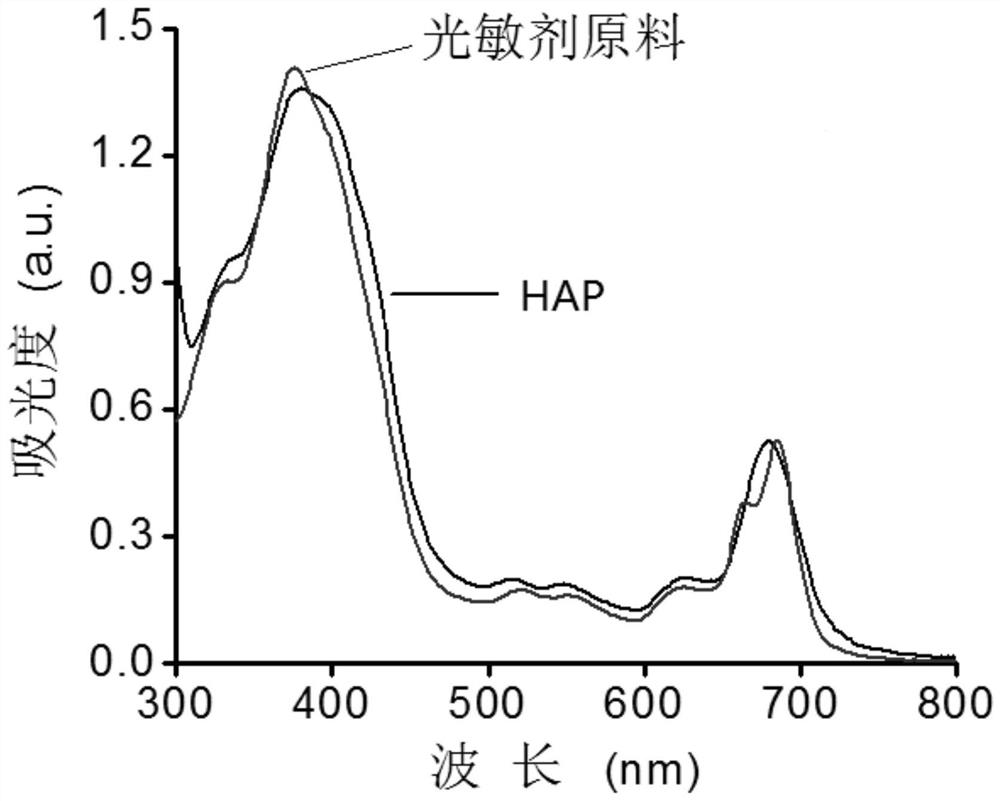
[0078] Compound 4 (25.0 mg) and PEI (600Da) (33.0 mg) were dissolved in 1 mL of methanol and stirred at 45°C in a nitrogen atmosphere protected from light for 48 hours. The mixture was dialyzed against deionized water (deionized water, MWCO = 1kDa) for 2 days and lyophilized to obtain compound 5 (TK-PEI), deuterated heavy water for NMR, figure 2 is the NMR image of compound 5, Figure 4 It is the GPC chart of compound 5, the molecular weight is 11 kDa.
Embodiment 3
[0080] Compound 4 (25.0 mg) and PEI (1800Da) (33.0 mg) were dissolved in 1 mL of methanol and stirred at 45°C in a dark nitrogen atmosphere for 48 hours. The mixture was dialyzed against deionized water (DI water, MWCO = 1 kDa) for 2 days and lyophilized to give compound 5 (TK-PEI) with a molecular weight of 18 kDa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com