Use of protein in preparation of drugs for prevention and treatment of Alzheimer's disease
A technology for Alzheimer's disease, uses, application in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of embodiment 1 sTREM2 protein
[0046] Construction of pFUSE-sTREM2-TEV-hIgG1-Fc1 expression plasmid: pFUSE-hIgG1-Fc1 (available for purchase) was selected as the eukaryotic expression vector, and EcoR Ⅰ and Xho Ⅰ restriction sites were selected as the cloning site to construct pFUSE-sTREM2-hIgG1- Fcl expression plasmid. The steps are as follows: Design upstream primer: 5'-CG GAATTC ATGGAGCCTCTCCGGCTGC-3'SEQ ID NO: 5 where the underline is the EcoR Ⅰ restriction site;
[0047] Downstream primer: 5'-CCG CTCGAG TT TGGGAAGGGGATTTCTC-3'SEQ ID NO: 6, where the underline is the Xho I restriction site; where the double underline corresponds to the coding sequence of the TEV restriction site: GAAAACCTGTATTTTTCAGGGA SEQ ID NO: 7, and the amino acid sequence of the TEV restriction site is: ENLYFQG SEQ ID NO: 8.
[0048] The nucleotide sequence of TREM2 is (purchased from Beijing Yiqiao Sino Biotechnology Co., Ltd., article number HG11084-M):
[0049] ATGGAGC...
Embodiment 2
[0053] Example 2 Preparation of sTREM2-Fc fusion protein
[0054] Construction of pFUSE-sTREM2-hIgG1-Fc1 expression plasmid: pFUSE-hIgG1-Fc1 was selected as the eukaryotic expression vector, and EcoR Ⅰ and Xho Ⅰ restriction sites were selected as the cloning site to construct the pFUSE-sTREM2-hIgG1-Fc1 expression plasmid. The steps are as follows: Design upstream primer: 5'-CG GAATTC ATGGAGCCTCTCCGGCTGC-3'SEQ ID NO: 5 where the underline is the EcoRI restriction site;
[0055] Downstream primer: 5'-CCG CTCGAG TTTGGGAAGGGGATTTCTC-3'SEQ ID NO: 9 where the underline is the XhoI restriction site;
[0056] Using cDNA (SEQ ID NO: 4) as a template, it can be artificially synthesized, and the sTREM2 expression gene can be cloned by PCR. Gel recovery and purification of the target product, and then restriction endonucleases EcoR Ⅰ and Xho Ⅰ digest pFUSE-hIgG1-Fc1 plasmid, and gel recovery of large fragments. The PCR product was digested with the same enzyme, and the digested prod...
Embodiment 3
[0058] Example 3 Identification of sTREM2 protein and sTREM2-Fc fusion protein
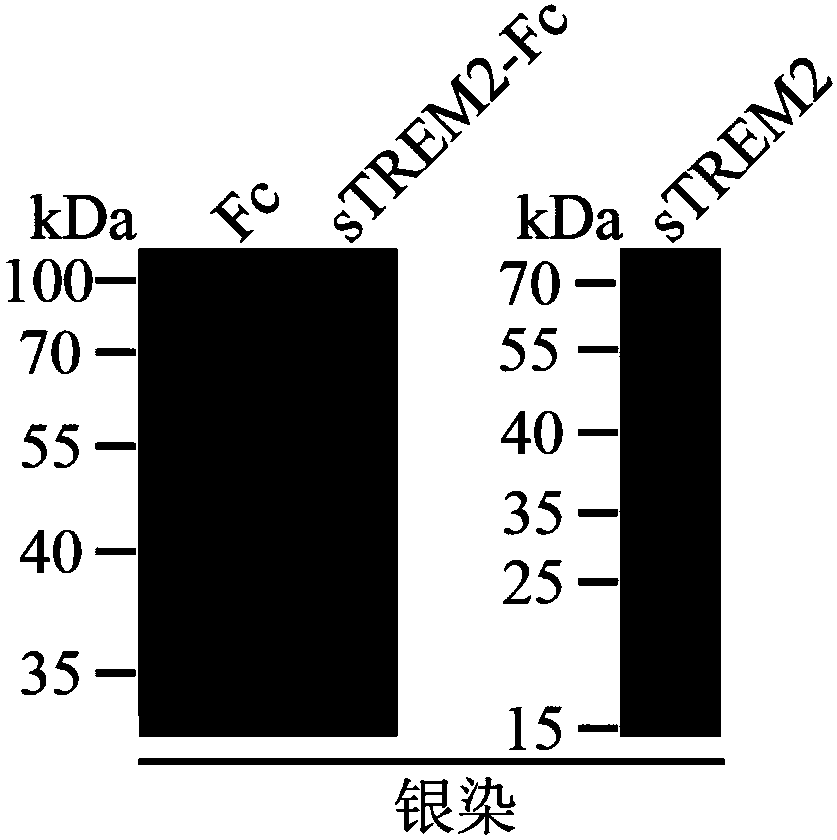
[0059] Identification of protein purity: The sTREM2-Fc fusion protein obtained in Example 2 and the sTREM2 protein obtained in Example 1 were subjected to SDS-PAGE electrophoresis, followed by silver staining to identify their purity. Identification results such as image 3 As shown, the sTREM2-Fc fusion protein and sTREM2 protein have single bands and high purity.
[0060] Identification of protein types: The sTREM2-Fc fusion protein obtained in Example 2 and the sTREM2 protein obtained in Example 1 were transferred to PVDF membrane after SDS-PAGE electrophoresis, and an antibody specifically recognizing the extracellular region of human TREM2 (R&Dsystems, AF1828) were identified by western blot. Identification results such as Figure 4 As shown, both sTREM2-Fc fusion protein and sTREM2 can be recognized by the antibody that recognizes human TREM2, indicating that the constructed protein is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com