A microwave synthesis method of 1,2,3,5-tetrasubstituted azocene compounds
A compound and azecene technology, which is applied in the field of microwave synthesis of 1,2,3,5-tetra-substituted azecene compounds, can solve the problems of low reaction efficiency, low atom utilization, and inability to popularize and apply industrially.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
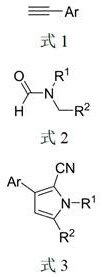
[0056] Following examples 1~4 all react by following reaction equation:
[0057]
[0058] The specific operation steps are: in a 50mL round bottom flask, add alkynes (30mmol), trimethylcyanosilane (30mmol), formamides (30mmol) and ammonium iodide (3mmol) successively, and the resulting mixture is heated in a microwave 400W in the reactor, stirring and reacting for 10 minutes under the condition of temperature control of 100°C. After the reaction, 30 mL of ethyl acetate was used to dissolve the reactant, the solution was washed with saturated brine, the liquid was separated, the organic phase liquid was concentrated in vacuo, dried in vacuo to calculate the weight.
Embodiment 1
[0060] raw material: TMSCN,
[0061] Target product:
[0062] Yield: 94%.
[0063] 1-ethyl-5-methyl-3-phenyl-1H-pyrrole-2-carbonitrile
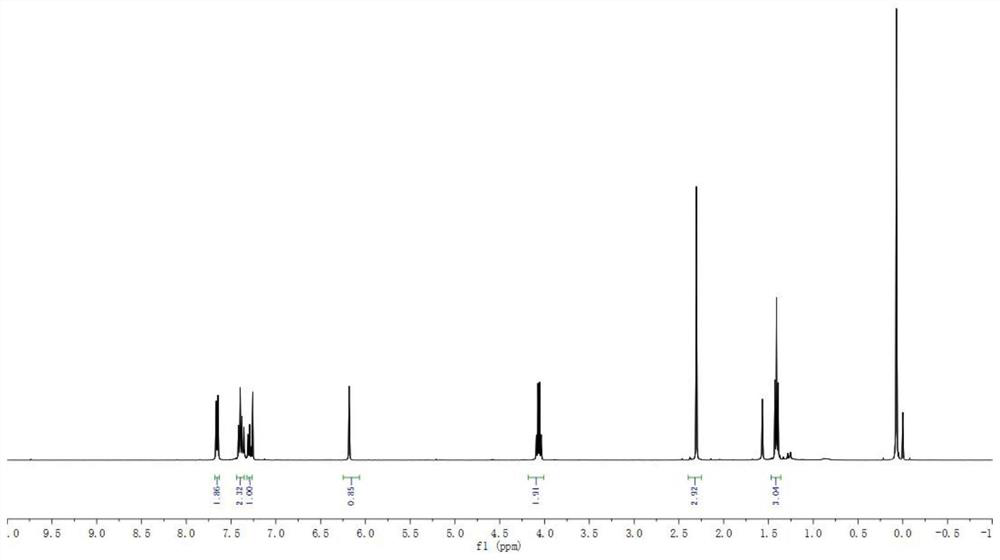
[0064] 1 H NMR (400MHz, CDCl 3 )δ7.69-7.62(m,2H),7.40(dd,J=15.9,8.1Hz,2H),7.29(t,J=7.4Hz,1H),6.17(s,1H),4.06(q,J =7.3Hz, 2H), 2.31(s, 3H), 1.42(t, J=7.3Hz, 3H).
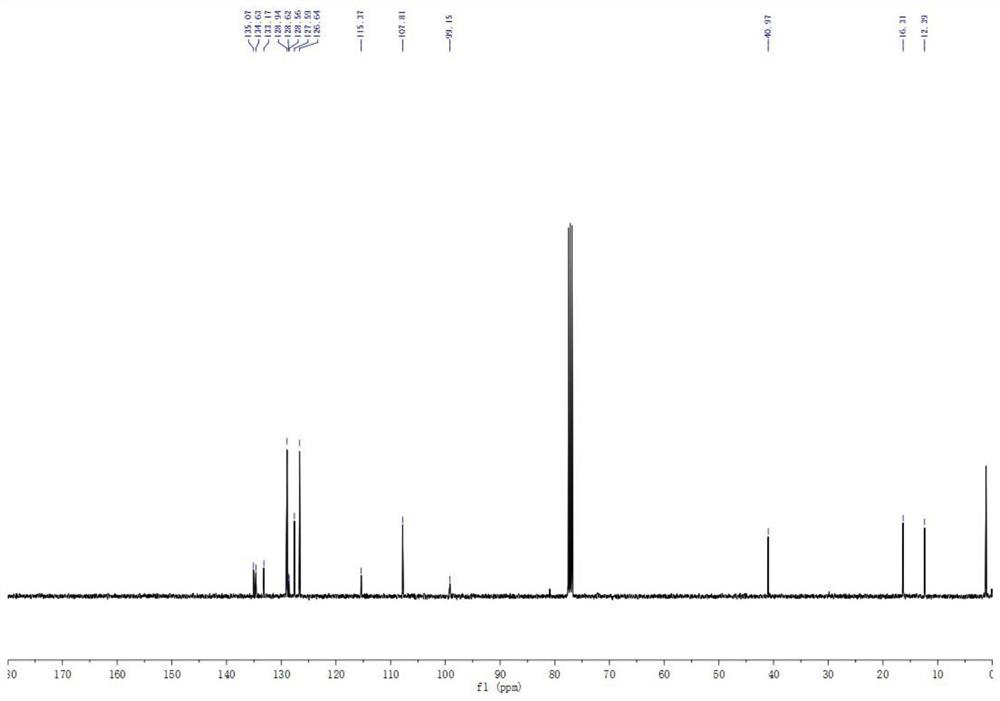
[0065] 13 C NMR (100MHz, CDCl 3 )δ135.1, 134.6133.2, 128.9, 128.6, 128.5, 127.6, 126.6, 115.4, 107.8, 99.2, 40.9, 16.3, 12.4.
Embodiment 2
[0067] raw material: TMSCN,
[0068] Target product:
[0069] 5-ethyl-3-phenyl-1-propyl-1H-pyrrole-2-carbonitrile
[0070] Yield: 95%.
[0071] 1 H NMR (400MHz, CDCl 3 )δ7.67(dd, J=8.4,1.6Hz,2H),7.39(t,J=7.2Hz,2H),7.36(s,1H),6.20(s,1H),3.98(t,J=7.6 Hz, 2H), 2.55(t, J=7.2Hz, 2H), 1.77-1.70(m, 4H), 1.44-1.40(m, 2H), 1.04(t, J=7.6Hz, 3H), 0.98(t ,J=7.6Hz,3H);
[0072] 13 C NMR (100MHz, CDCl 3 )δ134.3, 133.1, 128.8, 128.4, 128.4, 127.4, 126.5, 115.4, 106.4, 99.4, 45.7, 33.2, 28.4, 21.7, 19.9, 13.9, 13.7.
[0073] HRMS (ESI) calcd for C 16 h 19 N 2 [M+H] + :239.1548, found 239.1550.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com