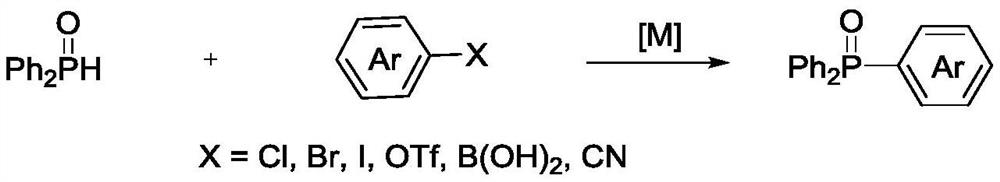Method for synthesizing trisubstituted arylphosphine oxide species by using diphenyl(tert-butyl)phosphine as substrate
A technology of diphenyl tertiary and aryl phosphine oxides, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., to reduce synthesis costs, avoid the use of precious metals, and achieve high yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
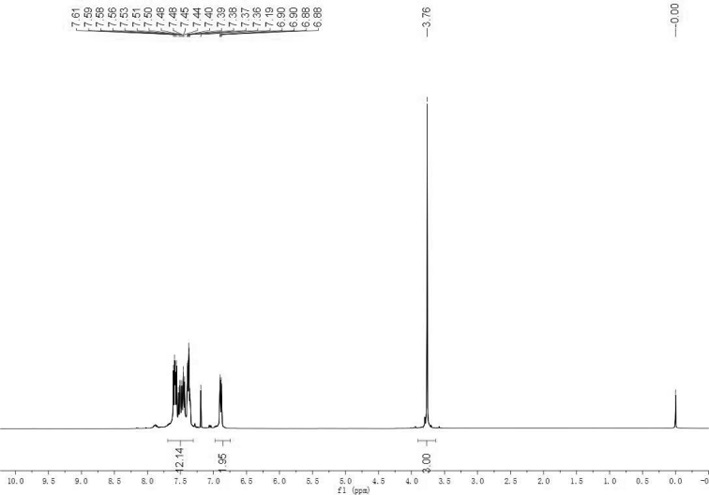
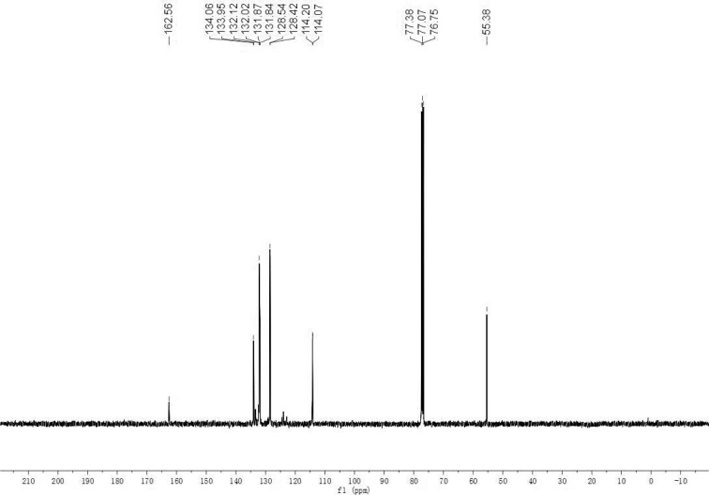
[0020] In a 25mL Schlenk tube, put 0.2mmol (48mg) of diphenyl tert-butylphosphine, 0.6mmol (83mg) of p-methoxyphenylhydrazine, 2.3mg of palladium acetate (5mmol%), 0.6mmol of sodium carbonate (64mg) and 2mL of water, fill the Shrek tube with oxygen; heat up to 100°C with stirring, stop the reaction after 24 hours, cool to room temperature and extract the organic matter with dichloromethane, spin the dichloromethane extract to dryness and pass through column chromatography 46 mg of white solid was isolated. The structure of the product was determined to be (4-methoxyphenyl)diphenylphosphine oxide by NMR, and the yield was 75%.
[0021] Different hydrazine compounds, palladium salt catalysts and different reaction conditions were selected, and trisubstituted arylphosphine oxide species were synthesized by the same method as in Example 1. The reaction results are shown in Table 1.
[0022] Table 1 Catalyzed synthesis of three substituted arylphosphine oxide species reaction resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com