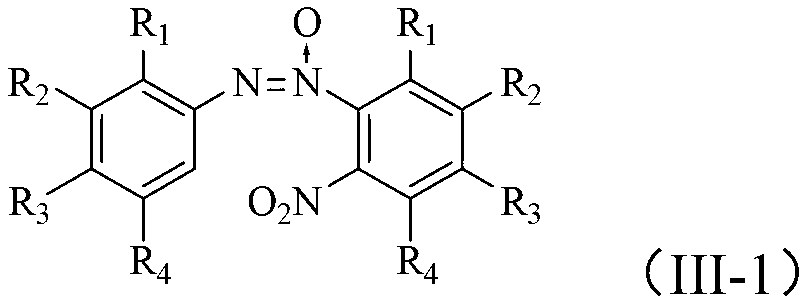
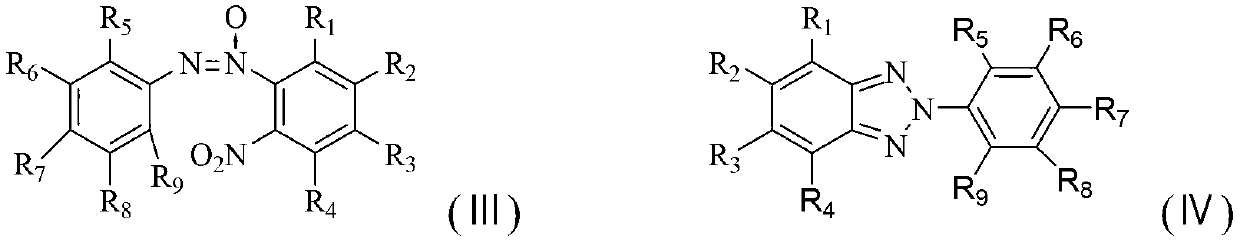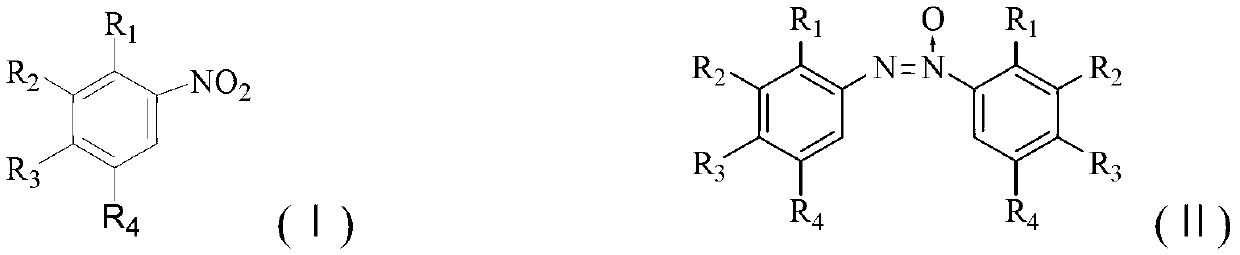Method for synthesizing benzotriazole compounds
A technology of benzotriazoles and synthetic methods, which is applied in the direction of organic chemistry, can solve the problems of uncontrollable reactions and ineffective effects, and achieve the effects of mild reaction conditions, low cost, and avoiding waste acid and a large amount of waste water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of 4,4'-dichloroazooxybenzene
[0037] Add 9.42g (60mmol) of p-nitrochlorobenzene, 30mL of 95% ethanol, and 6g (30mmol) of 20% NaOH solution in a three-neck flask, heat to 55°C, and add dropwise 77.1g of a 21% glucose solution (90mmol), reacted for 4 hours, cooled, added water, and suction filtered to obtain 7.82g of solid, with a yield of 97.6%. The measured melting point was 154-155°C (lit.153-154°C). MS: m / z=268.
Embodiment 2
[0038] Example 2 Synthesis of 4,4'-dimethylazooxybenzene
[0039] Add 1.37g (10mmol) of p-nitrotoluene, 5mL of 95% ethanol, and 1.2g (6mmol) of a 20% NaOH solution in a three-neck flask, heat to 55°C, and add dropwise 12.8g of a 21% glucose solution (15 mmol), reacted for 4 hours, cooled, added water, and filtered with suction to obtain 1.05 g of solid, with a yield of 92.6%. The measured melting point was 61-62°C (lit. 61.1-62.0°C). MS: m / z=227.
Embodiment 3
[0040] Example 3 Synthesis of 4,4'-diacetyl azooxybenzene
[0041] Add 1.65g (10mmol) of p-nitroacetophenone, 5mL of 95% ethanol, and 1.2g (6mmol) of 20% NaOH solution in a three-necked flask, heat to 55°C, and add dropwise a glucose solution with a mass fraction of 21%. 12.8g (15mmol), reacted for 4 hours, cooled, added water, and suction filtered to obtain 2.65g of solid, with a yield of 93.7%. The measured melting point was 191-192°C (lit. 190.8-191.5°C). MS: m / z=282.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com