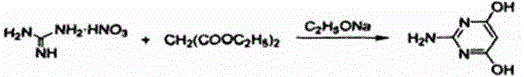Synthesis method of 2-amino-4, 6-dihydroxypyrimidine
A technology of dihydroxypyrimidine and amino group is applied in the field of synthesizing 2-amino-4,6-dihydroxypyrimidine, which can solve problems such as low yield and achieve the effects of improving purity, preventing decomposition and wide application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The method for synthesizing 2-amino-4,6-dihydroxypyrimidine in this example uses guanidine nitrate and diethyl malonate as raw materials for a one-step cyclization reaction. The specific steps of the synthesis method are as follows:
[0020] (1) Add 1.1 mol of 99% NaOH solid to 1 mol of 98% ethanol solution, and add 0.3 mol of 99% benzene to carry out azeotropic distillation to remove water, and finally remove Benzene, the sodium ethylate solution that obtains molar concentration is 2.8mol / L;
[0021] (2) Heat 1 mol of ammonium nitrate with a mass percentage concentration of 99% and 1.5 mol of dicyandiamide with a mass percentage concentration of 99% to a molten state, and then feed in liquid ammonia with a mass percentage concentration of 99%. Down heat preservation reaction, the temperature of heat preservation reaction is 200 ℃, and the reaction time is 30min, makes guanidine nitrate;
[0022] (3) Put 150 mL of 2.8 mol / L sodium ethoxide solution, 0.17 mol of 6.54 mo...
Embodiment 2
[0025] The method for synthesizing 2-amino-4,6-dihydroxypyrimidine in this example uses guanidine nitrate and diethyl malonate as raw materials for a one-step cyclization reaction. The specific steps of the synthesis method are as follows:
[0026] (1) Add 1.1 mol of 99% NaOH solid to 1 mol of 98% ethanol solution, and add 0.3 mol of 99% benzene to carry out azeotropic distillation to remove water, and finally remove Benzene, the sodium ethylate solution that obtains molar concentration is 2.8mol / L;
[0027] (2) Heat 1 mol of ammonium nitrate with a mass percentage concentration of 99% and 1.5 mol of dicyandiamide with a mass percentage concentration of 99% to a molten state, and then feed in liquid ammonia with a mass percentage concentration of 99%. Down heat preservation reaction, the temperature of heat preservation reaction is 180 ℃, and the reaction time is 45min, makes guanidine nitrate;
[0028] (3) Put 150 mL of 2.8 mol / L sodium ethoxide solution, 0.17 mol of 6.54 mo...
Embodiment 3
[0031] The method for synthesizing 2-amino-4,6-dihydroxypyrimidine in this example uses guanidine nitrate and diethyl malonate as raw materials for a one-step cyclization reaction. The specific steps of the synthesis method are as follows:
[0032] (1) Add 1.1 mol of 99% NaOH solid to 1 mol of 98% ethanol solution, and add 0.3 mol of 99% benzene to carry out azeotropic distillation to remove water, and finally remove Benzene, the sodium ethylate solution that obtains molar concentration is 2.8mol / L;
[0033] (2) Heat 1 mol of ammonium nitrate with a mass percentage concentration of 99% and 1.5 mol of dicyandiamide with a mass percentage concentration of 99% to a molten state, and then feed in liquid ammonia with a mass percentage concentration of 99%. Down heat preservation reaction, the temperature of heat preservation reaction is 190 ℃, and the reaction time is 38min, makes guanidine nitrate;
[0034] (3) Put 150 mL of 2.8 mol / L sodium ethoxide solution, 0.17 mol of 6.54 mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com