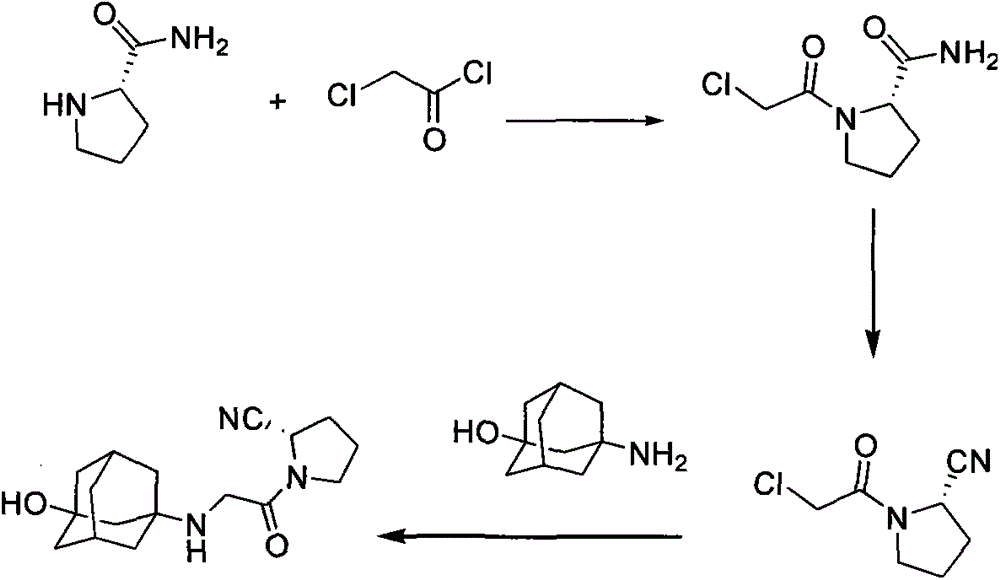
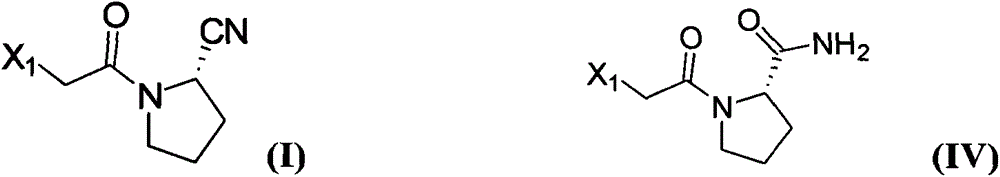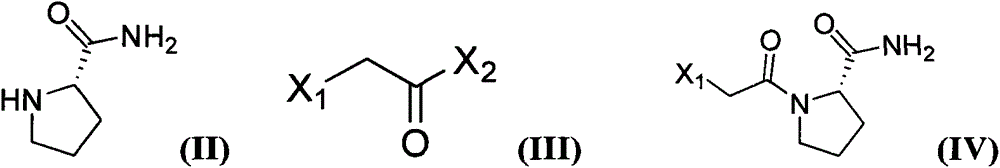Method for preparing substituted (S)-pyrrolidine-2-formonitrile and vildagliptin
A technology of pyrrolidine and nitrile, which is applied in the field of medicine, can solve the problems of product residue, high price, and difficult operation, and achieve the effects of environmental protection, increased yield, and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Method A
[0061] Synthesis of (S)-1-(2-chloroacetyl)pyrrolidine-2-carboxamide
[0062] Compound S-proline amide (11.2g, 100mmol) was added to compound chloroacetyl chloride (11.5g, 100mmol) tetrahydrofuran (200mL) solution pre-cooled to 0°C, the solution was kept at 0°C and stirred for 30 minutes, potassium carbonate (27.3 g, 200 mmol) was added into the mixed solution, and the resulting mixed solution was stirred at 0° C. for 1 hour, and then the reaction solution was warmed up to room temperature and stirred for 3 hours.
[0063] The end of the reaction was detected by TLC. After the compound S-prolinamide completely disappeared, the reaction system was filtered, the filter cake was washed with tetrahydrofuran (50 mL), and the filtrates were combined. The solvent was rotary evaporated to dryness, and the residual oil was dissolved in ethyl acetate. The obtained ethyl acetate solution was washed with water (50 mL×2), and dried over anhydrous sodium sulfate for 2 hou...
Embodiment 2
[0065] Method B
[0066] Synthesis of (S)-1-(2-bromoacetyl)pyrrolidine-2-carboxamide
[0067] Compound S-prolinamide (11.2g, 100mmol) was added to bromoacetyl bromide (20.0g, 100mmol) tetrahydrofuran (200mL) solution pre-cooled to 0°C, and the solution was stirred at 0°C for 30 minutes, potassium carbonate (27.3g, 200 mmol) was added into the mixed solution, and the resulting mixed solution was stirred at 0° C. for 1 hour, and then the reaction solution was warmed up to room temperature and stirred for 3 hours.
[0068] The end of the reaction was detected by TLC. After compound 2 disappeared completely, the reaction system was filtered, the filter cake was washed with tetrahydrofuran (50 mL), and the filtrates were combined. The solvent was rotary evaporated to dryness, and the residual oil was dissolved in ethyl acetate. The obtained ethyl acetate solution was washed with water (50 mL×2), and dried over anhydrous sodium sulfate for 2 hours. After removing the desiccant, i...
Embodiment 3
[0070] Method C
[0071] Synthesis of (S)-1-(2-bromoacetyl)pyrrolidine-2-carboxamide
[0072] Compound S-prolinamide (11.2g, 100mmol) was added to a solution of bromoacetyl chloride (15.6g, 100mmol) tetrahydrofuran (200mL) precooled to 0°C, and the solution was stirred at 0°C for 30 minutes. Potassium carbonate (27.3g, 200 mmol) was added into the mixed solution, and the resulting mixed solution was stirred at 0° C. for 1 hour, and then the reaction solution was warmed up to room temperature and stirred for 3 hours.
[0073] The end of the reaction was detected by TLC. After compound 2 completely disappeared, the reaction system was filtered, the filter cake was washed with tetrahydrofuran (50 mL), and the filtrates were combined. The solvent was rotary evaporated to dryness, and the residual oil was dissolved in ethyl acetate. The obtained ethyl acetate solution was washed with water (50 mL×2), and dried over anhydrous sodium sulfate for 2 hours. After removing the desicca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com