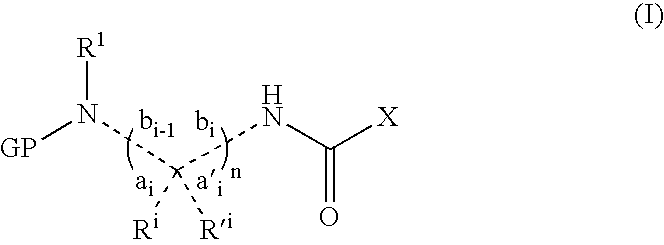
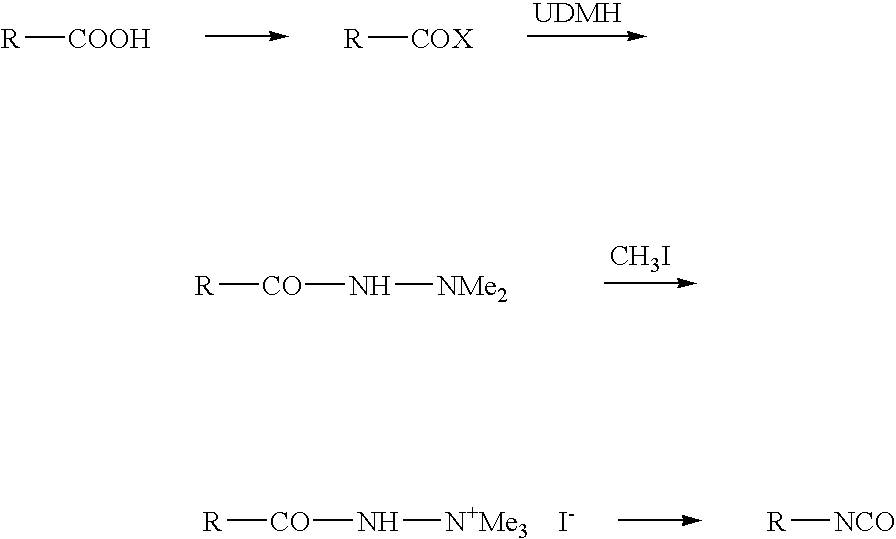Patents
Literature
36results about "Preparation from carboxylic acid nitrogen analogues" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
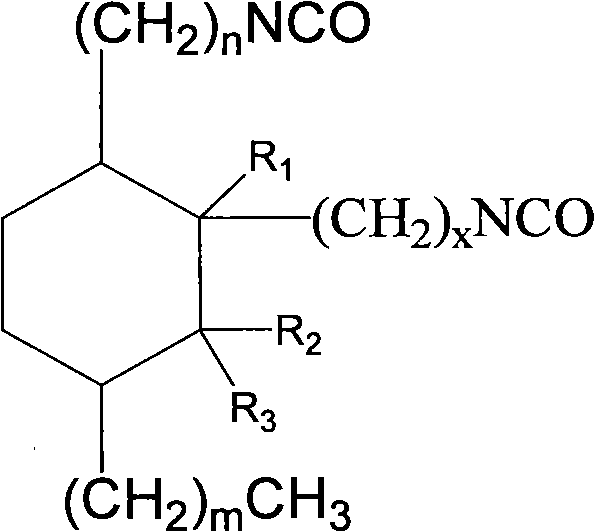
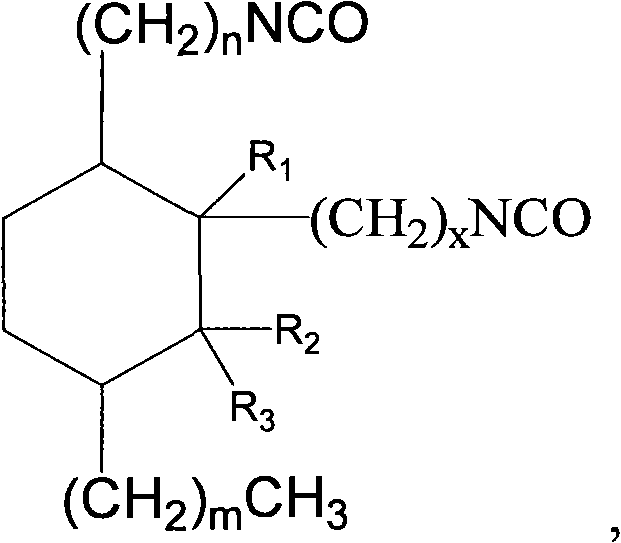
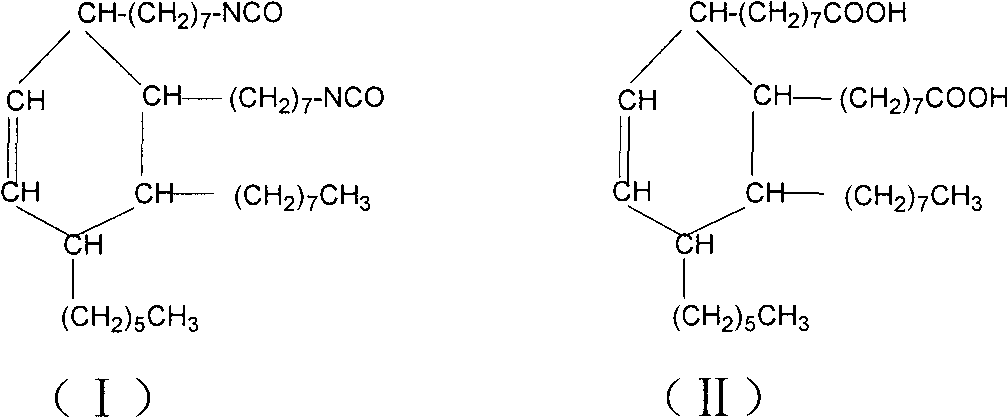
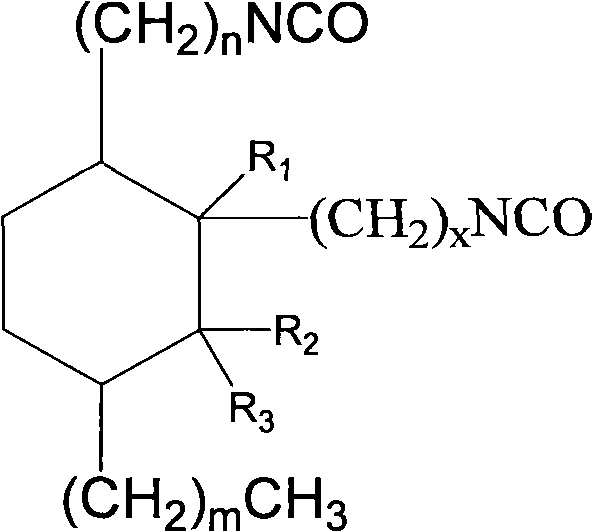
2-octyl-3,4-di(7-diisocyanateheptyl)-1-hexylcyclohexane and its preparation method and use
InactiveCN101100448AEasy to industrializeEasy to operatePreparation from carboxylic acid nitrogen analoguesElastomerIsomerization
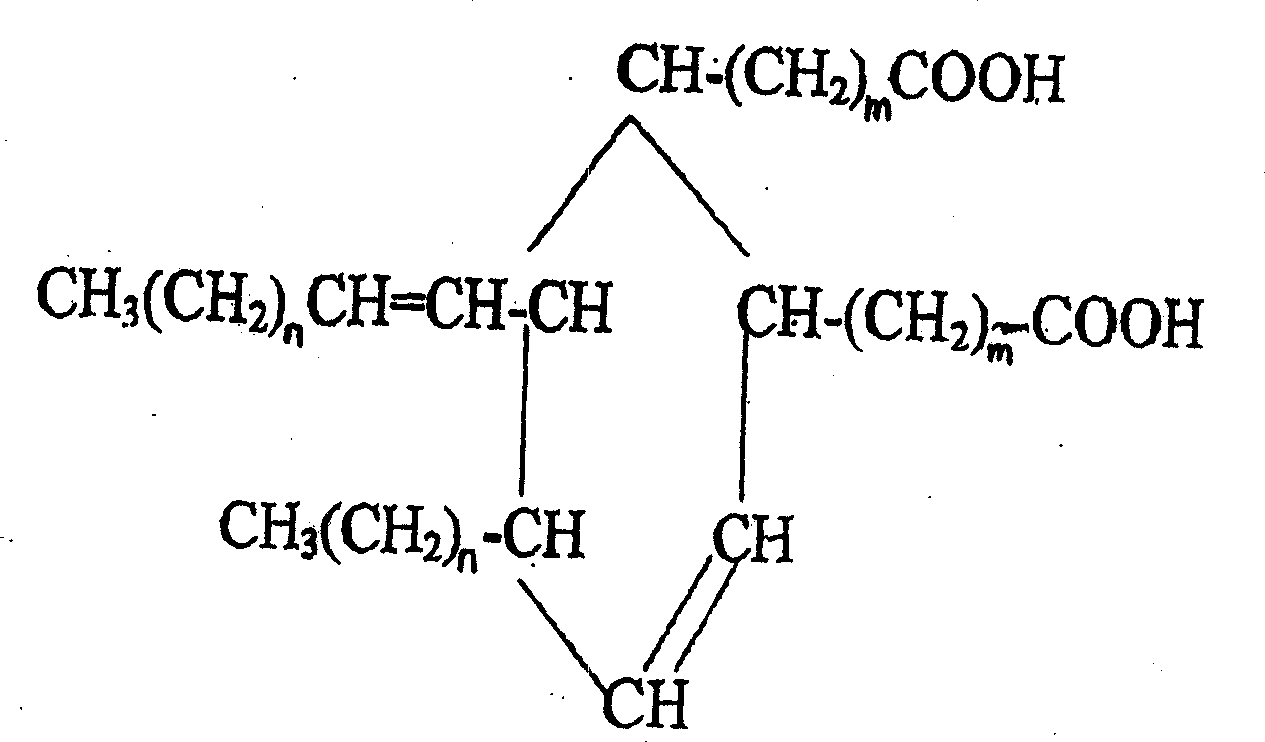
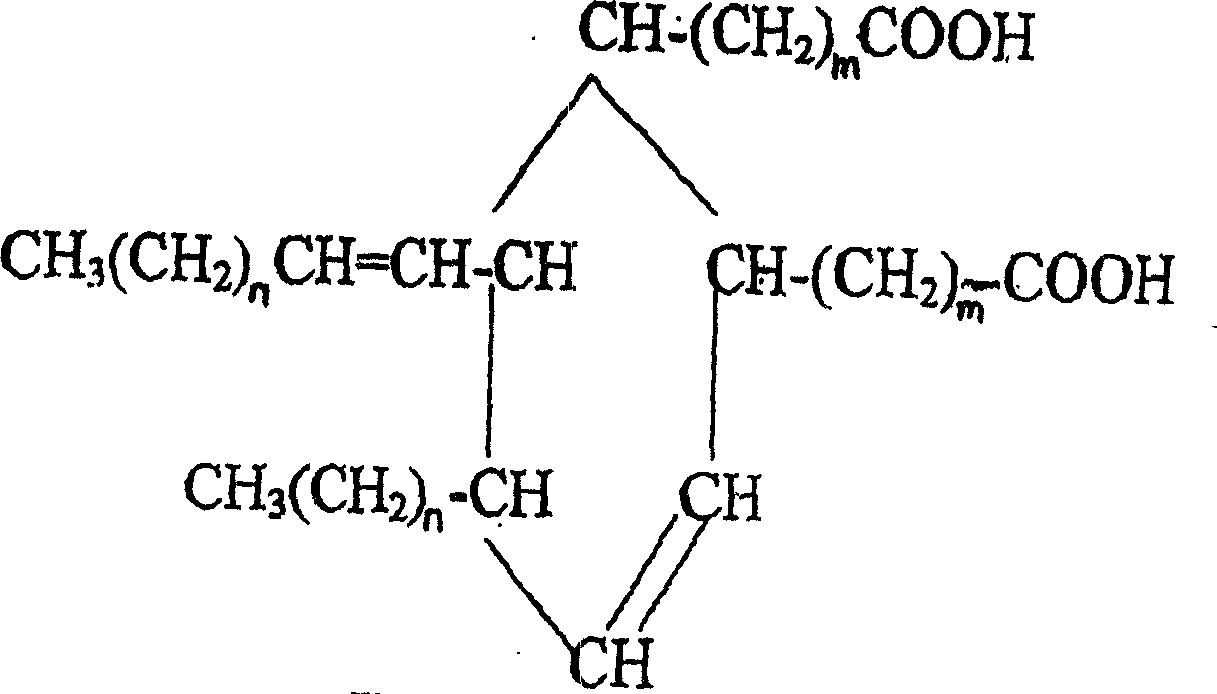
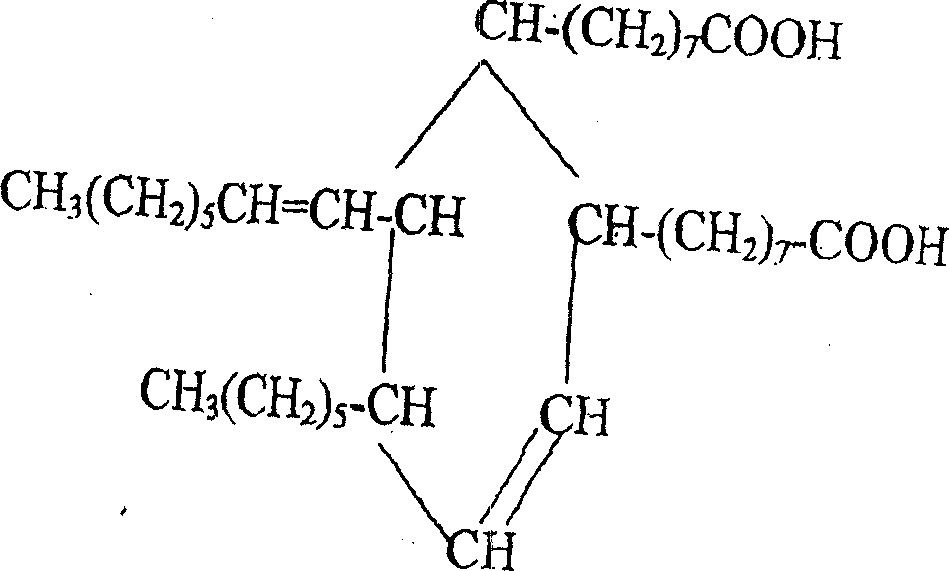
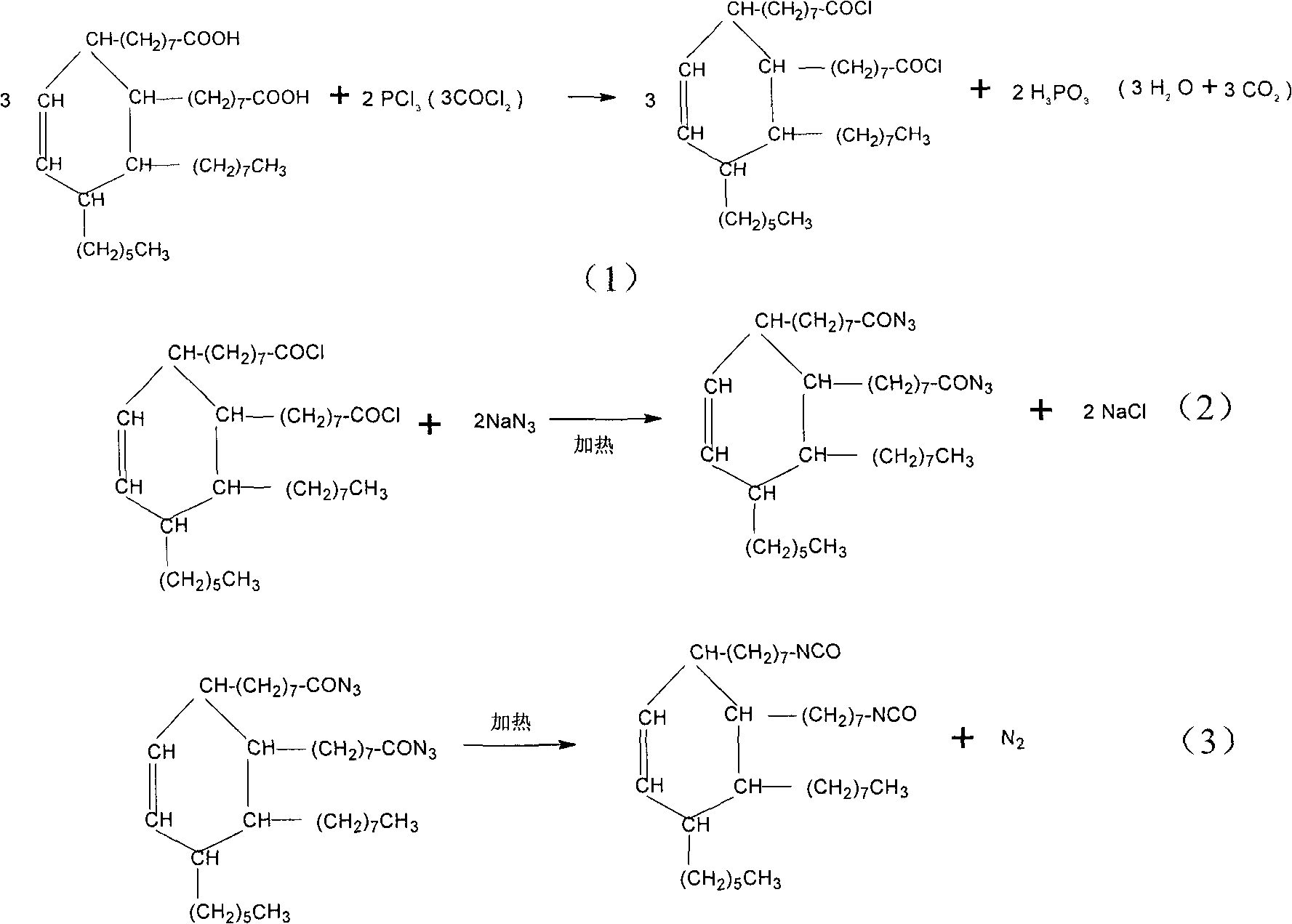
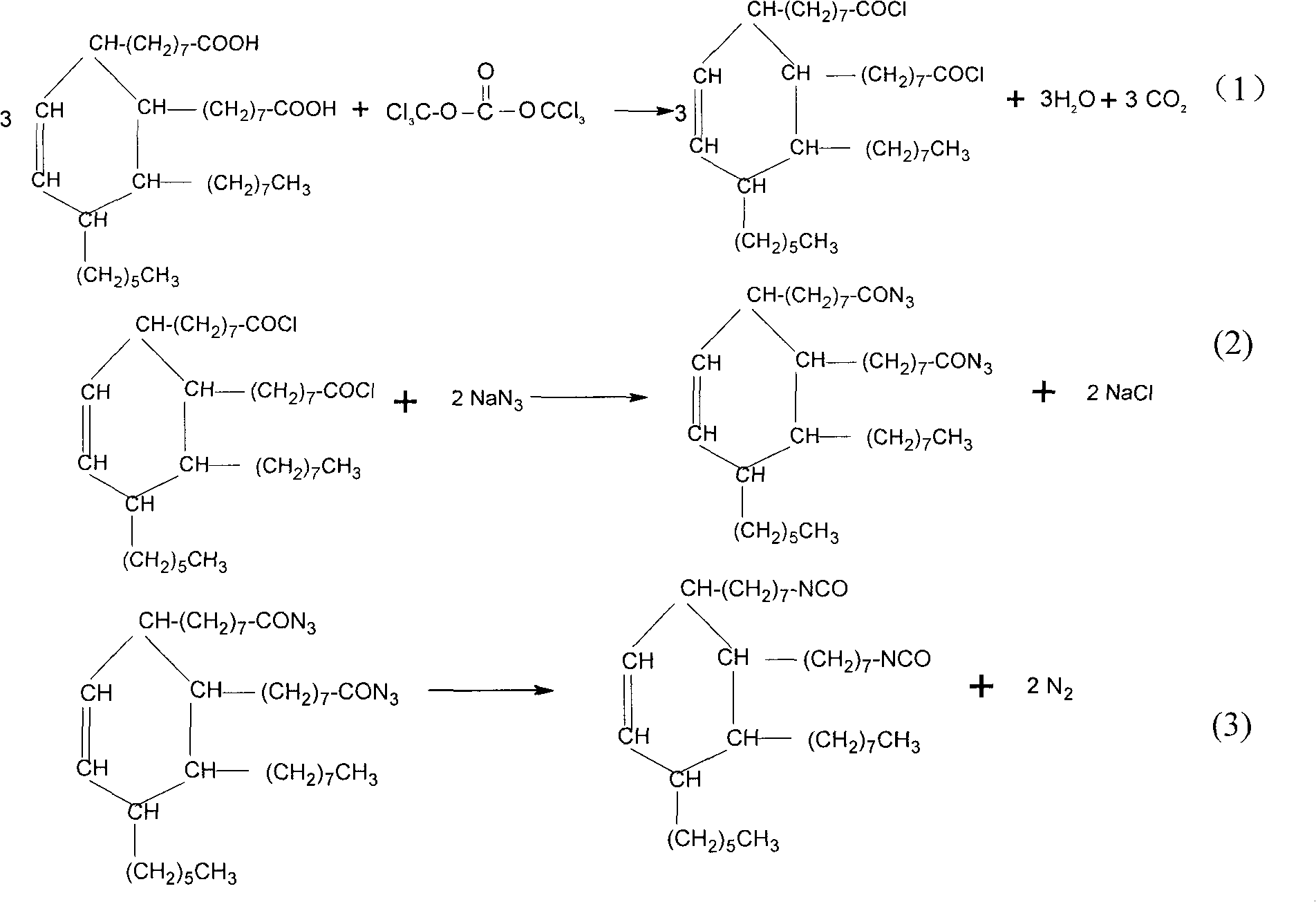
Production of 2-octyl-3,4-di(7-isocyanate caprylic)-1-hexyl cyclohexane and fatty isocyanate containing it is carried out by hydrogenation catalyzing for dimmer acid between room temperature to 280 deg. C, dewatering for generated saturated dimmer acid, reacting with sulfurous chloride, reacting dimeric acid acyl bromide with sodium azide to generate dimeric acid azide, isomerization decomposing to convert into final product. It can be used to produce polyurethane varnish, elastomer, adhesive, spinning finishing agent and rocket impeller.
Owner:刘林学 +1
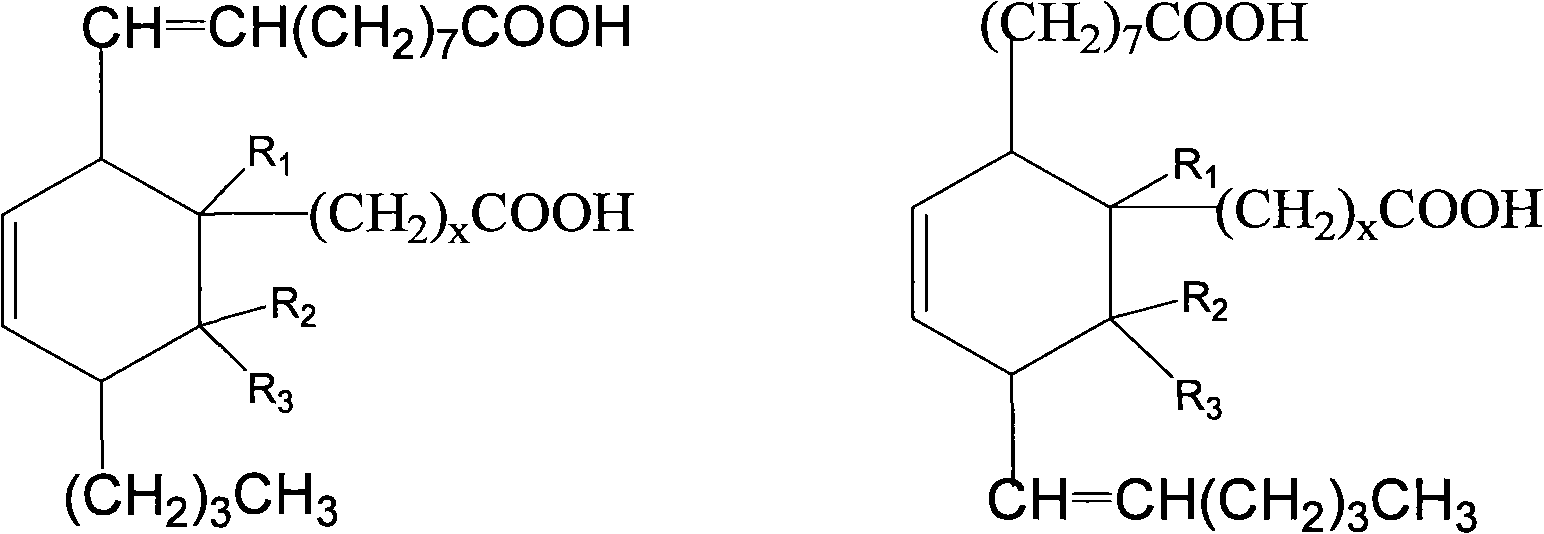
Aliphatic diisocyanate and preparation method and purposes thereof
ActiveCN101805270AHigh yieldEasy to operatePreparation from carboxylic acid nitrogen analoguesPolyureas/polyurethane adhesivesElastomerChemical structure
Owner:浙江优创材料科技股份有限公司
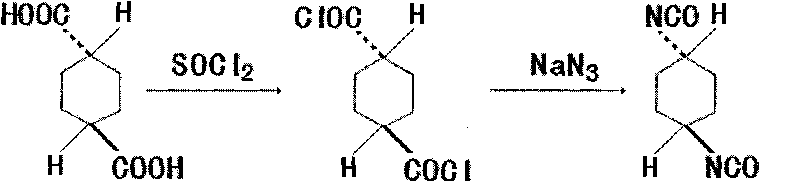
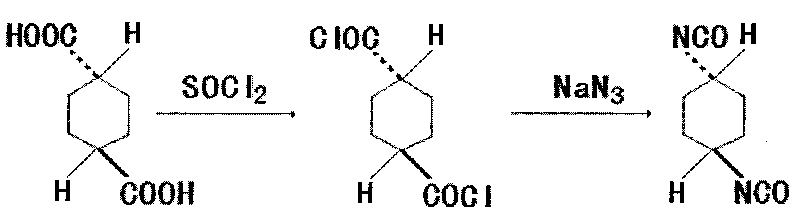
Method for synthesizing trans-1,4-cyclohexane diisocyanate
ActiveCN101735111ASimple process routeRaw materials are easy to getPreparation from carboxylic acid nitrogen analoguesCyclohexane diisocyanateSodium azide
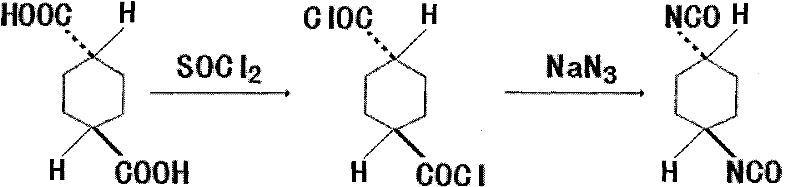
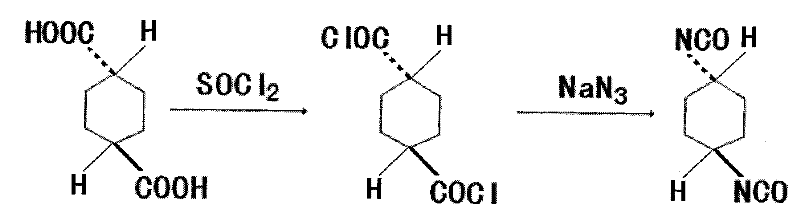
The invention discloses a method for synthesizing trans-1,4-cyclohexane diisocyanate, which comprises the following steps: (1) mixing trans-1,4-cyclohexanedicarboxylic acid with thionyl chloride, heating under reflux, decompressing and recovering the extra thionyl chloride for obtaining trans-1,4-cyclohexanedicarbonyl chloride; (2) adding toluene, benzene or xylene into the trans-1,4-cyclohexanedicarbonyl chloride, heating to 45-75 DEG C, and further adding sodium azide for obtaining mixture; and (3) keeping the temperature of the mixture for 0.5-1.5 hours at the temperature of 45-75 DEG C, filtering for removing insoluble matters, decompressing, recovering the toluene and distilling for obtaining the trans-1,4-cyclohexane diisocyanate. The synthesis method has the advantages of simple process route, easy obtainment of raw materials and short reaction period, thereby being applicable to industrial mass production.
Owner:江苏恒祥化学股份有限公司
Method for preparing diisocyanate by heat decomposition
ActiveCN101386585ANo pollution in the processGood catalyticPreparation from carboxylic acid nitrogen analoguesAlcoholDecomposition
The present invention discloses a method for preparing diisocyanate by thermally separating diamino formic ether. The method comprises the following steps that fatty group or alicyclic group diamino formic ether undergoes a thermal decomposition reaction for 0.5 to 2.5 hours in the presence of a catalyst at a reaction pressure of between 1.0 and 1.5 atmospheric pressure and a reaction temperature of between 50 and 240 DEG C so as to form fatty group or alicyclic group diisocyanate. The catalyst is easy to obtain and can be recycled, the reaction conditions are mild, the cost and the energy dissipation are reduced, the byproduct of the reaction is recoverable alcohol that does not cause contamination to environment, and thus the method is more suitable for the large scale industrial production.
Owner:WANHUA CHEM GRP CO LTD
Synthesis method of dimer(fatty acid)yl diisocyanate
InactiveCN101830832AHigh yieldPreparation from carboxylic acid nitrogen analoguesSynthesis methodsSodium azide
The invention discloses a synthesis method of dimer(fatty acid)yl diisocyanate, which comprises the steps of: adding a methylbenzene solution of dimer(tall oil acid) and dimethylformamide into a reaction bulb, dropping a methylbenzene solution containing di(trichloromethyl) carbonic ester under the stirring at a temperature of 70-80 DEG C, reacting for 1h, filtering, evaporating to remove methylbenzene to obtain dimer(tall oil acid) acyl chloride, wherein the mol ratio of the dimer(tall oil acid) to the di(trichloromethyl) carbonic ester is 3:1-1.5; adding sodium azide and deionized water into the reaction bulb, dropping an acetone solution dissolved with the dimer(tall oil acid) acyl chloride under the stirring, adding 300ml of normal hexane, dimixing, washing a normal hexane layer by using cold water, drying by using anhydrous sodium sulfate to obtain an anhydrous sodium sulfate solution of dimer(tall oil acid) acyl azide, wherein the mol ratio of the dimer(tall oil acid) acyl chloride to the sodium azide is 1:2-2.2; and adding 300ml of normal hexane into the reaction bulb, dropping the normal hexane solution under the stirring at a temperature of 65-70 DEG C, and continuing to react for 30min after the dropping to obtain a target product.
Owner:XIAN MODERN CHEM RES INST
Stabilized activated derivatives of carbamic acid, their process of preparation and their use for the preparation of ureas
Owner:CENT NAT DE LA RECHERCHE SCI +1
Isocyanate with inflaming retarding characteristic and preparation method and application thereof
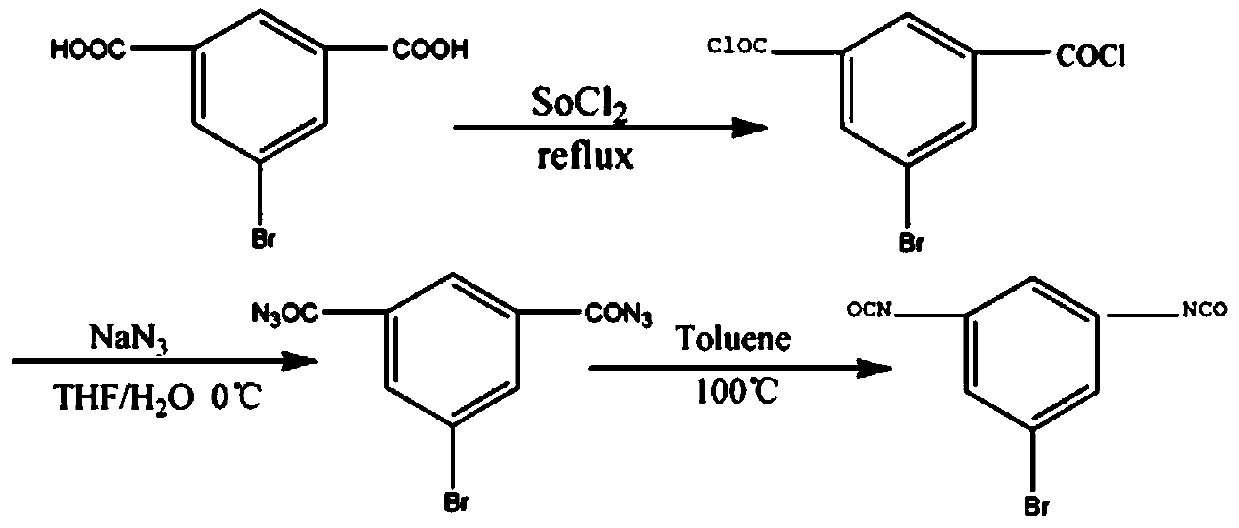
InactiveCN109970605AHas flame retardant propertiesNovel structureFireproof paintsPreparation from carboxylic acid nitrogen analoguesBenzeneBromine
The invention discloses isocyanate with an inflaming retarding characteristic and a preparation method and application thereof, and belongs to the technical field of inflaming retarding coatings. Isophthalic acid is taken as a raw material, and the 1-bromine-3,5-diisocyanate benzene with the inflaming retarding characteristic is synthesized. The preparation method comprises the following steps of(1) preparation of 5-bromine-isophthalyl chloride; (2) preparation of 5-bromine 2-nitrine; (3) preparation of the 1-bromine-3,5-diisocyanate benzene. According to the preparation method, based on thephysicochemical property of an original coating, the non-toxic and fire-proof effects are added for the coating, a production technology and the preparation method are simple, there are no complex processes, the quality of the prepared product is stable, and the isocyanate is suitable for large-scale production, wide in market prospect and high in social and economic benefits.
Owner:苏州塔霍新材料科技有限公司
Bio-based aromatic diisocyanates for preparation of polyurethanes
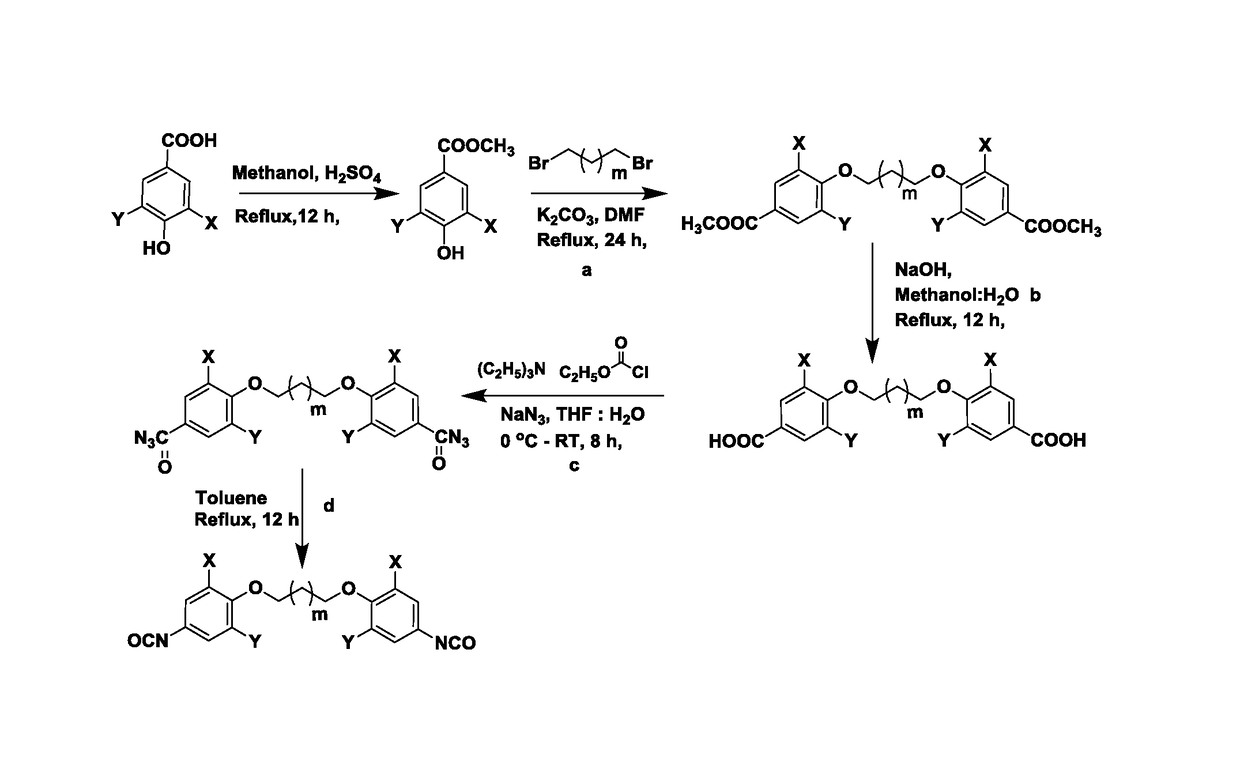
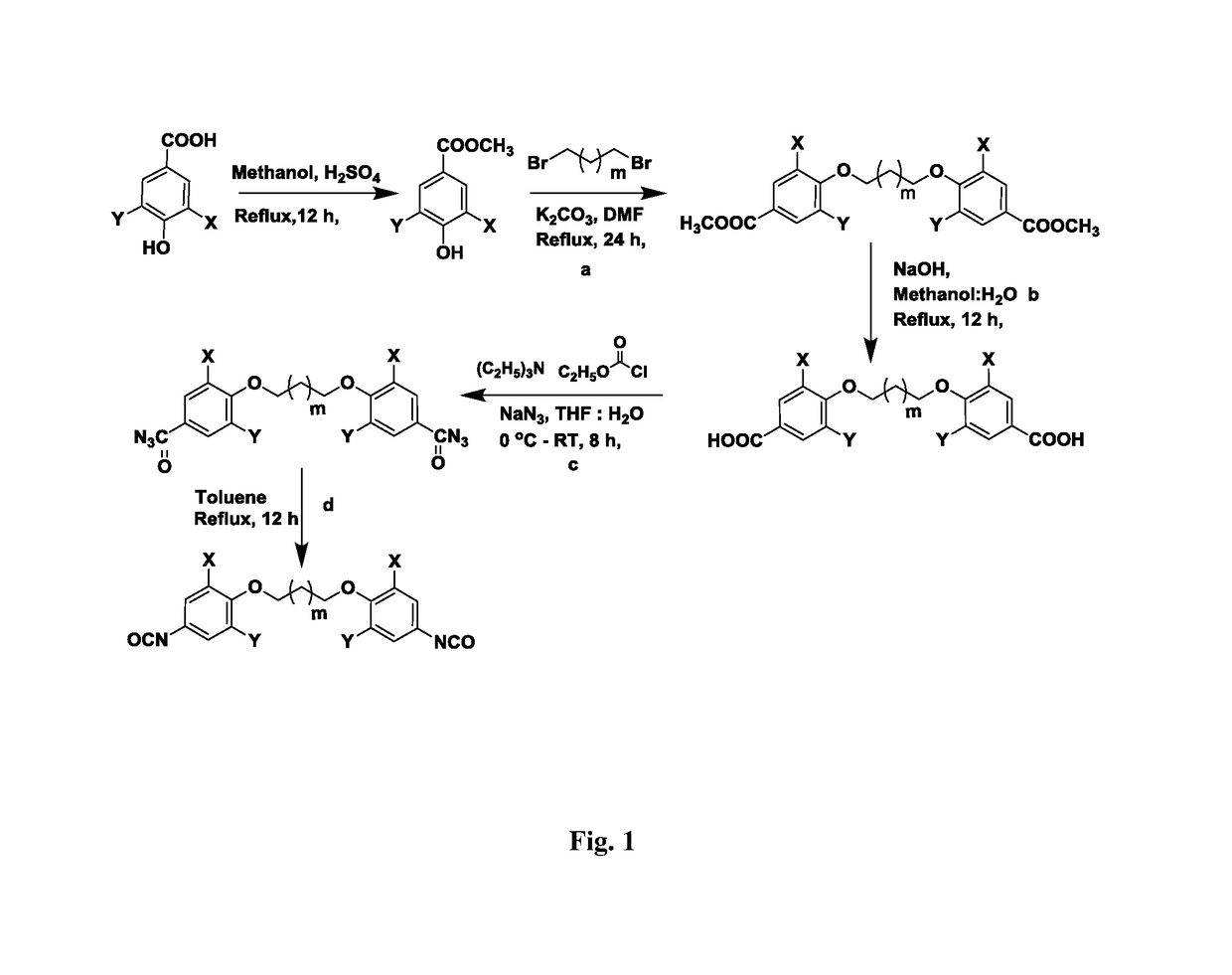
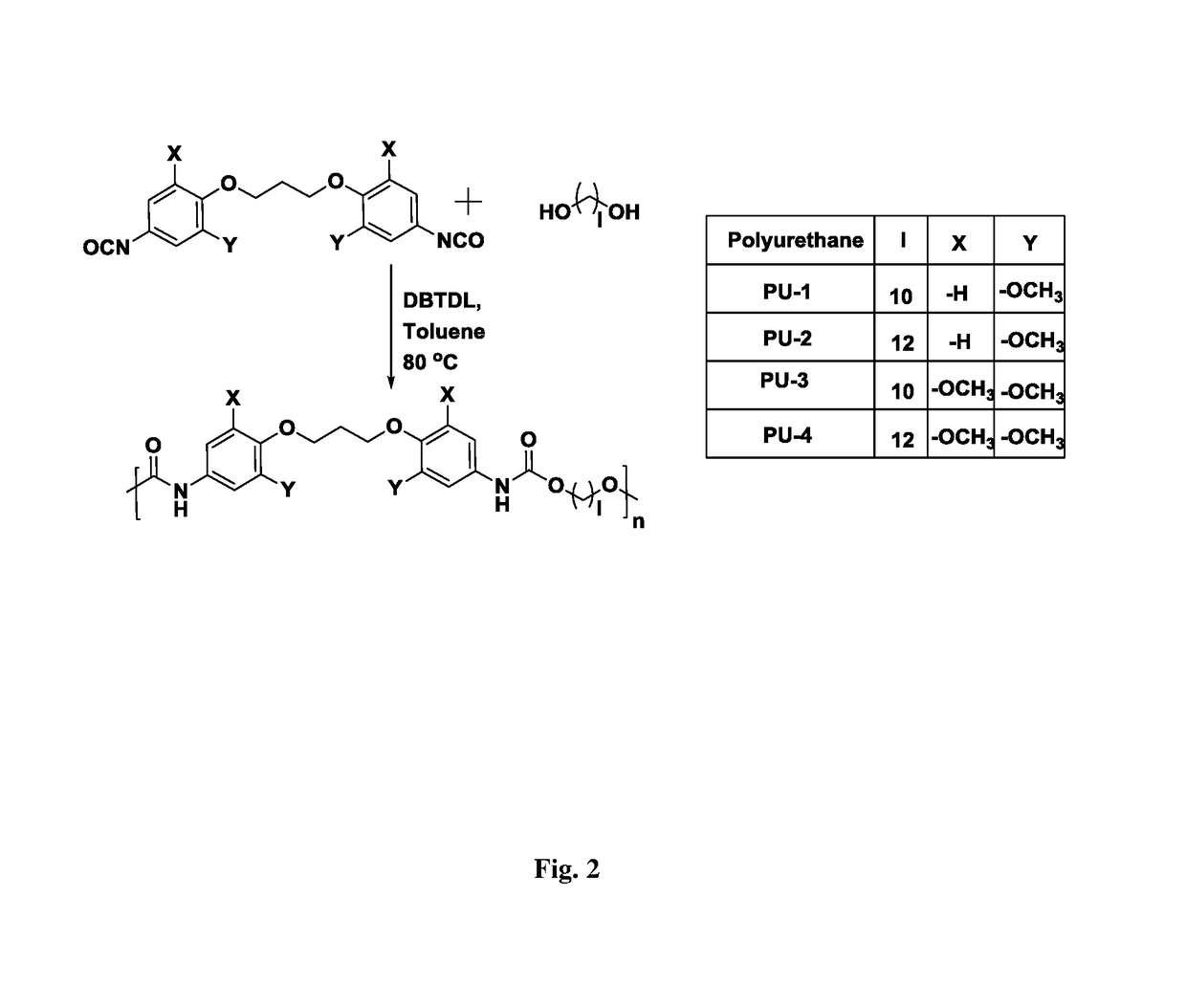
ActiveUS20170369427A1Use in synthesisPreparation from carboxylic acid nitrogen analoguesChemistryIsocyanate
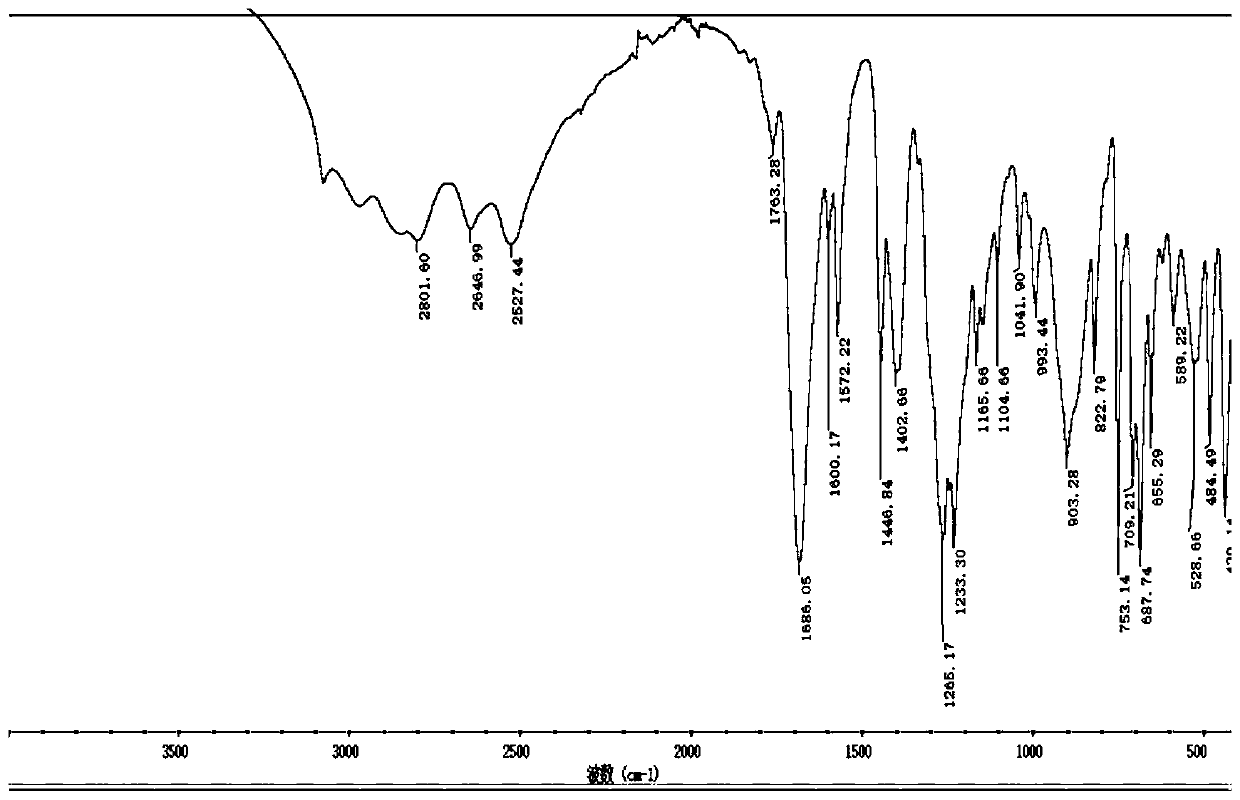
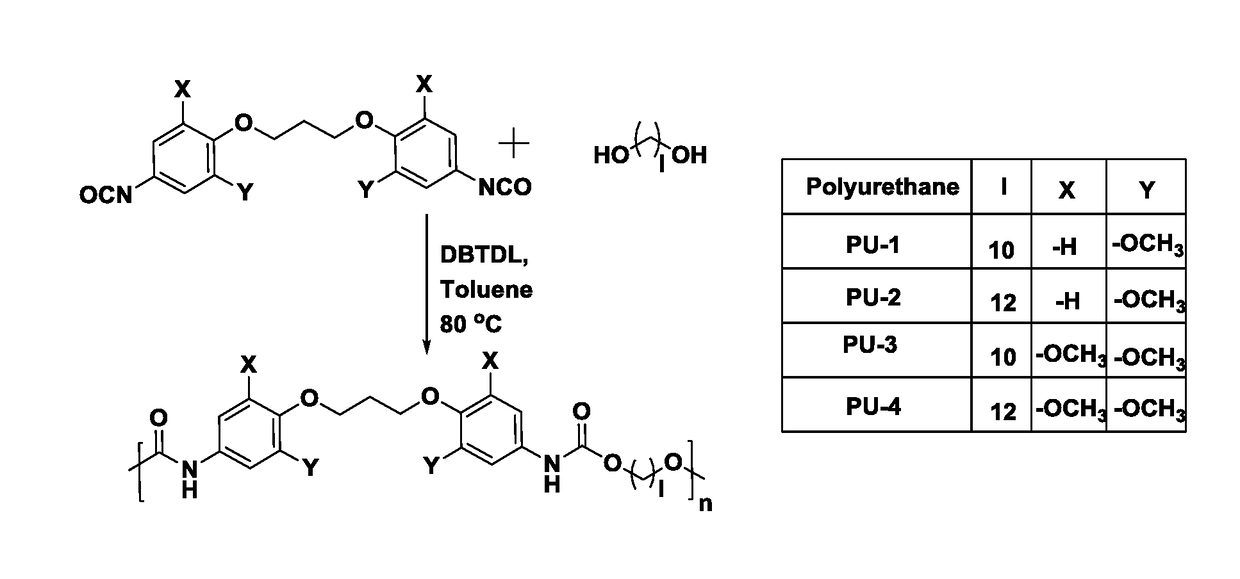
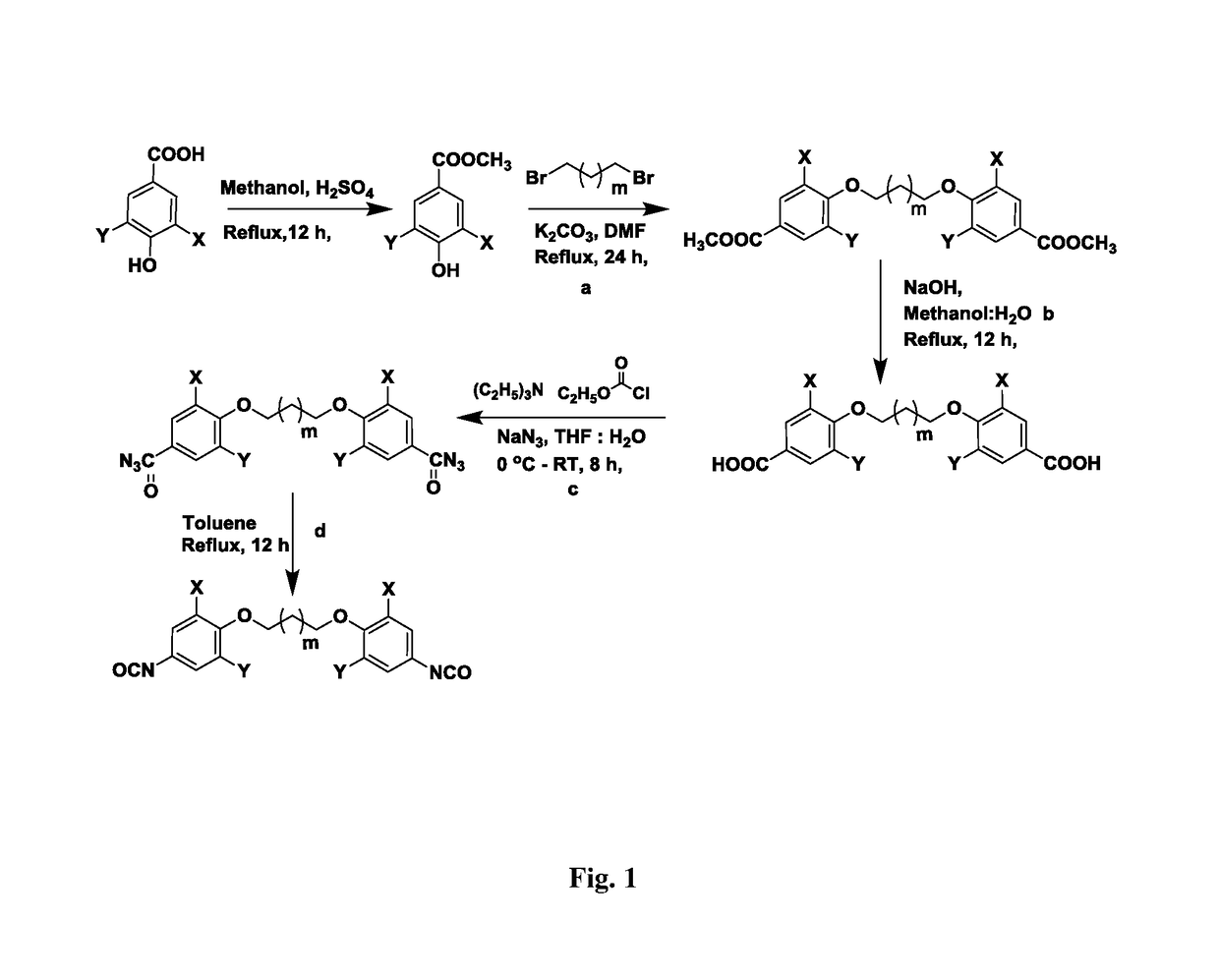
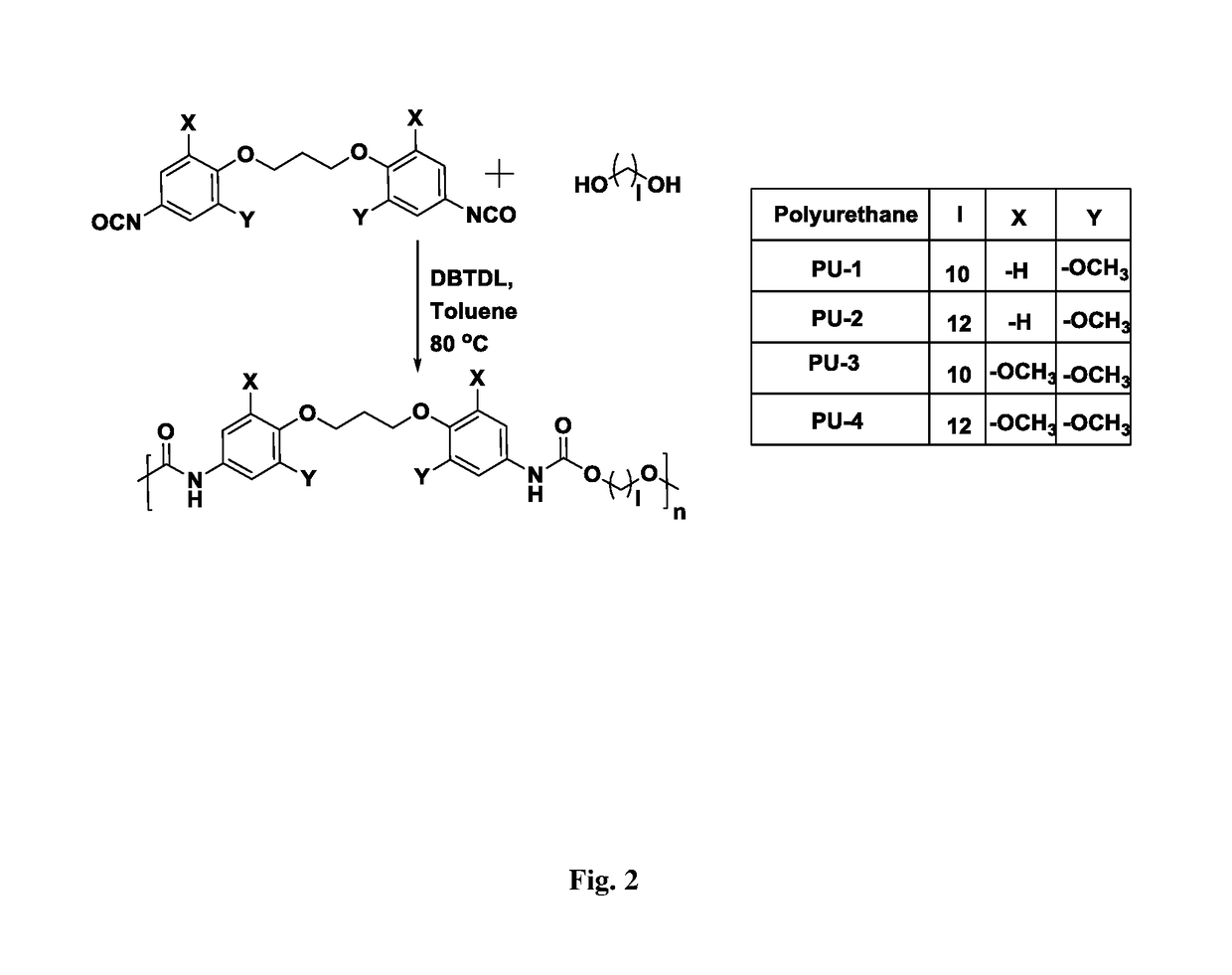
The present invention provides bio-based aromatic diisocyanate of formula (I). [Formula should be inserted here] wherein X is OCH3, Y is selected from —H or OCH3, and m=0-12. The present invention further provides a method for preparation of aromatic diisocyanate of formula (I) useful for preparation of polyurethane.
Owner:COUNCIL OF SCI & IND RES
Urethane compound and method for producing the same, and isocyanate and method for producing the same
ActiveUS20120010427A1Low costHigh yieldPreparation from carboxylic acid nitrogen analoguesCarbamic acid derivatives preparationAlcoholCarbamate
Owner:MITSUI CHEM INC
Bio-based aromatic diisocyanates for preparation of polyurethanes
The present invention provides bio-based aromatic diisocyanate of formula (I). [Formula should be inserted here] wherein X is OCH3, Y is selected from —H or OCH3, and m=0-12. The present invention further provides a method for preparation of aromatic diisocyanate of formula (I) useful for preparation of polyurethane.
Owner:COUNCIL OF SCI & IND RES
Method for synthesizing n-butyl isocyanate
InactiveCN105384659ALess side effectsNon-volatilePreparation from carboxylic acid nitrogen analoguesN-butylisocyanideEvaporation
The invention discloses a method for synthesizing n-butyl isocyanate. The method comprises the following steps: weighing n-valeric acid and thionyl chloride according to the mole ratio of 1: 1, putting n-valeric acid and thionyl chloride in a round-bottomed flask, enabling an opening of the round-bottomed flask to be connected to a reflux condensing tube, of which an upper opening is provided with a calcium chloride drying tube, carrying out magnetic stirring, heating the round-bottomed flask to the temperature of 60-70 DEG C in oil bath, and carrying out reaction for 4-5 hours, so as to obtain a n-valeryl chloride crude product; and dissolving the crude product and a catalyst in an anhydrous toluene solvent, heating the solution to the temperature of 60-80 DEG C in a three-necked flask, enabling the three-necked flask to be connected to a thermometer, a reflux condensing tube, of which an upper opening is provided with a calcium chloride drying tube, and a flask cork respectively, carrying out magnetic stirring for 5-8 minutes, then, slowly adding drying sodium azide, of which the mole is equal to that of n-valeric acid, carrying out reaction until no gas is produced, keeping the reaction for 10-15 minutes, then, filtering out insolubles, and carrying out rotary evaporation to remove toluene, thereby obtaining n-butyl isocyanate. The method has the advantages of high yield, mild reaction conditions, simplicity in operation, short reaction time and little environmental pollution.
Owner:ANHUI GUANGXIN AGROCHEM
Low chlorine, multi-staged method for producing cycloaliphatic disocyanates
InactiveUS8536370B2Reduce environmental pollutionSuppress formation of N-methylPreparation from carboxylic acid nitrogen analoguesOrganic compound preparationAlkaneAlcohol
Low chlorine, multi-staged method for producing cycloaliphatic diisocyanates. The invention relates to a multi-staged method for the continuous low-chlorine production of cycloaliphatic diisocyanates, comprising the synthesis of diaminodipheynl alkanes, the hydration thereof into the corresponding cycloaliphatic diamines and the subsequent conversion of cycloaliphatic diamines to the corresponding cycloalkylene biscarbamates and the thermal cleaving of the latter into the cycloaliphatic diisocyanates and alcohol.
Owner:EVONIK DEGUSSA GMBH
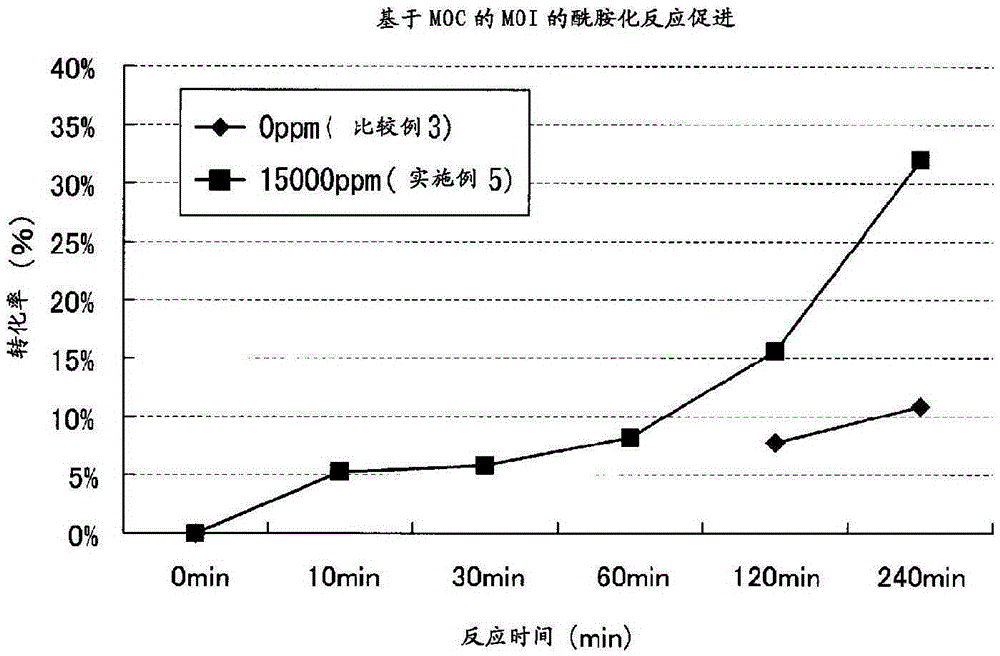
Reaction accelerator, urethane compound using same, thiourethane compound, and production method for amide compound or urea compound
ActiveCN105392562AEasy to preparePreparation from carboxylic acid nitrogen analoguesCarbamic acid derivatives preparationHydrogenHalide
Provided is a reaction accelerator used in a reaction between a compound having an isocyanate group that does not directly bond to an aromatic ring, in each molecule, and a compound having an active hydrogren-containing group. The reaction accelerator comprises a compound having a carbamoyl halide group. Also provided is a production method whereby: the compound having a isocyanate group that does not directly bond to an aromatic ring, in each molecule, and the compound having an active hydrogren-containing group are caused to react; and a urethane compound, a thiourethane compound, an amide compound, or a urea compound are produced. The production method is characterized by said reaction occurring in the presence of the reaction accelerator.
Owner:株式会社力森诺科
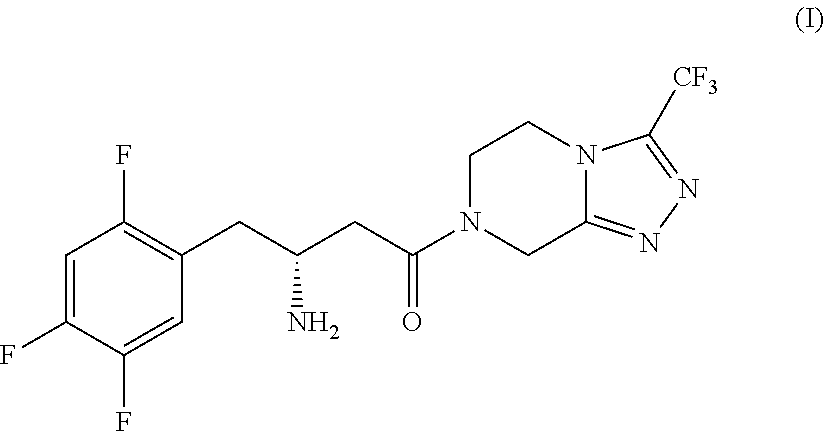
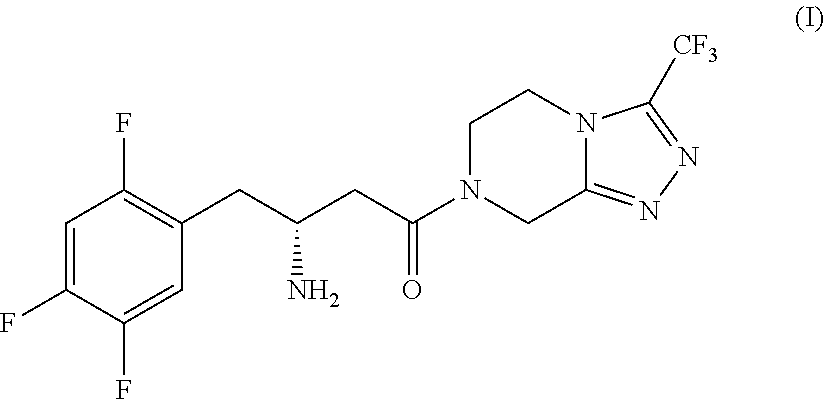
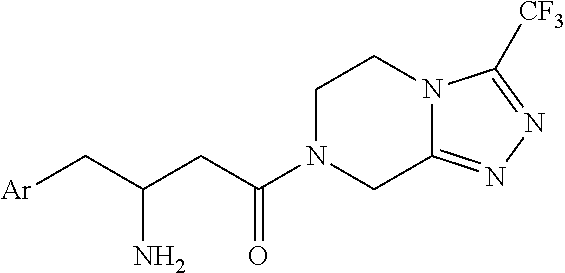
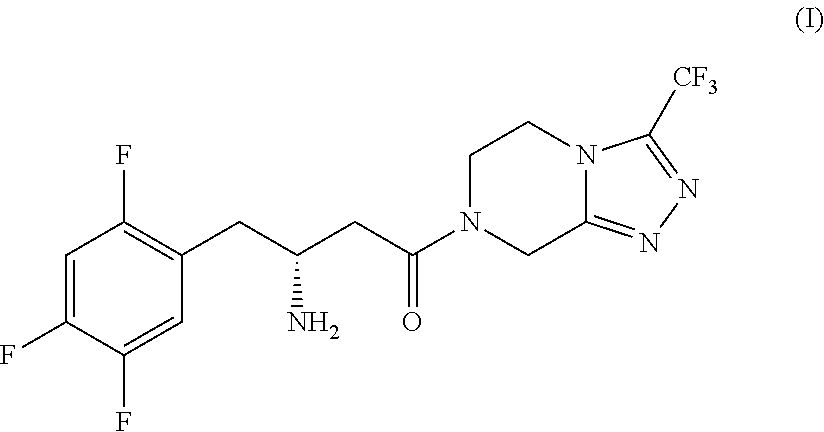
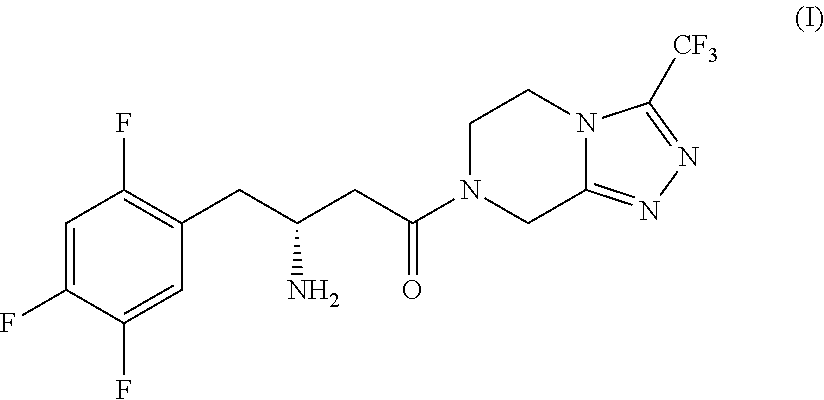
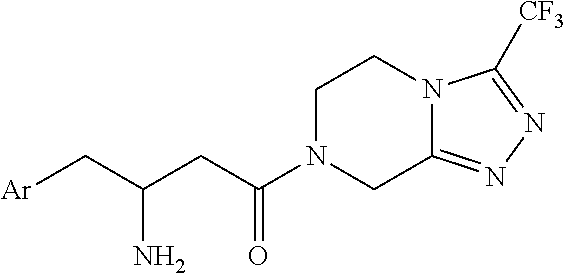
Process for the production of sitagliptin
InactiveUS8912327B2Easily isolableEasily purifiablePreparation from carboxylic acid nitrogen analoguesCarbamic acid derivatives preparationSitagliptinPyrazine
Owner:QUIMICA SINTETICA
Multistage continuous preparation of cycloaliphatic diisocyanates
InactiveUS7420080B2Speed up the processImprove availabilityPreparation from carboxylic acid nitrogen analoguesOrganic compound preparationPolymer sciencePhosgene
The invention relates to a multistage process for continuous and phosgene-free preparation of cycloaliphatic diisocyanates.
Owner:EVONIK DEGUSSA GMBH
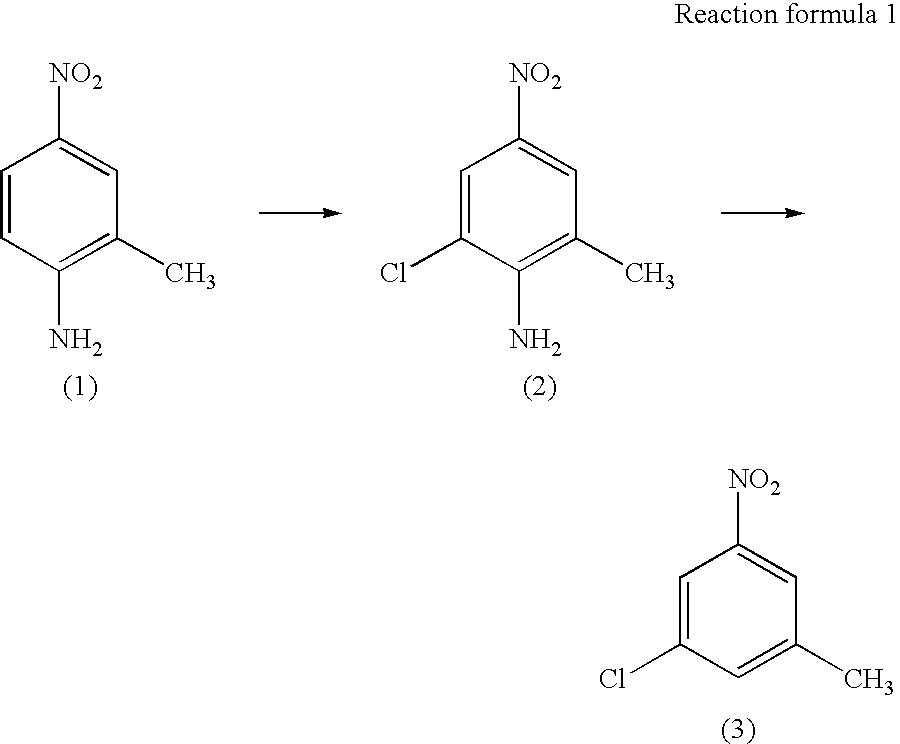
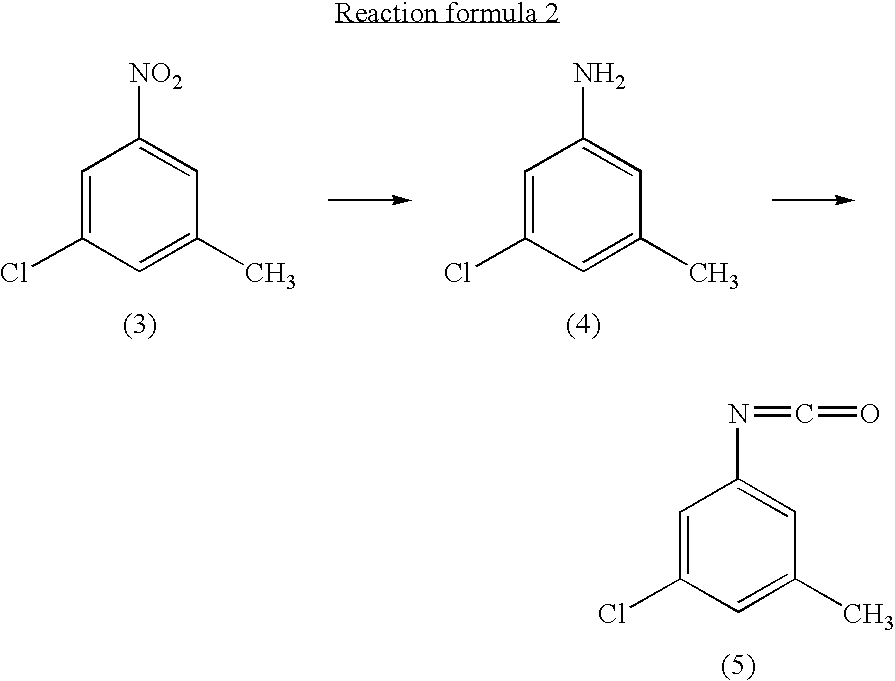
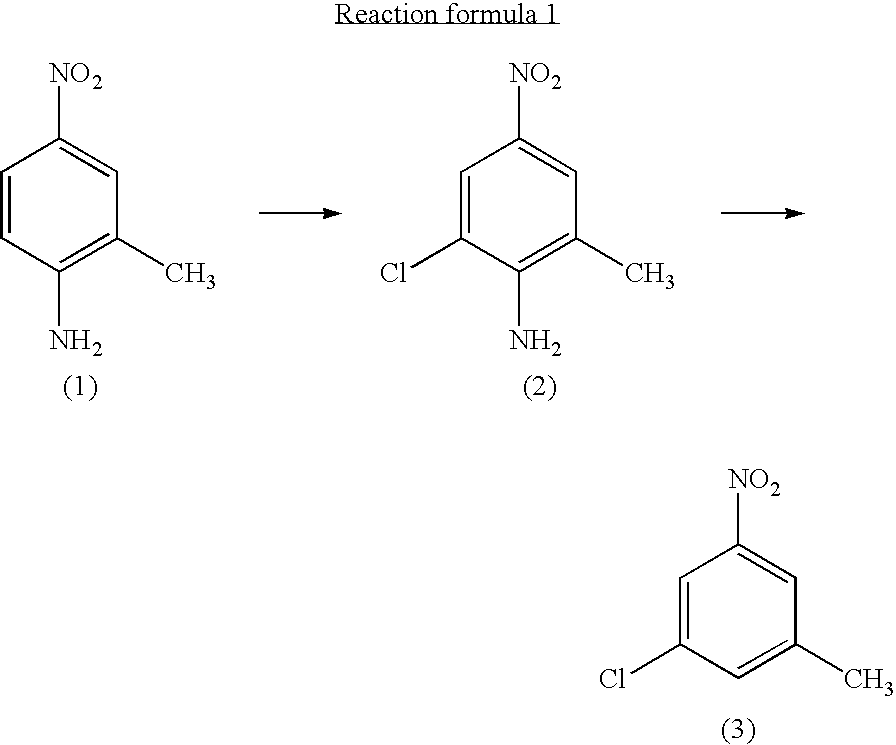
Process for preparing 3-chloro-5-nitrotoluene
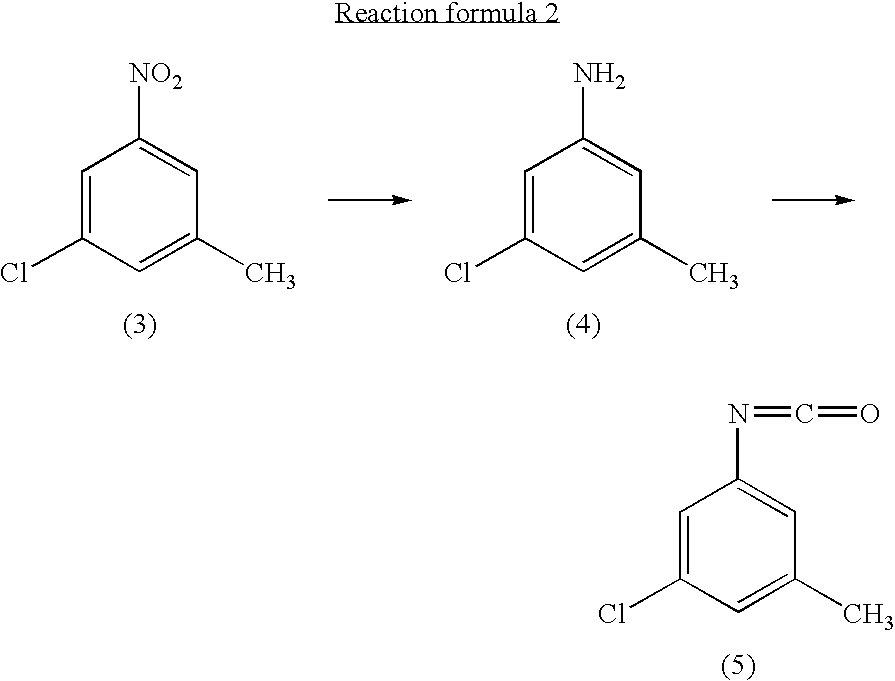
InactiveUS20040147776A1Preparation from carboxylic acid nitrogen analoguesOrganic compound preparationHypochloriteChemistry
A preparation process of 3-chloro-5-nitrotoluene is provided in mild conditions. It is a process for preparing 3-chloro-5-nitrotoluene, which comprises reacting 2-methyl-4-nitroaniline with a chlorinating agent such as 5-butyl hypochlorite in a neutral condition to obtain 2-chloro-4-nitro-6-methylaniline and deaminating the 2-chloro-4-nitro-6-methylaniline to obtain 3-chloro-5-nitrotoluene.
Owner:DAICEL CHEM IND LTD
Process for preparing 3-chloro-5-nitrotoluene
InactiveUS7081548B2Preparation from carboxylic acid nitrogen analoguesOrganic compound preparationMethylanilineHypochlorite
A preparation process of 3-chloro-5-nitrotoluene is provided in mild conditions. It is a process for preparing 3-chloro-5-nitrotoluene, which involves the steps of reacting 2-methyl-4-nitroaniline with a chlorinating agent such as 5-butyl hypochlorite in a neutral condition to obtain 2-chloro-4-nitro-6-methylaniline and deaminating the 2-chloro-4-nitro-6-methylaniline to obtain 3-chloro-5-nitrotoluene.
Owner:DAICEL CHEM IND LTD
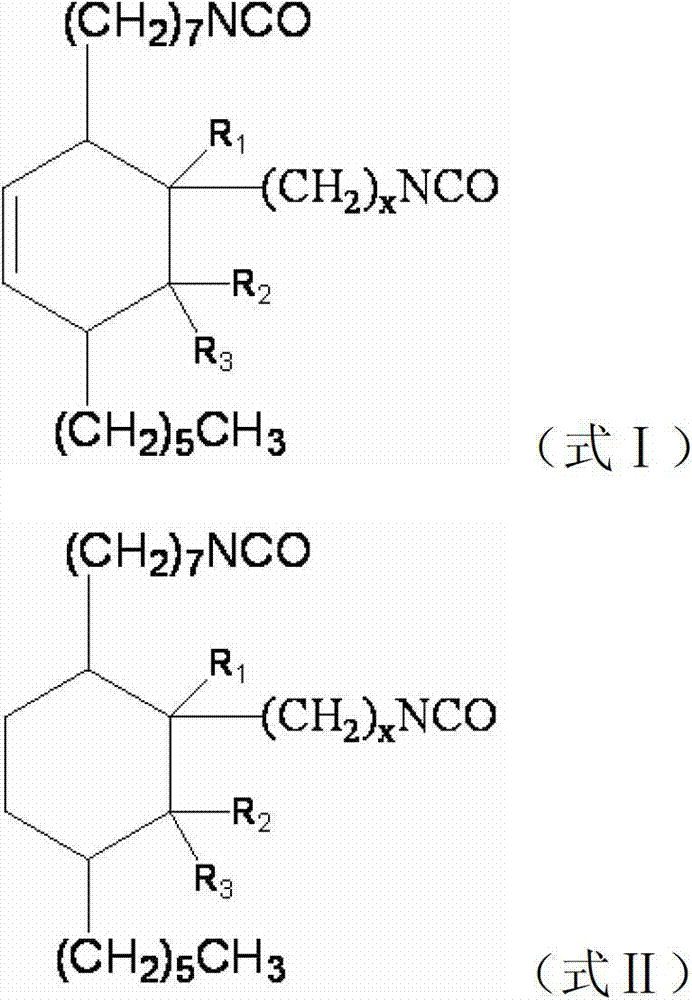
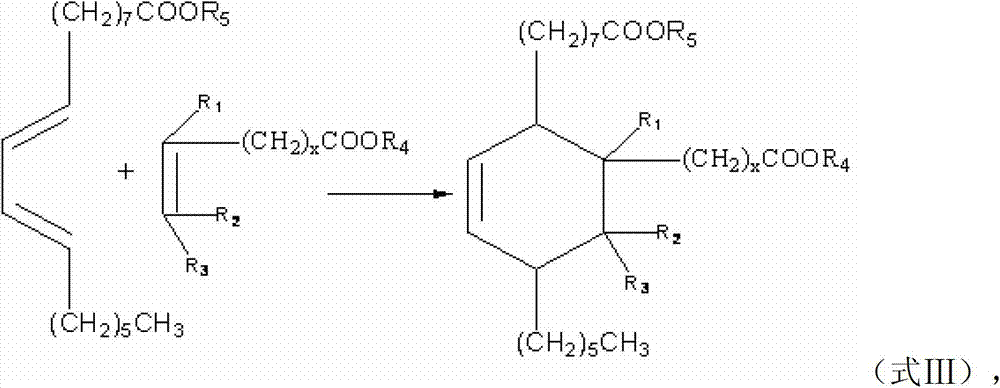
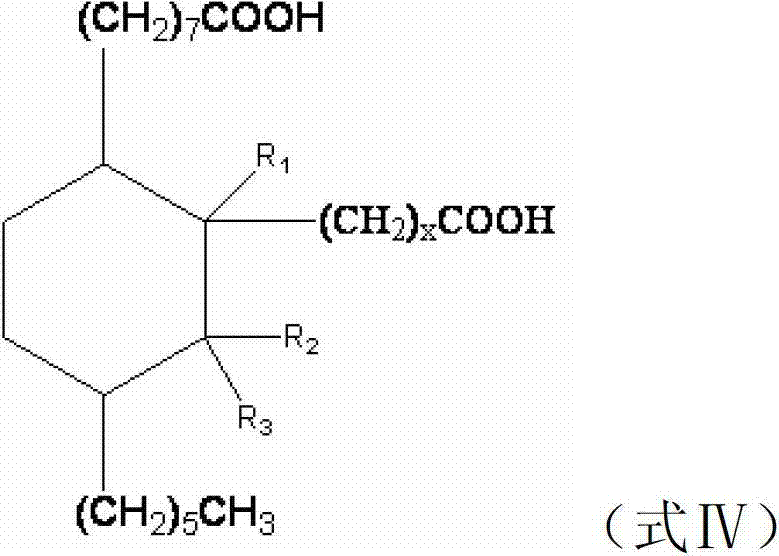
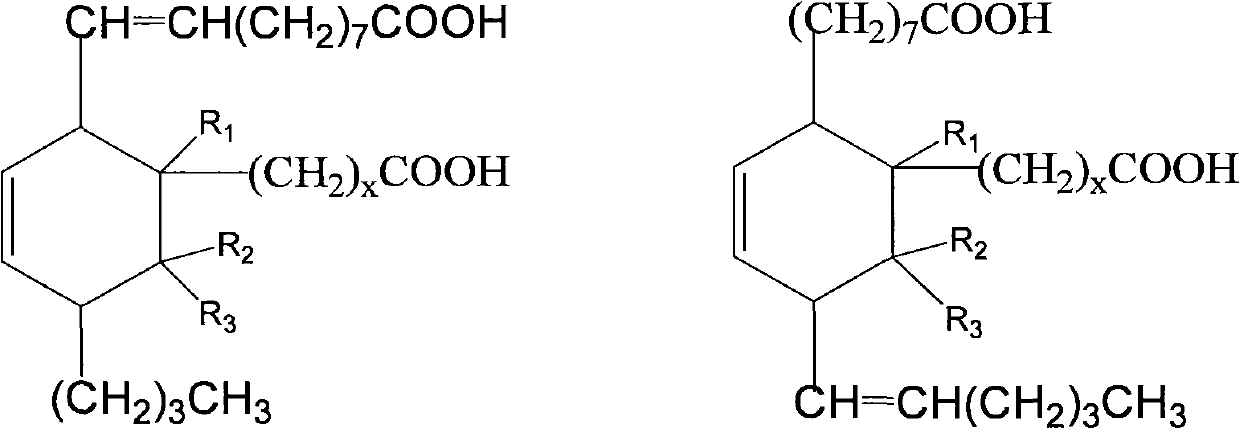
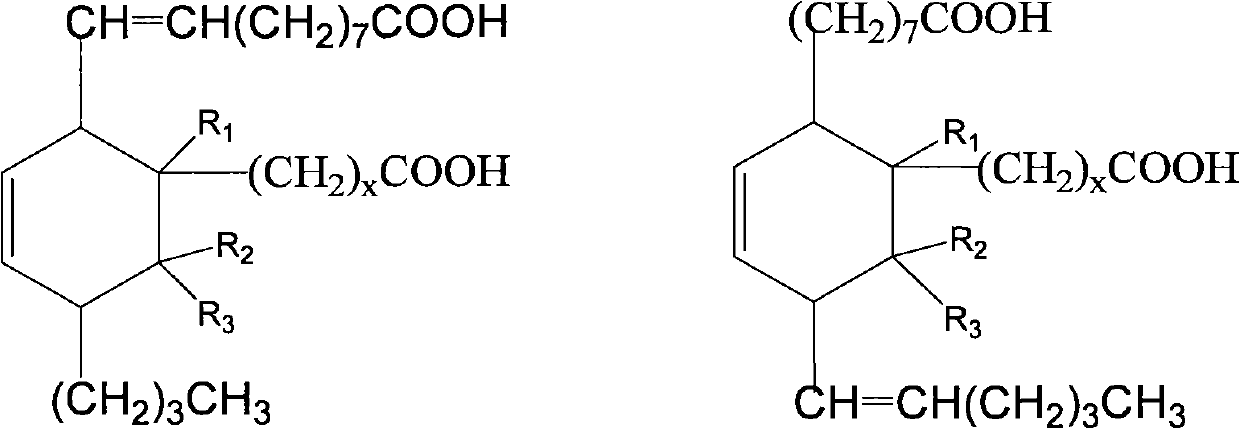
Preparation method of C21-36 alicyclic diisocyanate and use
ActiveCN103113262ASingle configurationLess side effectsPreparation from carboxylic acid nitrogen analoguesChemical recyclingHydrogenation reactionDouble bond
The invention provides a preparation method of C21-36 alicyclic diisocyanate and use. The preparation method comprises the following steps of: preparing an unsaturated alicyclic binary acid based on dehydrated ricinoleic acid or ester and a double bond containing fatty acid or ester as raw materials; preparing a saturated alicyclic binary acid through hydrogenation reaction; preparing acyl chloride of the alicyclic binary acid by acylating chlorination; preparing a trinitride of alicyclic binary acid by nitriding reaction; and finally preparing the alicyclic diisocyanate through isomerized decomposition reaction. According to the invention, acid or ester extracted from castor oil is used as a basic material, so that the production raw material is easy to get and low in cost, and prepared alicyclic diisocyanate is single in structure, less in side reaction and small in production difficulty, and the molecular weight is within a range of 300-500. According to the method, a same solvent is used in later three steps to the benefit of industrialization. The whole process is simple to operate, mild in condition, environment-friendly, safe in production, and is very beneficial for industrial popularization and application.
Owner:陕西聚泰新材料科技有限公司
Process for the production of sitagliptin
InactiveUS20140081026A1Easily isolableEasily purifiablePreparation from carboxylic acid nitrogen analoguesCarbamic acid derivatives preparationSitagliptinPyrazine
A novel process is described for the synthesis of Sitagliptin, IUPAC name 7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazine, of formula (I).
Owner:QUIMICA SINTETICA
Environment-friendly polyisocyanate synthesis method suitable for industrial production
InactiveCN111333547AGood choiceMethod environmentally friendlyPreparation from carboxylic acid nitrogen analoguesToxic gasPtru catalyst
The invention provides a method for synthesizing polyisocyanate. The method comprises the following steps: carrying out a reaction on R1OOC-R-COOR1 in the presence of NaOH and NH2HCl, and neutralizingto obtain dihydroxamic acid HO-NH-CO-R-CO-NH-OH, wherein R1 is selected from C1-C15 aliphatic alkyl, and R is selected from one or more of phenylene, substituent-containing C6-C30 arylene, unsubstituted C6-C30 arylene, C6-C20 sub-aliphatic hydrocarbon group, C6-C20 cycloaliphatic hydrocarbon group and a naphthylene group; and rearranging the dihydroxamic acid under an alkaline condition to obtainthe polyisocyanate OCN-R-NCO. According to the method, highly toxic gases are not adopted, corrosive gases or intermediates are not generated in the reaction process, and noble metal catalysts are not needed, so that the method is environment-friendly and simple, the product selectivity in the reaction process is good, side reactions are few, and the yield is high.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Multistage continuous preparation of cycloaliphatic diisocyanates
InactiveUS20050267310A1Speed up the processAvoid disadvantagesPreparation from carboxylic acid nitrogen analoguesOrganic compound preparationPhosgeneDi-isocyanate
The invention relates to a multistage process for continuous and phosgene-free preparation of cycloaliphatic diisocyanates.
Owner:EVONIK DEGUSSA GMBH
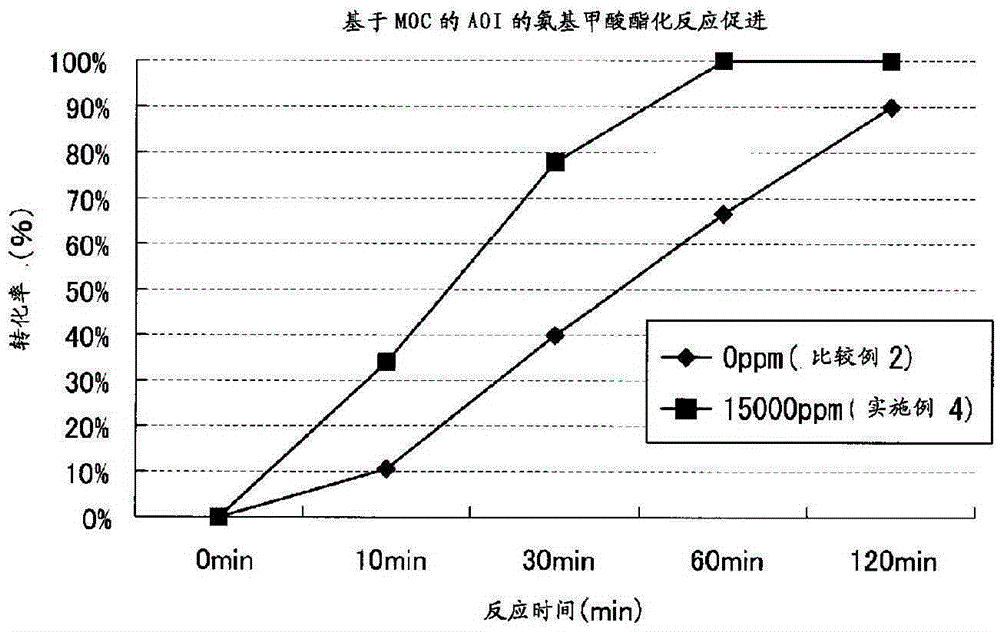
Preparation method of teriflunomide and intermediate thereof
ActiveCN103848756AHigh yieldReduce manufacturing costPreparation from carboxylic acid nitrogen analoguesCarboxylic acid nitrile preparationElectrophilic additionIsomerization
The invention belongs to the technical field of medicinal chemistry and discloses a preparation method of teriflunomide and an intermediate thereof. The preparation method of the teriflunomide provided by the invention comprises the following steps: getting a compound with a structure as shown in formula I and an azide in an organic solvent to generate nucleophilic substitution reaction to generate a compound with the structure as shown in formula II; getting and mixing the compound with the structure as shown in the formula II with the organic solvent, heating to obtain the compound with the structure as shown in formula III; under inert-gas protection and alkaline conditions, getting the compound with the structure as shown in the formula III and the compound with the structure as shown in the formula IV to generate electrophilic addition and isomerization reaction in the organic solvent to obtain the teriflunomide. According to the preparation method of the teriflunomide provided by the invention, the reaction intermediate is not needed to be purified, operation is simple, yield of the teriflunomide is improved, production cost of the teriflunomide is lowered, and therefore, the preparation method is more beneficial to industrial production of the teriflunomide.
Owner:HYBIO PHARMA WUHAN CO LTD
Method for preparing diisocyanate by heat decomposition
ActiveCN101386585BNo pollution in the processGood catalyticPreparation from carboxylic acid nitrogen analoguesAlcoholDecomposition
Owner:WANHUA CHEM GRP CO LTD
Preparation method and application of isocyanate
InactiveCN103724230AHigh bonding strengthImprove physicsPreparation from carboxylic acid nitrogen analoguesNon-macromolecular adhesive additivesFiberOrganic synthesis
The invention relates to a preparation method and application of isocyanate, particularly relates to a preparation method of C4-C6 aliphatic isocyanate prepared from nylon acid as a raw material, and an application of the C4-C6 aliphatic isocyanate, and belongs to the technical fields of organic synthesis, adhesive chemistry and composite materials. The C4-C6 aliphatic isocyanate is prepared from urea and nylon acid as raw materials in manners of firstly preparing C4-6 aliphatic amide and reacting the C4-6 aliphatic amide with oxalyl chloride. The isocyanate is applied to preparation of polyurethane foaming plastic, rubber, elastic fibers, coatings, and bonding wood based composite materials.
Owner:UNIV OF JINAN
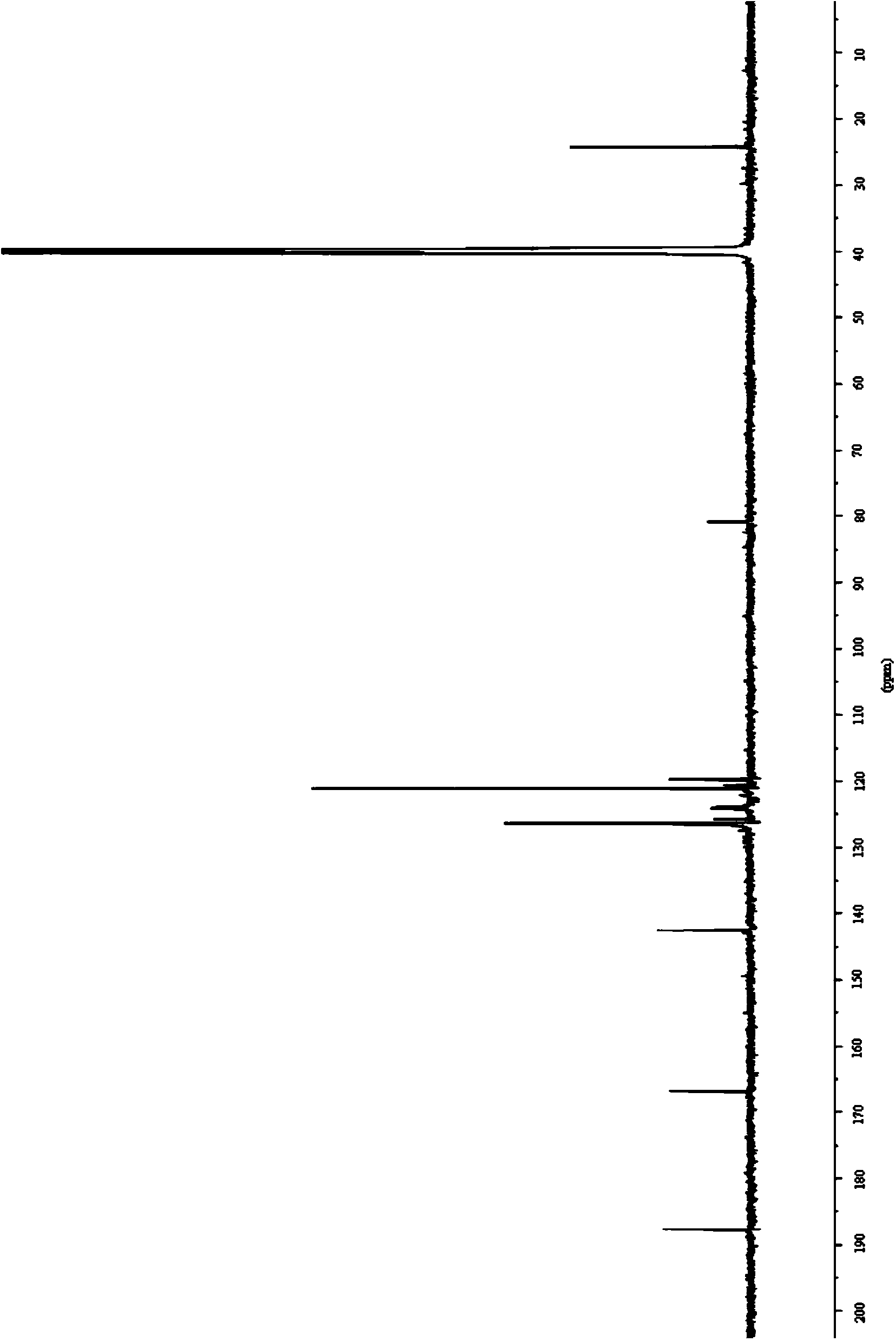
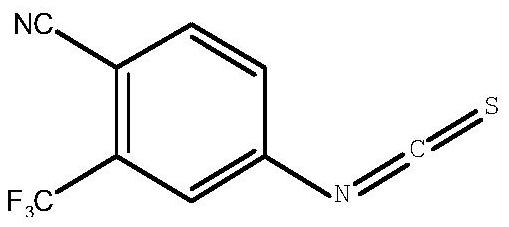
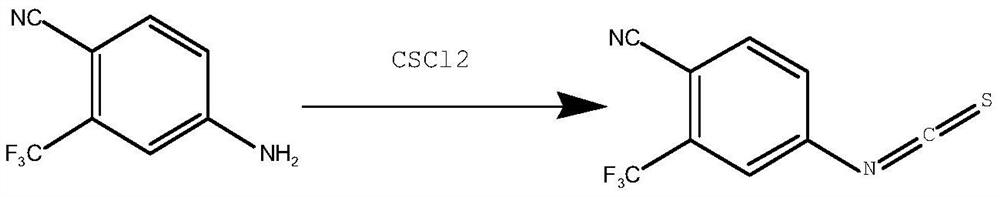
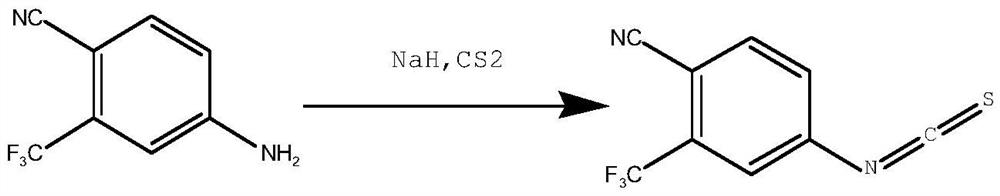
Synthesis method of 4-isothiocyanato-2-(trifluoromethyl)benzonitrile
PendingCN112876391AThe synthetic route is simpleEasy to operatePreparation from carboxylic acid nitrogen analoguesCarboxylic acid nitrile preparationPhosphoric acidTrifluoromethyl
The invention discloses a synthesis method of 4-isothiocyanato-2-(trifluoromethyl)benzonitrile. The synthesis method comprises the following steps: with 3-trifluoromethyl-4-cyanobenzoic acid as an initial raw material, firstly, subjecting 3-trifluoromethyl-4-cyanobenzoic acid to reacting with diphenyl azidophosphate under an anhydrous condition to generate 4-isocyanato-2-(trifluoromethyl)benzonitrile, and then subjecting 4-isocyanato-2-(trifluoromethyl)benzonitrile to reacting with a Lawson reagent to obtain the compound 4-isothiocyanato-2-(trifluoromethyl)benzonitrile. The method has the advantages of simple synthetic route, one-pot reaction, mild reaction conditions, simple post-treatment, high product yield (wherein total yield is greater than or equal to 83.9%), and easy industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
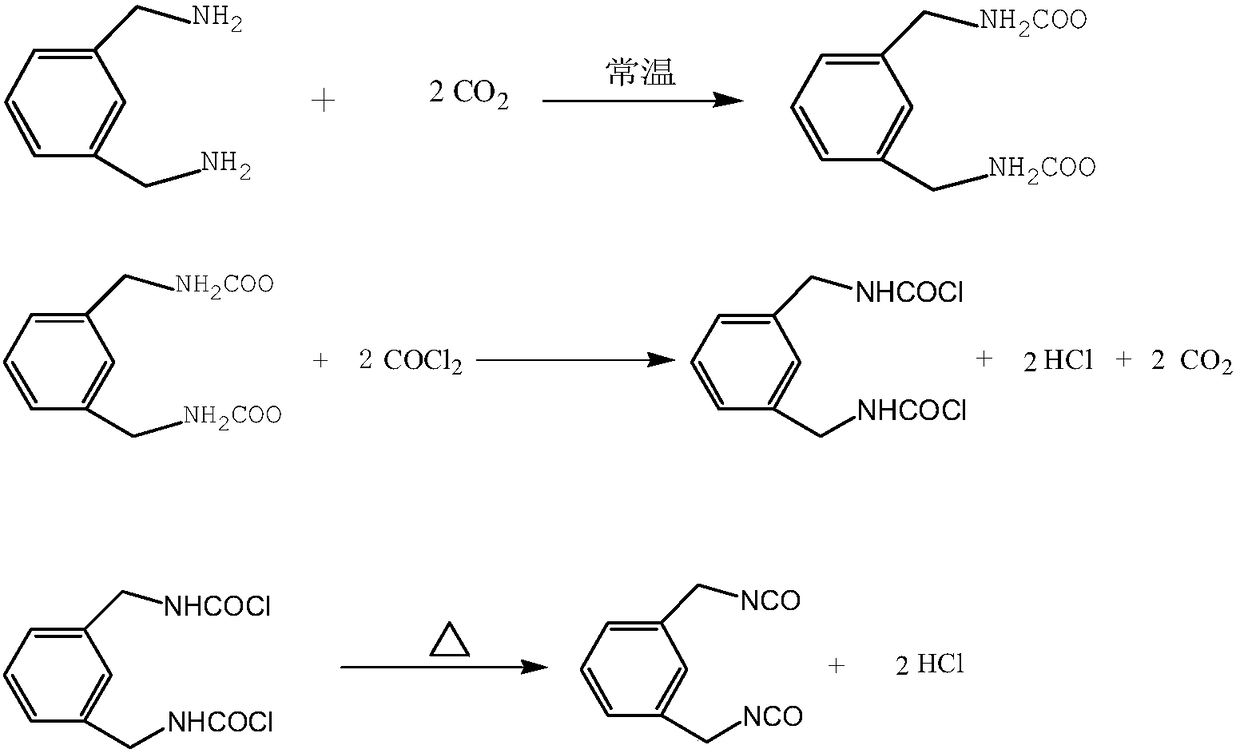
Method for preparing high-content 1,3-bis(isocyanatomethyl)benzene
InactiveCN108658809ATo avoid the generationAvoid corrosionPreparation from carboxylic acid nitrogen analoguesCarbamic acid derivatives preparationFormateSolvent
The invention discloses a method for preparing high-content 1,3-bis(isocyanatomethyl)benzene. M-xylylenediamine is used as a raw material, and under the presence of a solvent, the m-xylylenediamine and carbon dioxide are subjected to pressurization for salt forming reaction to prepare amido formate; the amido formate is reacted with phosgene to prepare the 1,3-bis(isocyanatomethyl)benzene; a method of combining cryogenic crystallization and high vacuum distillation is adopted for the 1,3-bis(isocyanatomethyl)benzene, and then the high-purity 1,3-bis(isocyanatomethyl)benzene is obtained. According to the method, the technology is green and environmentally friendly, and no large quantity of waste acid is produced; a liquid-phase salt-forming phosgenation method is adopted, so that generationof a by-product urea is avoided; a method of low-temperature crystallization and then distillation is adopted, so that the phenomenon that a high-temperature continuous rectification method is adopted to cause polymerization, and thus the product content is reduced is avoided; according to the technology, three wastes are fewer, the product content is high, the yield is high, and the economic andsocial benefits of the method are significant.
Owner:JIANGSU KUAIDA AGROCHEM
Aliphatic diisocyanate and preparation method and purposes thereof
ActiveCN101805270BHigh yieldEasy to operatePreparation from carboxylic acid nitrogen analoguesFibre treatmentElastomerPolymer science
The invention provides a type of aliphatic diisocyanate with a novel chemical structure, which is C21-36 aliphatic diisocyanate, has the same excellent performance with other traditional aliphatic isocyanates, and is used for preparing polyurethane varnish, coating, elastomer, adhesive, textile finishing agent, rocket propellant and the like. The invention also provides a preparation method of this type of substance, which comprises the following steps that: (a) unsaturated dicarboxylic acid with a special structure or the ester solution thereof are catalyzed and hydrogenised to prepare saturated diacid; (b) the saturated diacid is dissolved into inert solvent and added with chloride agent to react and prepare binary acid chloride; (c) sodium azide is added in to react and prepare the corresponding azide; and (d) the azide is isomerized and decomposed to prepare an aliphatic diisocyanate crude product. The method is characterized by lower condition requirements, safety, environmental-friendliness, fewer side effects, high yield and easy wide popularization in industry.
Owner:浙江优创材料科技股份有限公司
Synthetic method of trans-1,4-cyclohexane diisocyanate
ActiveCN101735111BSimple process routeRaw materials are easy to getPreparation from carboxylic acid nitrogen analoguesFiltrationDistillation
The invention discloses a synthesis method of trans-1,4-cyclohexane diisocyanate, which includes the following steps: (1) Mix trans-1,4-cyclohexanedicarboxylic acid and thionyl chloride, and heat Reflux and recover excess thionyl chloride under reduced pressure to obtain trans-trans-1,4-cyclohexanedicarboxyl chloride; (2) Add toluene to trans-1,4-cyclohexanedicarboxyl chloride , benzene or xylene, heat to 45-75°C, then add sodium azide to obtain a mixture; (3) Insulate the mixture at a temperature of 45-75°C for 0.5-1.5 hours; filter to remove insoluble matter and recover under reduced pressure Toluene is distilled to obtain trans-1,4-cyclohexane diisocyanate. The synthesis method of the present invention has a simple process route, easy-to-obtain raw materials, short reaction cycle, and is suitable for industrial large-scale production.
Owner:江苏恒祥化学股份有限公司
Preparation method and application of isocyanate
InactiveCN103724230BHigh bonding strengthImprove physicsPreparation from carboxylic acid nitrogen analoguesNon-macromolecular adhesive additivesFiberOrganic synthesis
The invention relates to a preparation method and application of isocyanate, particularly relates to a preparation method of C4-C6 aliphatic isocyanate prepared from nylon acid as a raw material, and an application of the C4-C6 aliphatic isocyanate, and belongs to the technical fields of organic synthesis, adhesive chemistry and composite materials. The C4-C6 aliphatic isocyanate is prepared from urea and nylon acid as raw materials in manners of firstly preparing C4-6 aliphatic amide and reacting the C4-6 aliphatic amide with oxalyl chloride. The isocyanate is applied to preparation of polyurethane foaming plastic, rubber, elastic fibers, coatings, and bonding wood based composite materials.
Owner:UNIV OF JINAN
A kind of method for preparing dimer acid diisocyanate
ActiveCN104193652BLow costMild process conditionsPreparation from carboxylic acid nitrogen analoguesPolyurethane elastomerDimer acid
The invention discloses a method for preparing dimer acid diisocyanate. The method comprise the steps of preparing dimer acid dihydrazide by reacting a dimer acid diester and an aqueous solution of hydrazine, reacting the dimer acid dihydrazide with the aqueous solution of sodium nitrite to generate dimer acid diacyl azide, and pyrolyzing the dimer acid diacyl azide to obtain the dimer acid diisocyanate. The method has the advantages of mild process conditions, good product appearance, low synthesis cost and the like. A polyurethane elastomer having the elongation percentage of 1000% and the tensile strength of 10MPa at a normal temperature can be prepared by reacting the dimer acid diisocyanate with hydroxyl-terminated polybutadiene and butanediol.
Owner:LIMING RES INST OF CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com