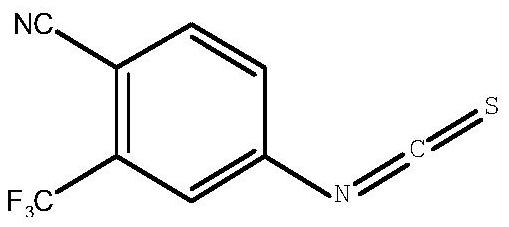
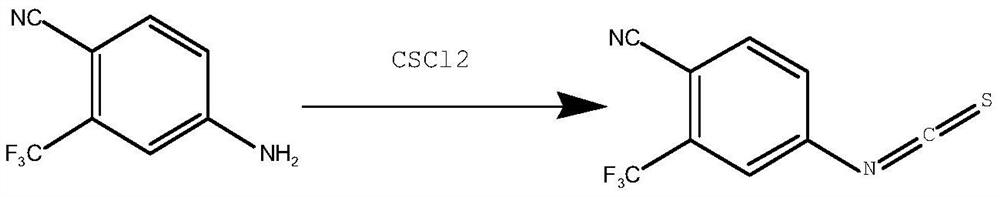
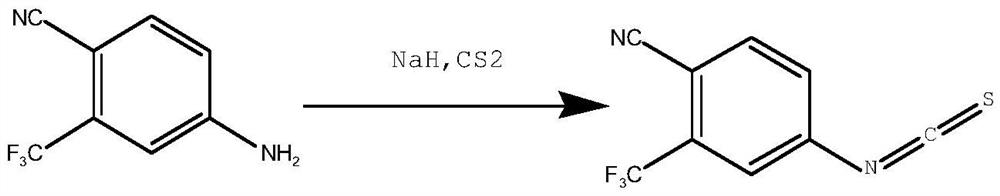
Synthesis method of 4-isothiocyanato-2-(trifluoromethyl)benzonitrile
A technology of trifluoromethyl and isothiocyanate, applied in the field of drug synthesis, can solve the problems of environmental hazards, cumbersome operations, and low yields, and achieve the effects of mild reaction conditions, simple synthetic routes, and high product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Synthesis of 4-isocyanate-2-(trifluoromethyl)benzonitrile
[0028] Add 20 g of 3-trifluoromethyl-4-cyanobenzoic acid and 28.14 g of diphenylphosphoryl azide into 80 ml of toluene, raise the temperature to 45 ° C, stir for 3.5 h, and TLC detects that the reaction is complete (TLC, ethyl acetate : n-hexane=1:1), the reaction solution is down to room temperature for stand-by, to obtain 4-isocyanic acid-2-(trifluoromethyl)benzonitrile toluene solution;
[0029] 2) Synthesis of 4-isothiocyanato-2-(trifluoromethyl)benzonitrile
[0030] Add 41.36 g of Lawson's reagent to the 4-isocyanic acid-2-(trifluoromethyl)benzonitrile toluene solution, raise the temperature to 35°C and keep it warm for 4.5h, and TLC detects that the reaction is complete (TLC, ethyl acetate: di Chloromethane=1:1), add 50ml of saturated sodium bicarbonate to wash, wash with water, evaporate the solvent to dryness, add 150ml of n-heptane, beat and stir for 0.5 hours, filter, wash the filter cake with n-h...
Embodiment 2
[0032] 1) Synthesis of 4-isocyanate-2-(trifluoromethyl)benzonitrile
[0033] Add 20 g of 3-trifluoromethyl-4-cyanobenzoic acid and 28.10 g of diphenylphosphoryl azide into 80 ml of toluene, heat up to 50°C, stir for 3 hours, and TLC detects that the reaction is complete (TLC, ethyl acetate: n-Hexane=1:1). The reaction solution was lowered to room temperature for use to obtain 4-isocyanic acid-2-(trifluoromethyl)benzonitrile toluene solution.
[0034] 2) Synthesis of 4-isothiocyanato-2-(trifluoromethyl)benzonitrile
[0035] Add 41.30 g of Lawson's reagent to the 4-isocyanic acid-2-(trifluoromethyl)benzonitrile toluene solution, raise the temperature to 40°C and keep it warm for 4 hours, and TLC detects that the reaction is complete (TLC, ethyl acetate: dichloro Methane=1:1), add 50ml of saturated sodium bicarbonate to wash, wash with water, evaporate the solvent to dryness, add 150ml of n-heptane, beat and stir for 0.5 hours, filter, wash the filter cake with n-heptane, and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com