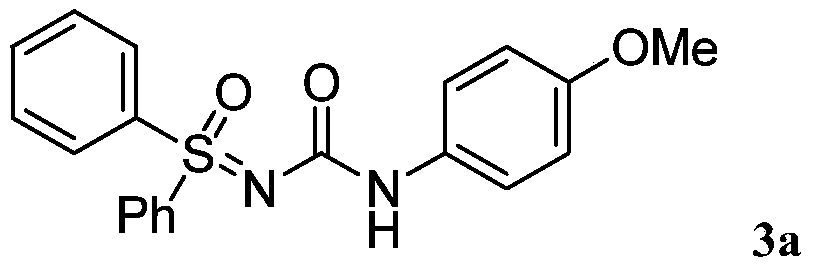
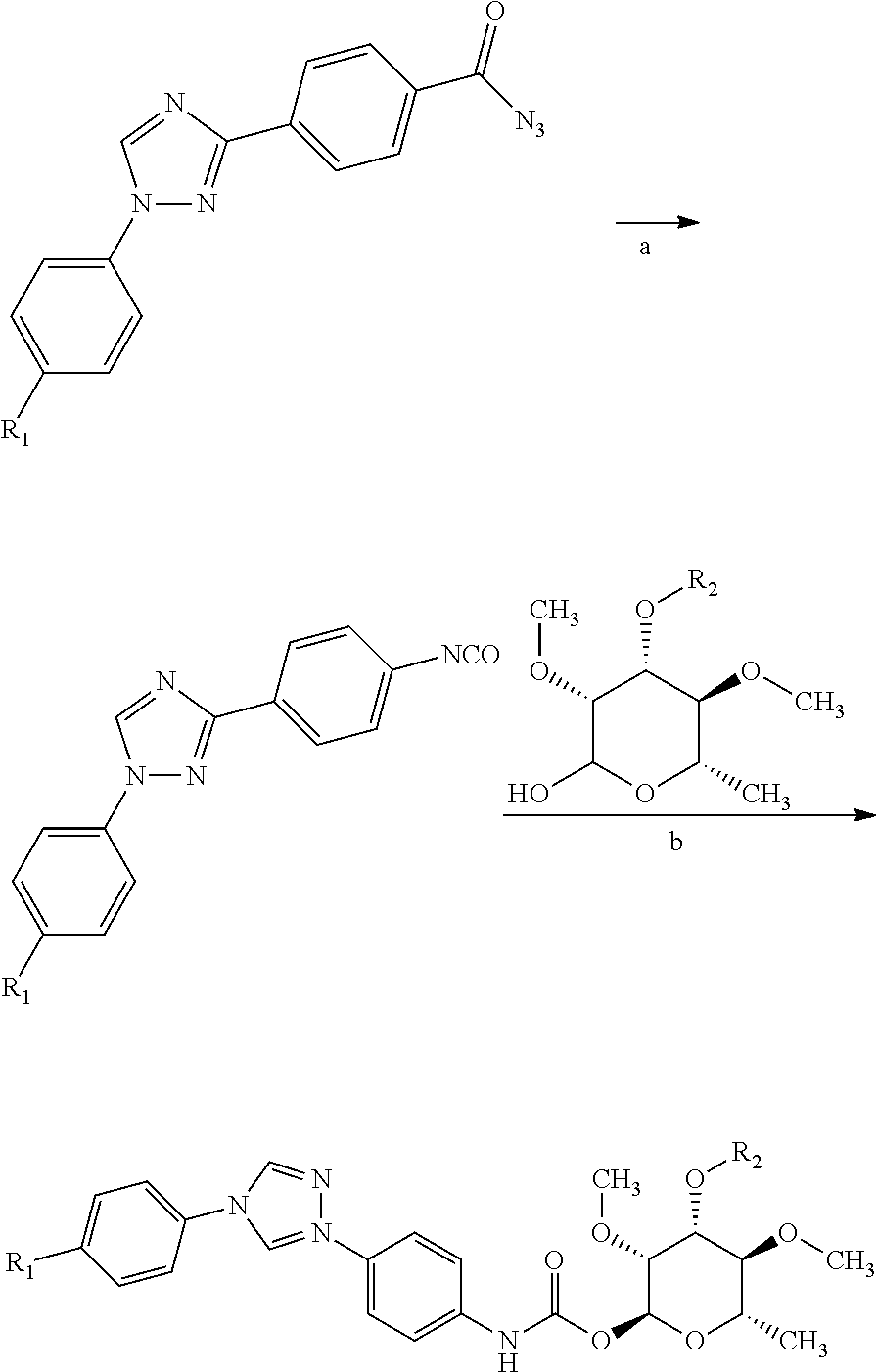
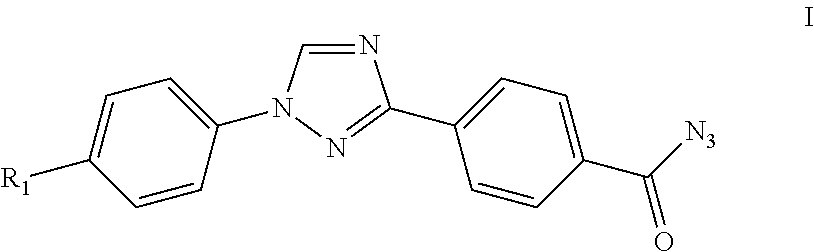
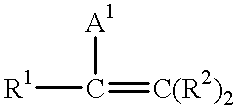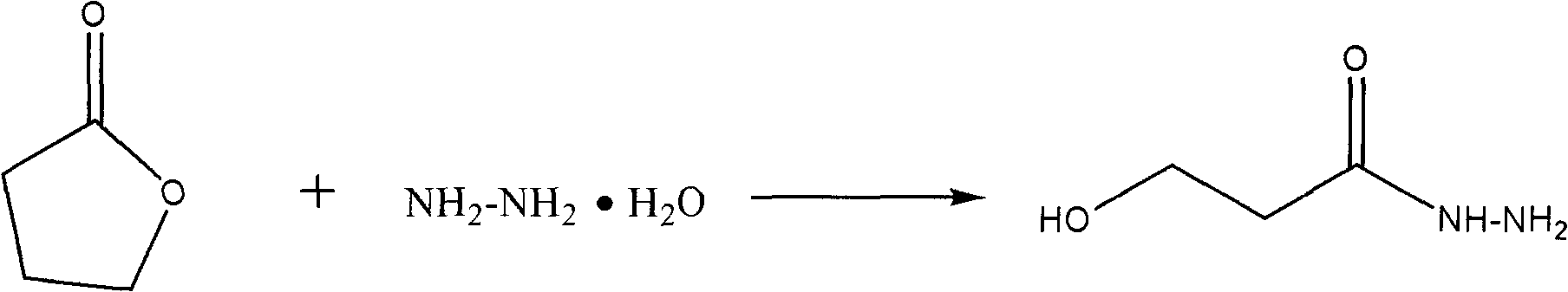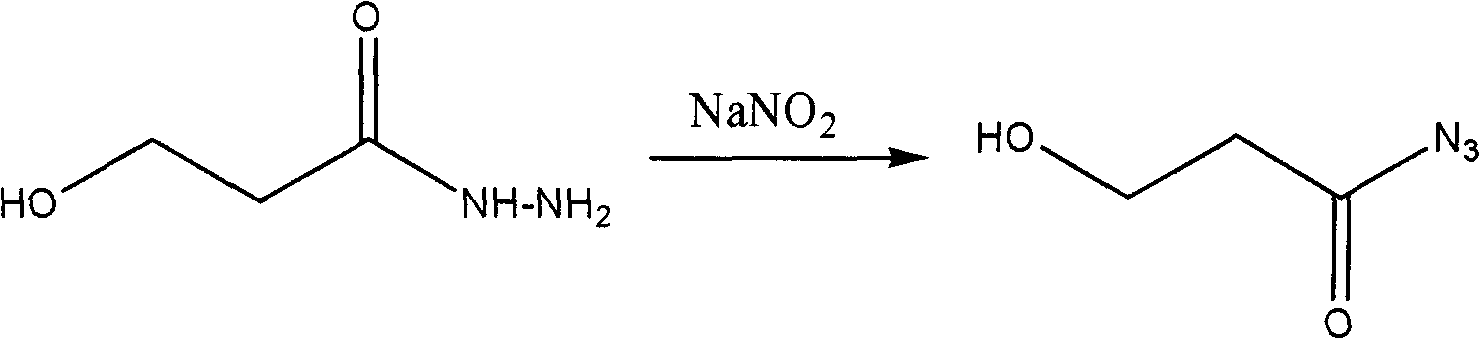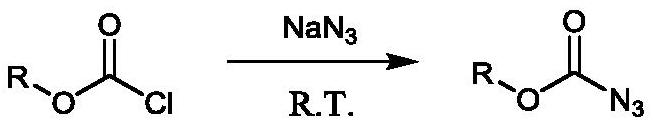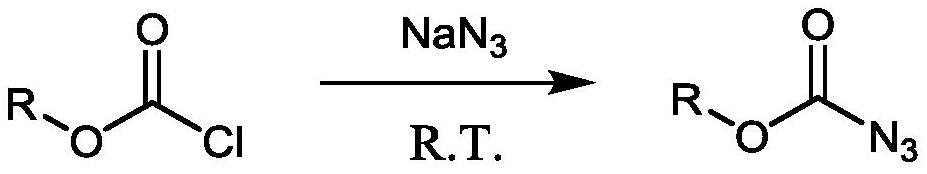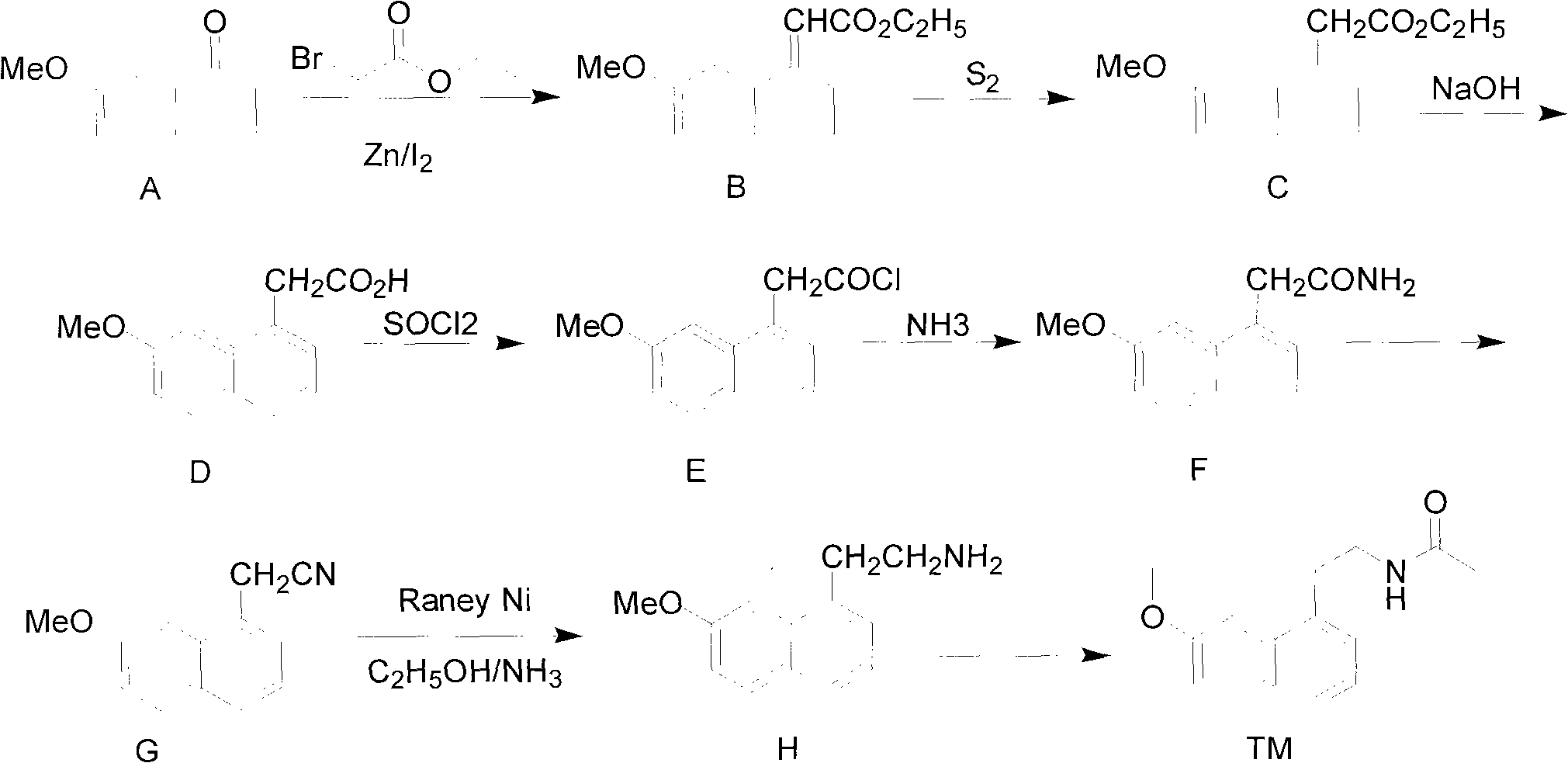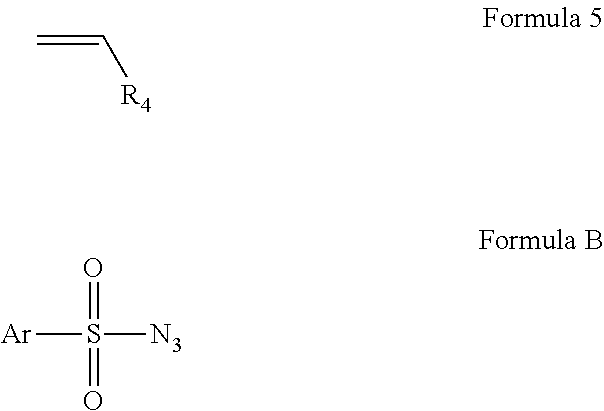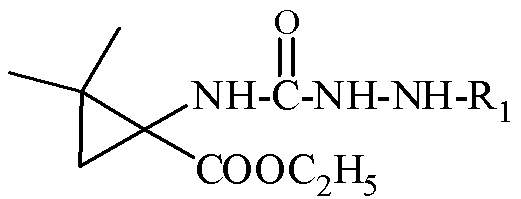Patents
Literature
38 results about "Acyl azide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acyl azides are carboxylic acid derivatives with the general formula RCON₃.
Process for preparing thermoplastic vulcanizates
This invention includes a process for forming a thermoplastic vulcanizate comprising: (a) admixing a C-H insertion curing agent with at least one elastomeric phase polymer to form a first admixture; (b) admixing at least one non-elastomeric polyolefin with the first admixture to form a second admixture; and (c) heating the second admixture to a temperature at least the decomposition temperature of the curing agent to crosslink the elastomeric phase while mixing the admixture to an extent sufficient to result in the formation of a thermoplastic material, hereinafter referred to as a thermoplastic vulcanizate, and optionally including an additional step (d) of shaping the resulting thermoplastic vulcanizate, especially by heating and foaming or molding the TPV. The C-H insertion curing agent is preferably selected from alkyl and aryl azides (R-N3), acyl azides (R-C(O)N3), azidoformates (R-O-C(O)-N3), sulfonyl azides (R-SO2-N3), phosphoryl azides ((RO)2-(PO)-N3), phosphinic azides (R2-P(O)-N3) and silyl azides (R3-Si-N3), with poly(sulfonyl azide) most preferred. Additionally, the invention includes a thermoplastic vulcanizate comprising a blend of: (1) an elastomeric phase crosslinked using a C-H insertion curing agent dispersed in; (2) at least one non-elastomeric thermoplastic polyolefin. The invention also includes a foamable composition comprising (1) an elastomeric phase crosslinked using a C-H insertion curing agent dispersed in; (2) at least one non-elastomeric thermoplastic polyolefin; and (3) from about 0.1 to about 25 percent by weight based on the combined weight of components (1) and (2) of at least one foaming agent as well as a fabricated part, cable jacket, cable insulation, or foam comprising the thermoplastic vulcanizate or the invention or resulting from the process of the invention.
Owner:THE DOW CHEM CO
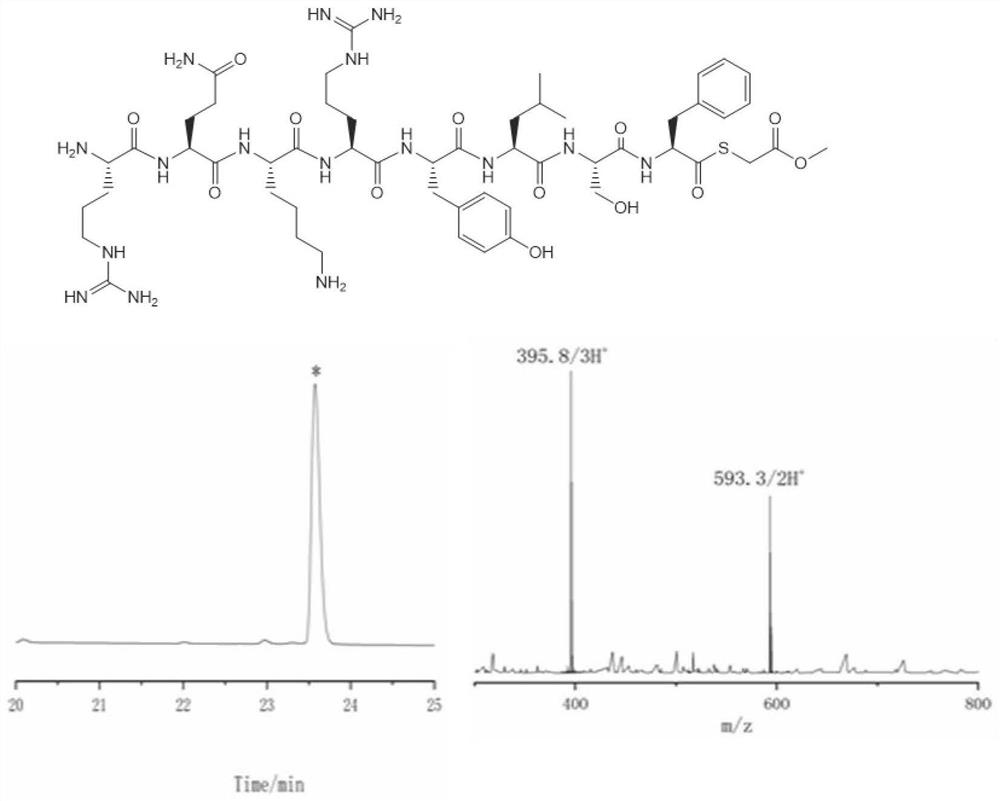
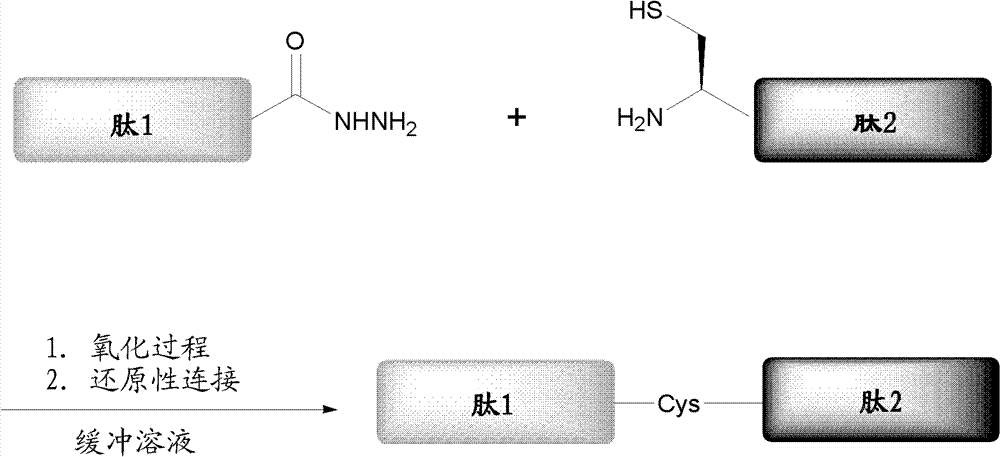
Protein preparation method
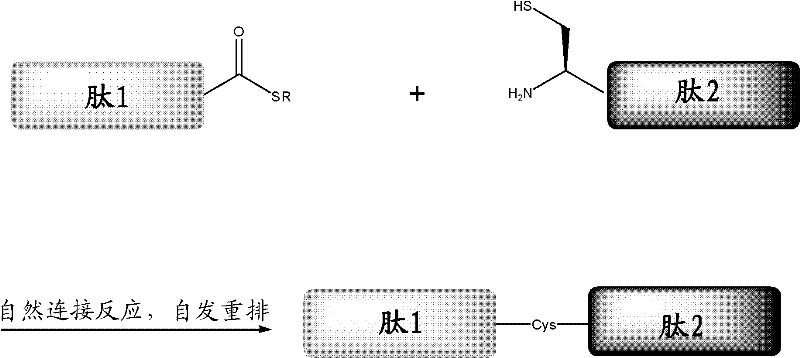
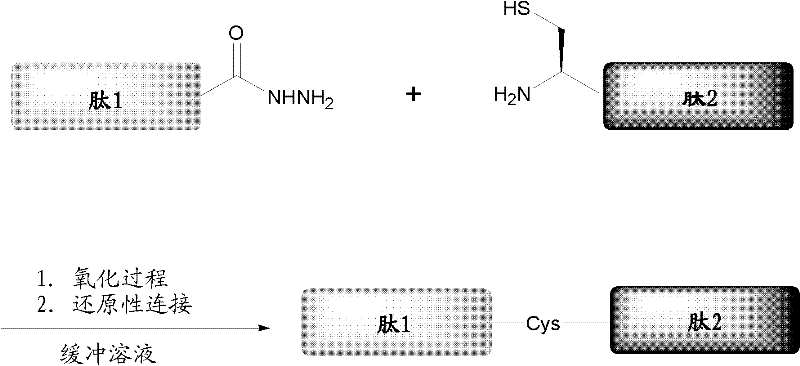
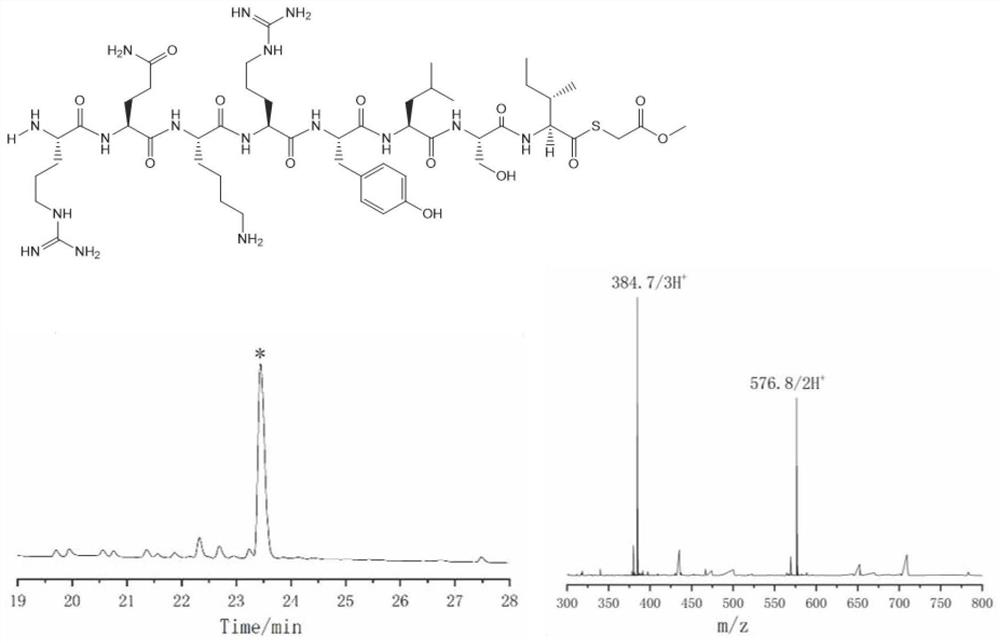
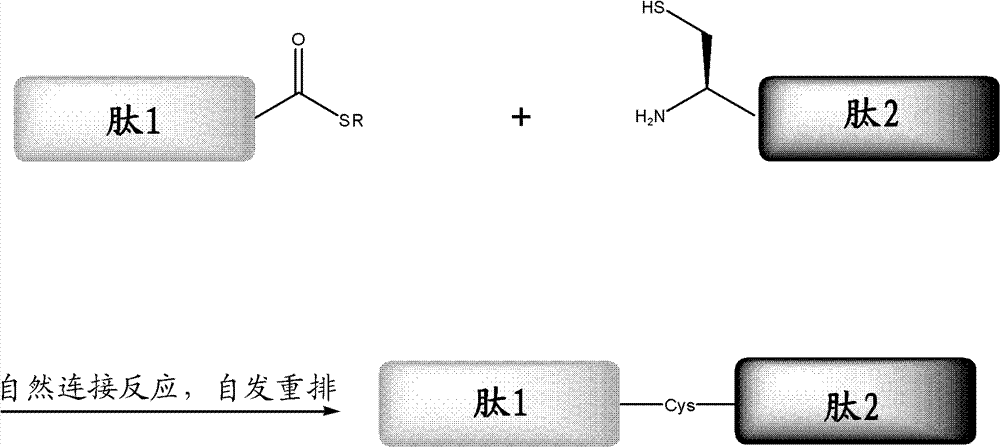
ActiveCN102199214AEffective peptide linkagePeptide preparation methodsHybrid peptidesThioester synthesisCrystallography
A protein preparation method provided by the invention comprises the following steps to connect a first peptide with hydrazide group on C terminus and a second peptide with cysteine on N terminus: mixing the first peptide and the second peptide in a solution containing NaNO2 and adjusting the mixed solution pH to an acidic pH so as to converting the hydrazide group of the first peptide into acyl azide group to obtain a first reaction mixture; adding a solution containing a reducing agent into the first reaction mixture and adjusting the pH to a neutral pH, wherein the reducing agent contains mercapto group, so as to convert the acyl azide group of the first peptide into thioester group, and maintaining the neutral pH so as to conducting a connection reaction between the thioester on the Cterminus of the first peptide and the N terminus of the second peptide to produce proteins. A method for preparing polypeptide thioester compounds is also provided. According to the protein preparation method provided by the invention, proteins can be effectively synthesized.
Owner:TSINGHUA UNIV
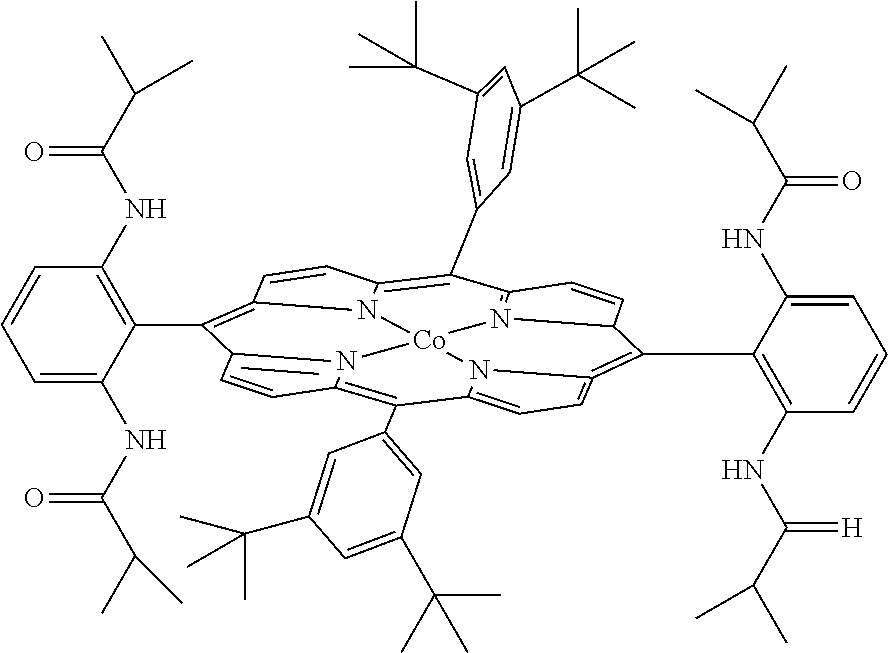
Intramolecular C-H amination with sulfonyl azides
InactiveUS20100063277A1Good yieldFunctional group formation/introductionOrganic chemistryPyrenesulfonyl azideNitrogen gas
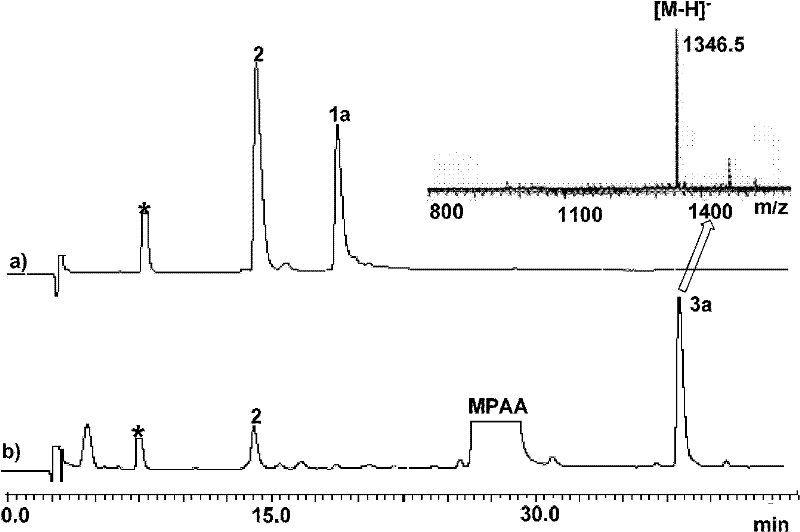
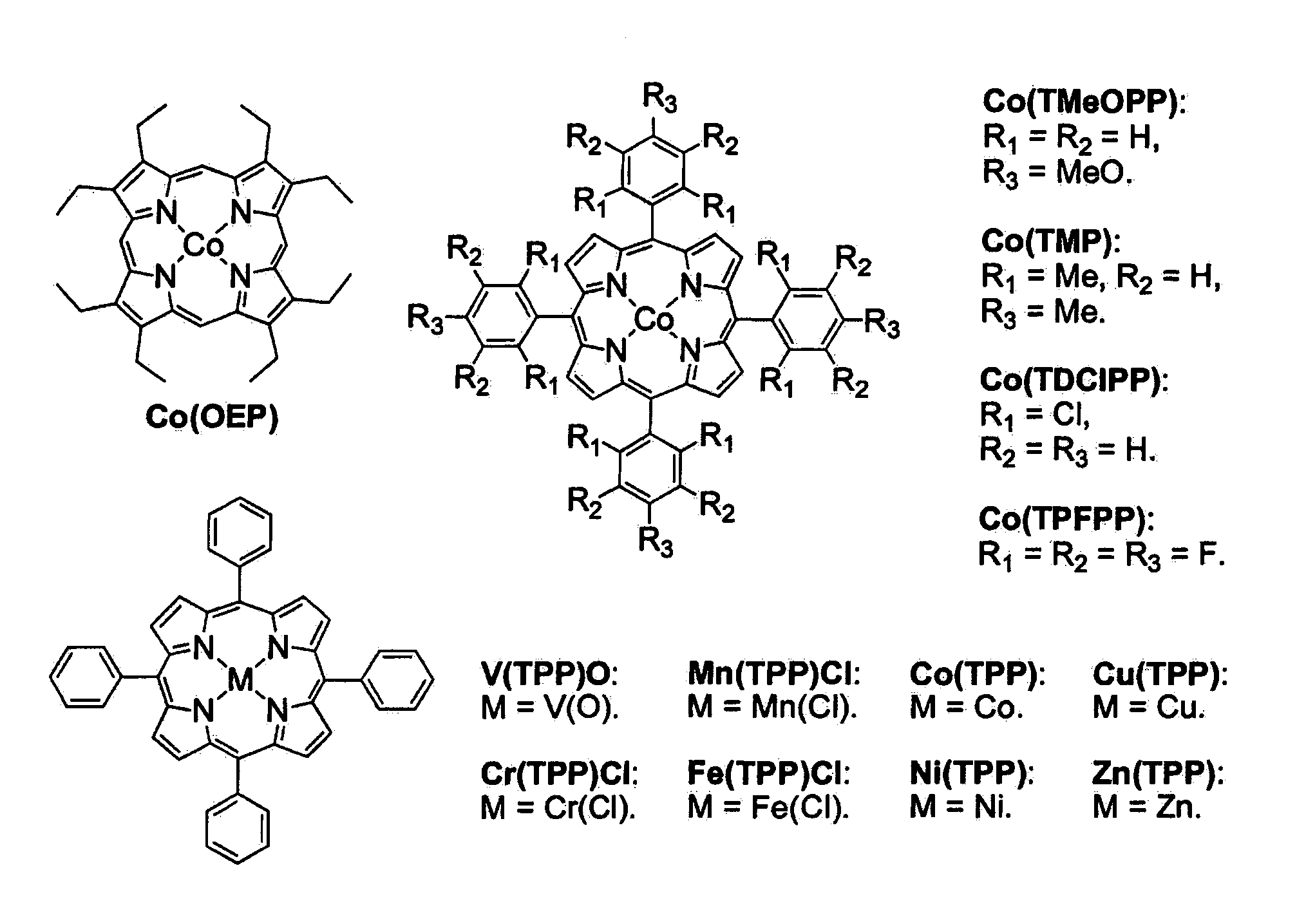
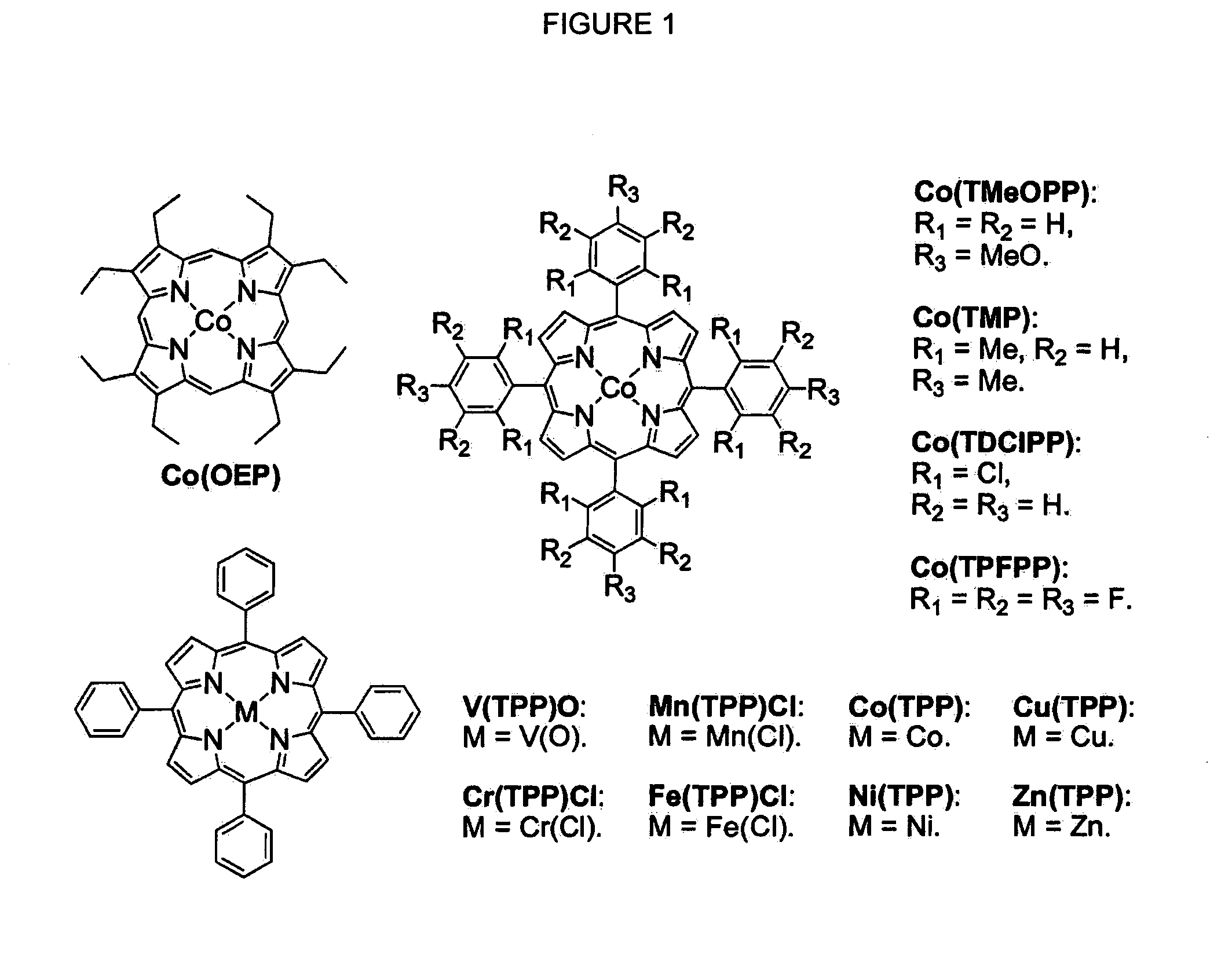
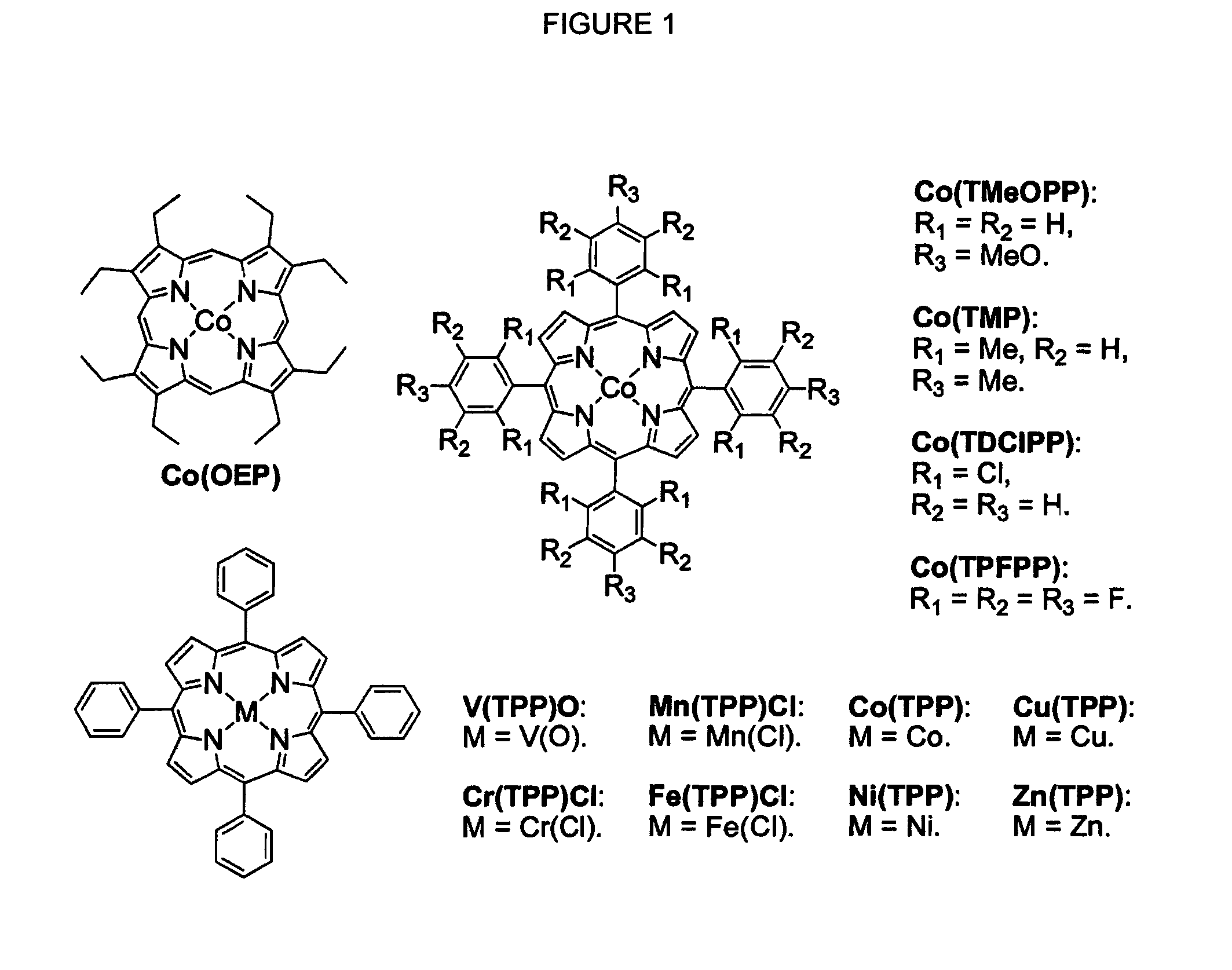
Cobalt (II) complexes of porphyrins are effective catalysts for intramolecular nitrene insertion of C—H bonds with arylsulfonyl azides. The cobalt-catalyzed process can proceed efficiently under mild and neutral conditions in low catalyst loading without the need of other reagents or additives, generating nitrogen gas as the only byproduct. Using the simple tetraphenylporphyrin (TPP) as the ligand, the cobalt-catalyzed intramolecular amidation can be applied to primary, secondary, and tertiary C—H bonds and suitable for a broad range of arylsulfonyl azides, leading to the syntheses of various benzosultam derivatives in excellent yields
Owner:UNIV OF SOUTH FLORIDA
Process for synthesizing an aminopropanol
InactiveCN103012165ASimple stepsEasy to operateOrganic compound preparationAmino-hyroxy compound preparationAminopropanolsAqueous solution
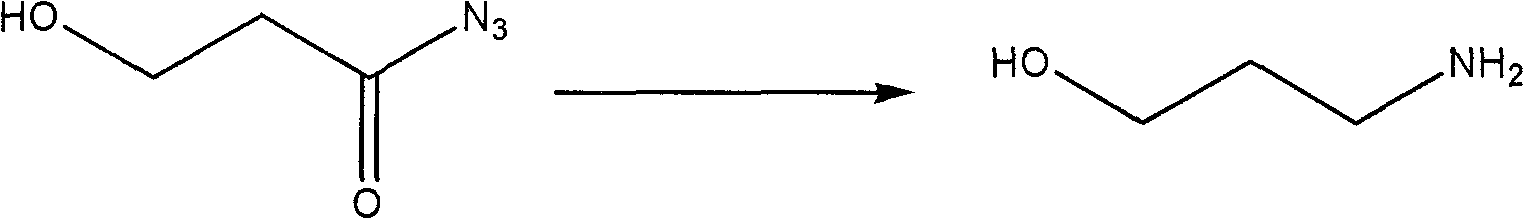
The invention relates to a process for synthesizing a medicinal intermediate and particularly relates to a process for synthesizing an aminopropanol, which has the advantages of safe raw materials, high yield and mild reaction condition. According to the technical scheme of the invention, ring opening is carried out on 1,4-butyrolactone, serving as a raw material, under the action of hydrazine hydrate, the aqueous solution of sodium nitrite is added to generate acyl azide, and finally the rearranging is carried out to generate 3-aminopropanol.
Owner:李兰心
Rare-earth metal luminous sensitizing agent, rare earth complex thereof, synthesis and use
ActiveCN101497586ASolid-state devicesSemiconductor/solid-state device manufacturingChemical synthesisFluorescence
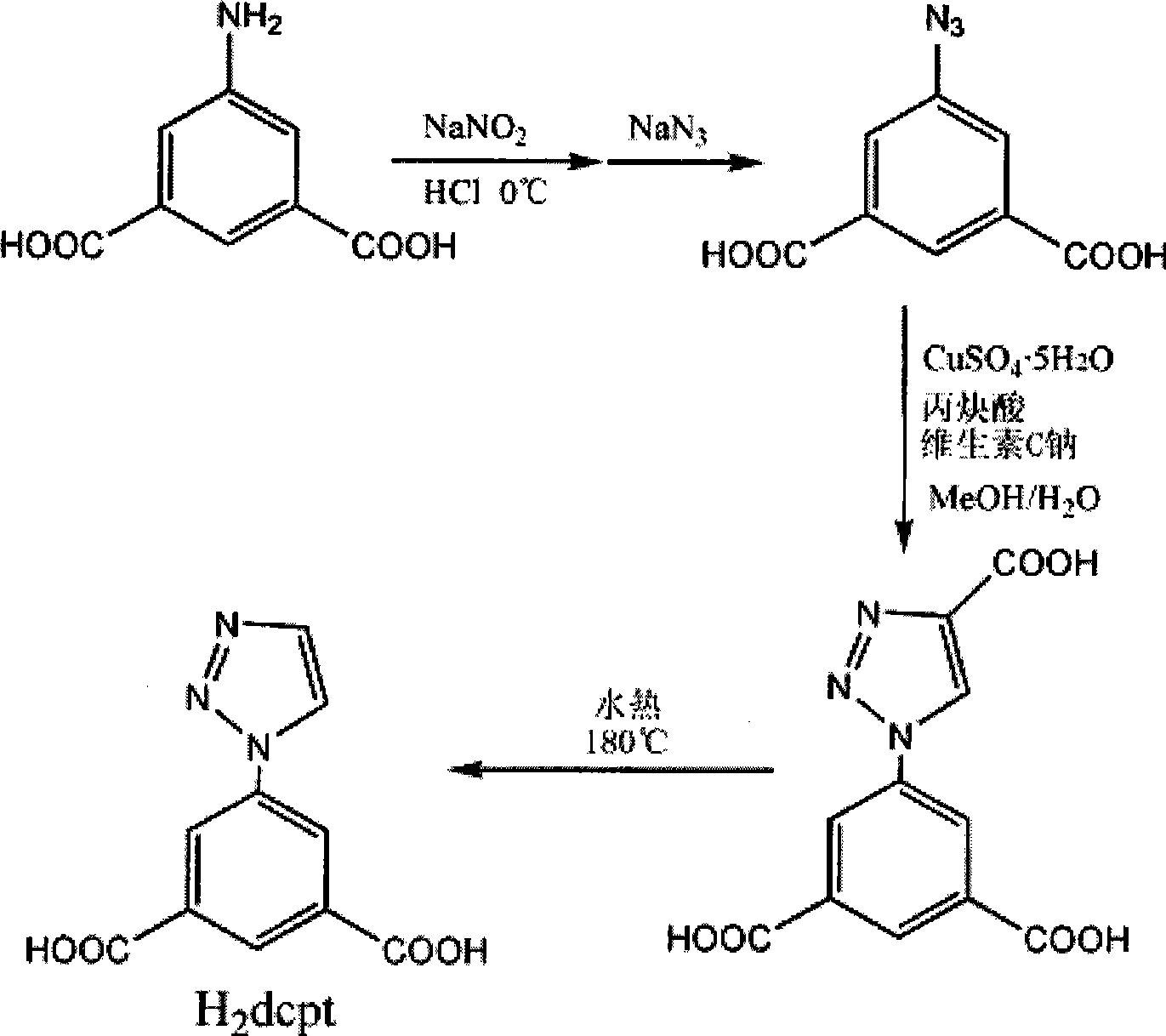
The invention provides a rare earth metal luminous sensitizer, a rare earth complex thereof and synthesis and application thereof, and relates to the field of chemical synthesis. The sensitizer is prepared by four steps which are diazo reaction, acyl azide substitution, cycloaddition and hydrothermal decarboxylation. A luminous material of the rare earth complex is prepared by hydrothermal reaction and slow cooling. The mol ratio of the materials of Ln(NO3)3.5H2O, H2dcpt and NaOH is 1:1:1, H2O is used as a solvent, the temperature in the hydrothermal reaction is raised to be 180 DEG C, and the cooling speed is 1 DEG C / hour. Tests show that H2dcpt is a quite effective sensitizer, and can improve the fluorescence radiation of the rare earth metal. The rare earth complex can be used to produce luminescent devices.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Synthesis method of dimer(fatty acid)yl diisocyanate
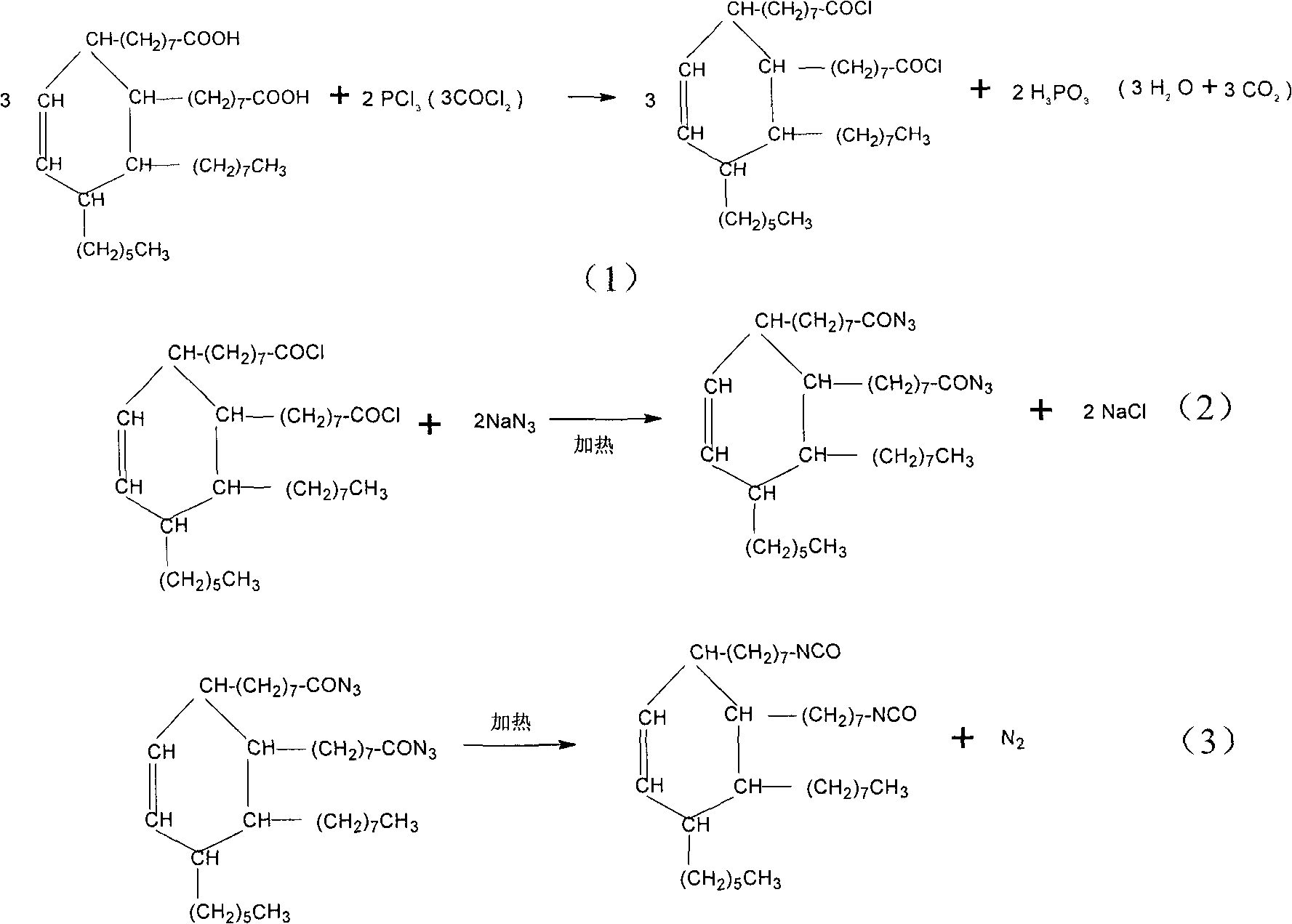
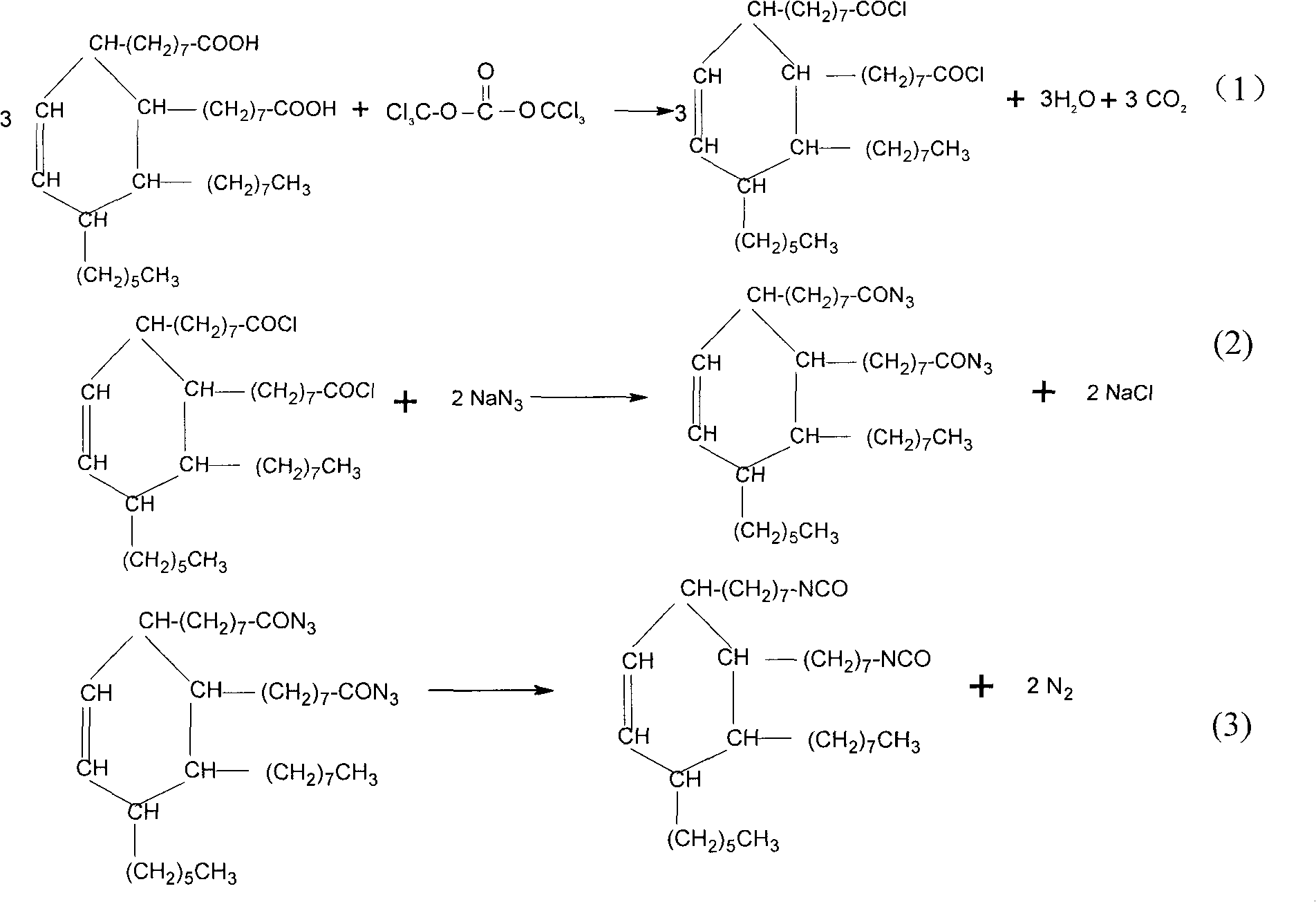
InactiveCN101830832AHigh yieldPreparation from carboxylic acid nitrogen analoguesSynthesis methodsSodium azide
The invention discloses a synthesis method of dimer(fatty acid)yl diisocyanate, which comprises the steps of: adding a methylbenzene solution of dimer(tall oil acid) and dimethylformamide into a reaction bulb, dropping a methylbenzene solution containing di(trichloromethyl) carbonic ester under the stirring at a temperature of 70-80 DEG C, reacting for 1h, filtering, evaporating to remove methylbenzene to obtain dimer(tall oil acid) acyl chloride, wherein the mol ratio of the dimer(tall oil acid) to the di(trichloromethyl) carbonic ester is 3:1-1.5; adding sodium azide and deionized water into the reaction bulb, dropping an acetone solution dissolved with the dimer(tall oil acid) acyl chloride under the stirring, adding 300ml of normal hexane, dimixing, washing a normal hexane layer by using cold water, drying by using anhydrous sodium sulfate to obtain an anhydrous sodium sulfate solution of dimer(tall oil acid) acyl azide, wherein the mol ratio of the dimer(tall oil acid) acyl chloride to the sodium azide is 1:2-2.2; and adding 300ml of normal hexane into the reaction bulb, dropping the normal hexane solution under the stirring at a temperature of 65-70 DEG C, and continuing to react for 30min after the dropping to obtain a target product.
Owner:XIAN MODERN CHEM RES INST
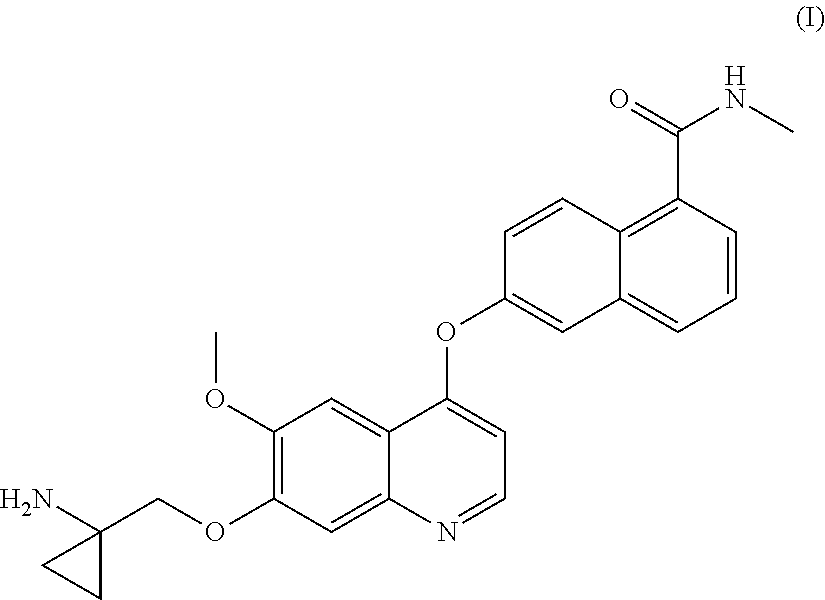
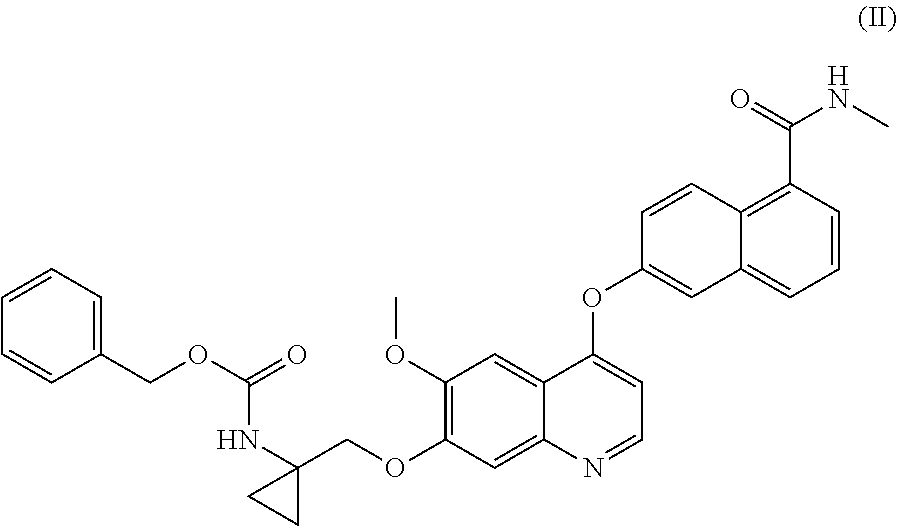
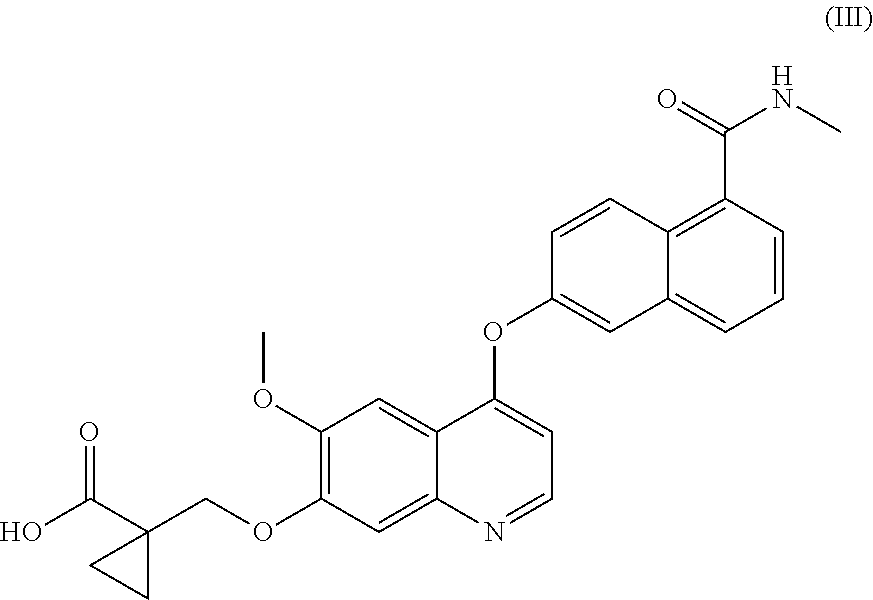
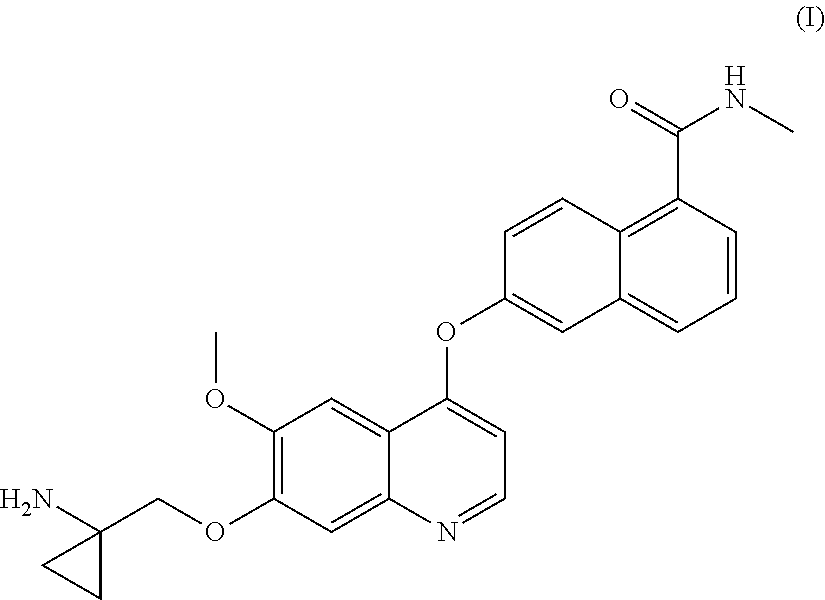
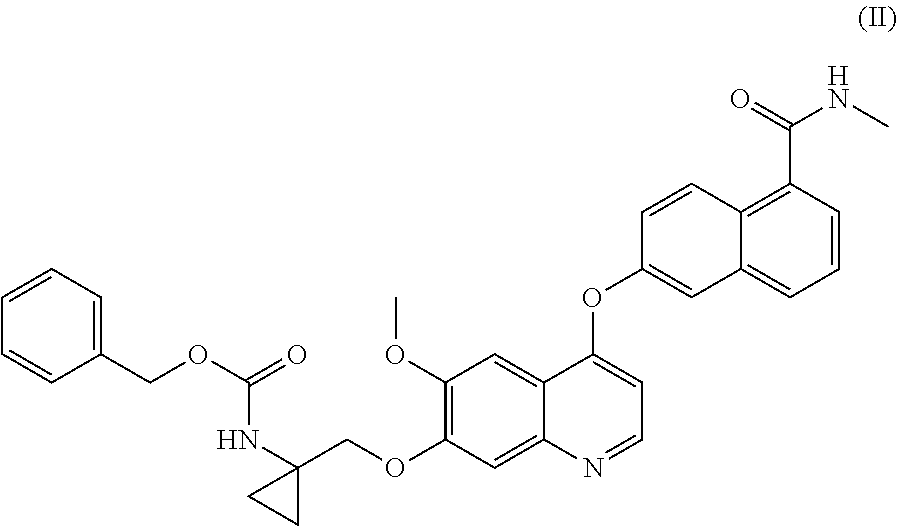
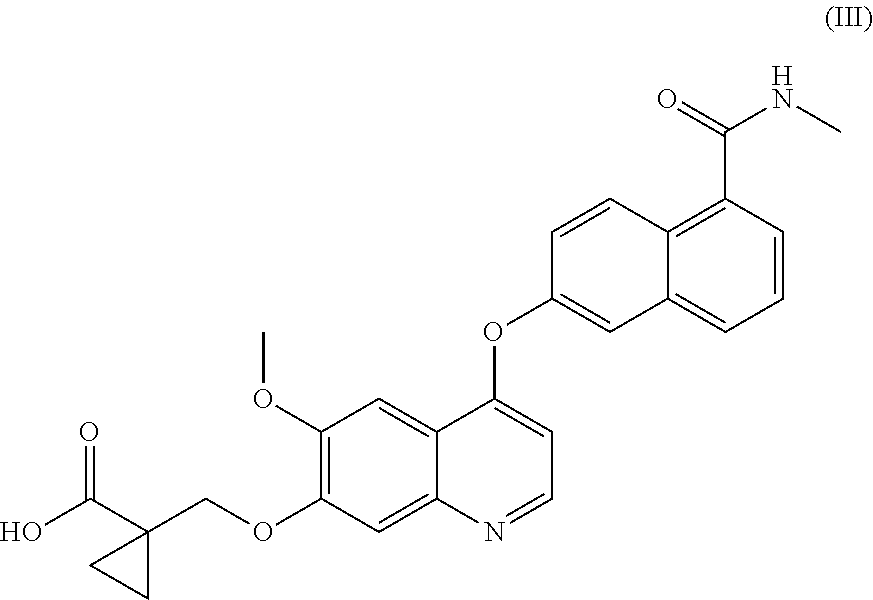
Process for the preparation of 6-(7-((1-aminocyclopropyl)methoxy)-6-methoxyquinolin-4-yloxy)-N-methyl-1-naphthamide and synthetic intermediates thereof
ActiveUS8642767B2High purityHigh yieldCarbamic acid derivatives preparationOrganic compound preparationCombinatorial chemistryMethyl group
Owner:CLOVIS ONCOLOGY ITAL SRL
Method for preparing amine compounds based on novel catalytic Curtius rearrangement reaction
ActiveCN112028814AEfficient synthesisQuick buildCarbamic acid derivatives preparationOrganic compound preparationArylNatural product
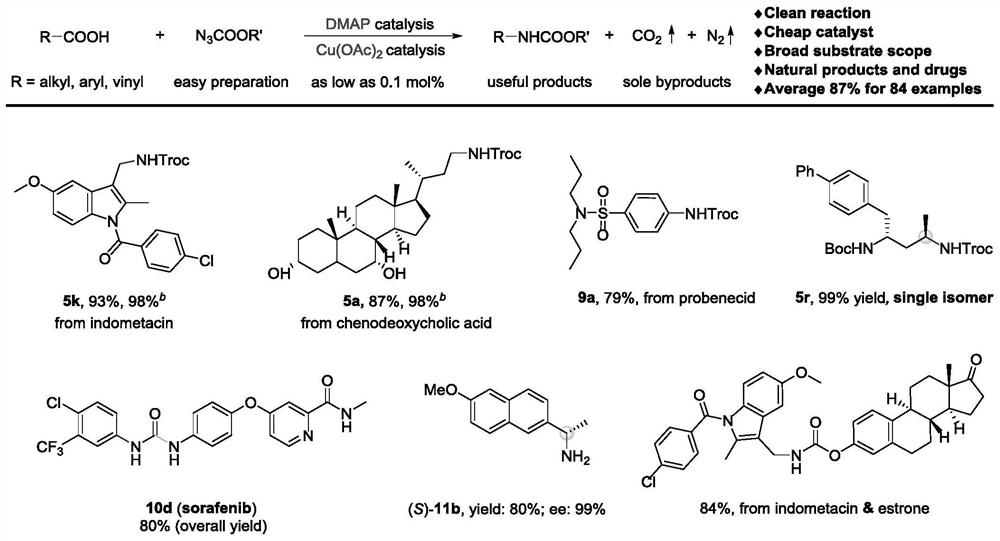
The invention relates to a method for preparing amine compounds based on a novel catalytic Curtius rearrangement reaction. Transition metal catalyzed formation of sp<2> C-N bond is an effective methodfor synthesizing arylamine, a coupling reaction for catalyzing sp<3> C-N bond is also reported, but a method for simultaneously realizing generation of sp<2> C-N bond and sp<3> C-N bond is relativelynot fully developed. According to the method, organic carboxylic acid with rich resources is used as a carbon source, alkyl / aryloxy acyl azide easy to prepare is used as a nitrogen source, under thecatalysis of DMAP and Cu(OAc)2 as low as 0.1mol%, gas N2 and CO2 are used as unique byproducts, and protected alkyl, alkenyl and aryl amine compounds are generated through a one-pot method. The reaction can be applied to later functionalization of natural products and drug molecules, synthesis of chiral alkylamine and rapid construction of different ureas and primary amines. Mechanism research shows that the reaction is carried out through cascade carboxylic acid activation, azidation, Curtius rearrangement and nucleophilic addition reaction.
Owner:NANJING UNIV
Modified polyaryletherketone (PAEK) polymer and method for obtaining the same
The invention relates to a modified polyaryletherketone (PAEK) polymer having surfaces chemically modified with azides, alkynes, thiols, maleimides, sulphonyl azides or thio acids, suitable for click reactions, and to a method for obtaining it. The invention further concerns conjugated biomaterials derived therefrom, PAEK-type materials having surfaces modified with an RGD (Arg-Gly-Asp) and / or OGP 10-14 (Tyr-Gly-Phe-Gly-Gly) peptidomimetic, and to a method for obtaining it. These materials are particularly useful for manufacturing medical devices. Finally, the invention also relates to a fluorescent PAEK material.
Owner:UNIV DEL PAIS VASCO EUSKAL HERRIKO UNIBERTSITATEA +2
Method for preparing buserelin by liquid-phase total synthesis
InactiveCN107602668AHigh yieldLow costLuteinising hormone-releasing hormonePeptide preparation methodsDiseaseProstate cancer
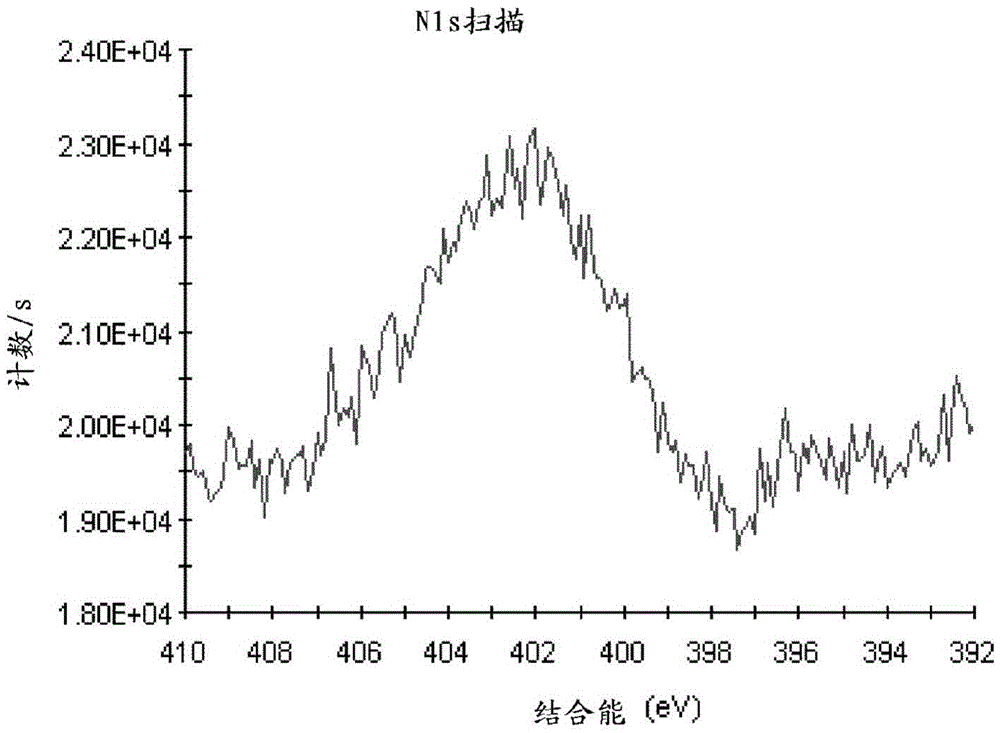
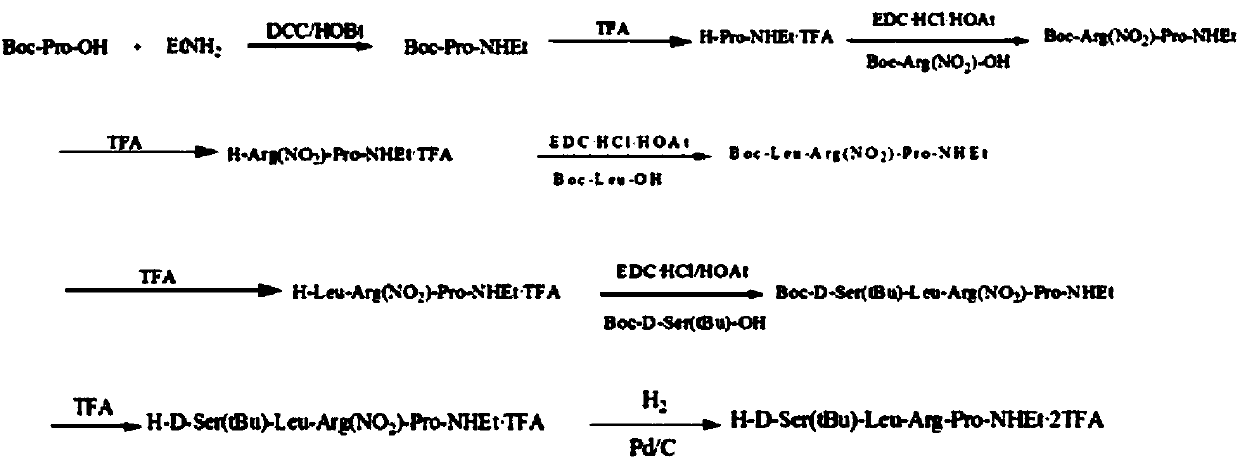
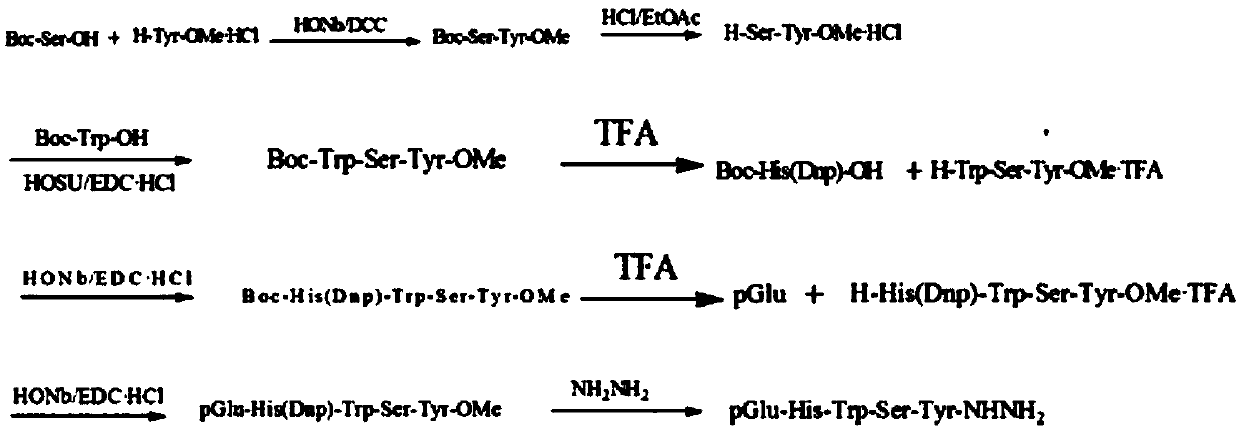
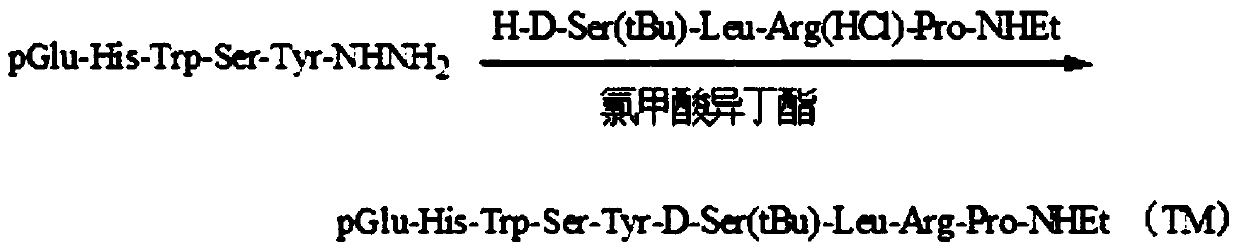
The invention belongs to the technical field of pharmacy, relates to a polypeptide type medical compound, and in particular relates to a method for preparing buserelin by liquid-phase total synthesis.The buserelin is obtained by totally synthesizing tetrapeptide H-D-Ser(tBu)-Leu-Arg-Pro-NHEt and pentapeptide PGlu-His-Trp-Ser-Tyr-NHNH2 in an acyl azide coupling manner through combined protection by Boc and Fmoc. A C terminal tetrapeptide (H-D-Ser(tBu)-Leu-Arg-Pro-NHEt) of the buserelin is subjected to technical improvement; Boc-D-Ser(tBu)-OH is applied to liquid-phase synthesis of the buserelin for the first time; Boc removal is realized under an acidic condition and tert-butyl is not influenced; finally, Boc-Pro-OH, Boc-Arg(NO2)-OH, Boc-Leu-OH and Boc-D-Ser(tBu)-OH are used as basic reagents, so that the yield of the tetrapeptide is improved, and the cost is remarkably is reduced; reaction conditions and the cost are relatively proper. A result shows that the yield of synthesizing a tetrapeptide segment is easy to improve and the purification is facilitated by a manner of removing nitryl again after tetrapeptide synthesis is finished. The prepared buserelin can be used for clinically treating diseases including breast cancer, prostate cancer, endometriosis, infertility and the like, can be widely applied to the pharmaceuticals industry and has extremely high economic value andsocial value.
Owner:HEFEI NORMAL UNIV
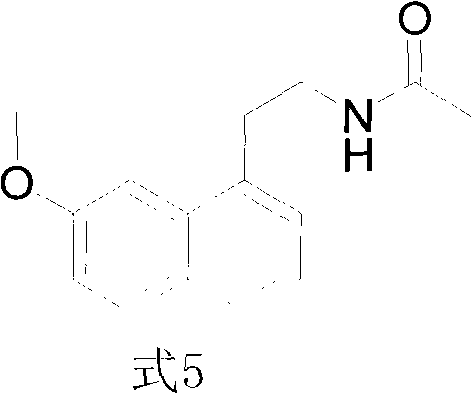
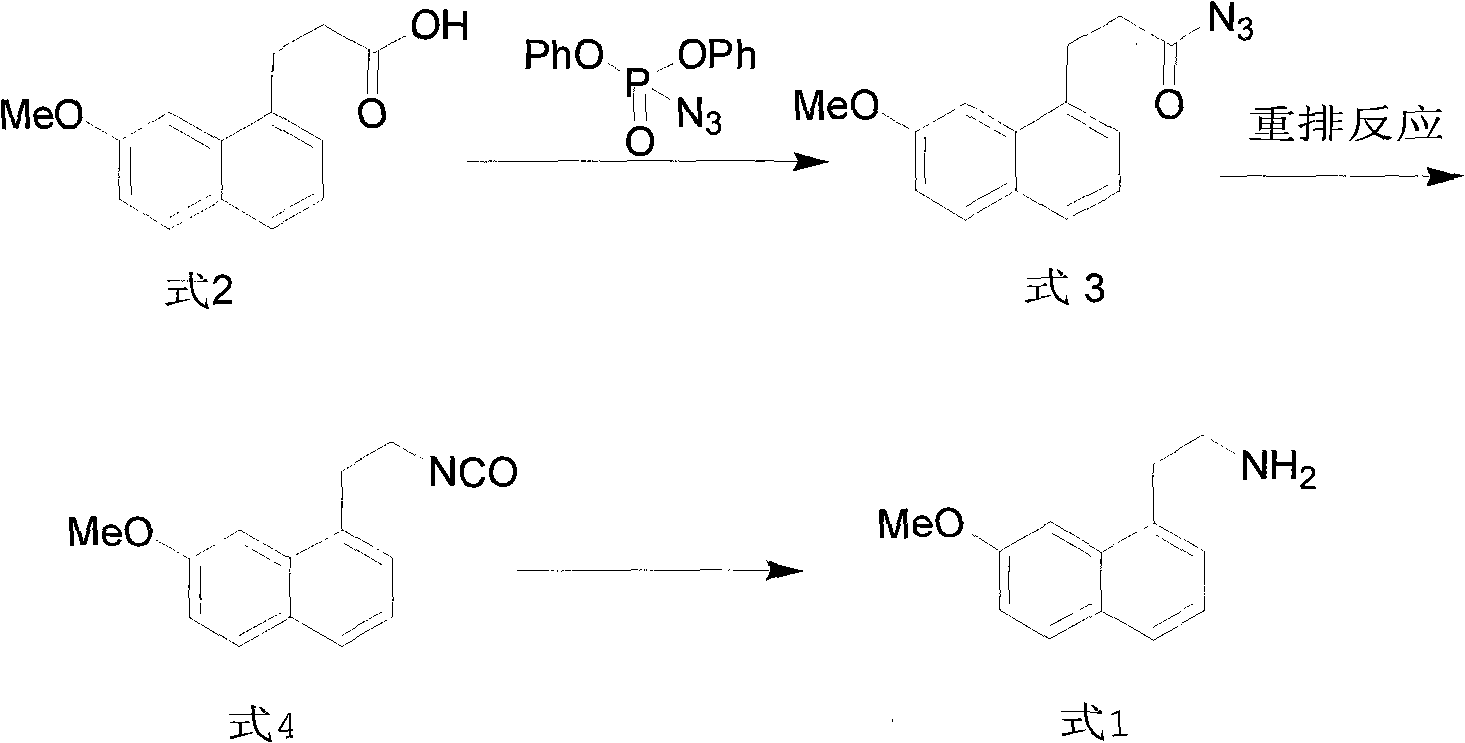
Synthesis method of valdoxan intermediate 2-(7-methoxy-1-naphthyl)ethylamine
ActiveCN101973897ALow costShort routeOrganic compound preparationAmino-hyroxy compound preparationPropanoic acidSynthesis methods
The invention provides a preparation method of a valdoxan intermediate 2-(7-methoxy-1-naphthyl)ethylamine, which has the advantages of simple and convenient operation, moderate conditions, short reaction time, high yield and high purity. In the method, 3-(7-methoxy-1-naphthyl)propionic acid reacts with diphenylphosphoryl azide to generate an acyl azide, and basic hydrolysis is carried out on the acyl azide to obtain the target product without separation and purification. The method is applicable to industrial production.
Owner:NANTONG BOTAO CHEM
Metal Porphyrin Catalyzed Olefin Aziridination with Sulfonyl Azides
InactiveUS20110112288A1Organic-compounds/hydrides/coordination-complexes catalystsCobalt organic compoundsHydrogenPyrenesulfonyl azide
Cobalt(II) complex of P1 [Co(P1)], a new porphyrin that was designed on the basis of potential hydrogen bonding interactions in the metal-nitrene intermediate, is a highly active catalyst for olefin aziridination with azides. The [Co(P1)]-based system can be effectively employed for different combinations of aromatic olefins and arysulfonyl azides, synthesizing various sulfonylated aziridines in excellent yields. Besides its mild catalytic conditions, the Co-catalyzed aziridination process enjoys several attributes associated with the relatively low cost of cobalt and widely accessible arylsulfonyl azides. Furthermore, it generates stable dinitrogen as the only by-product.
Owner:UNIV OF SOUTH FLORIDA
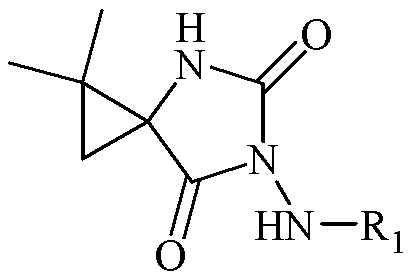
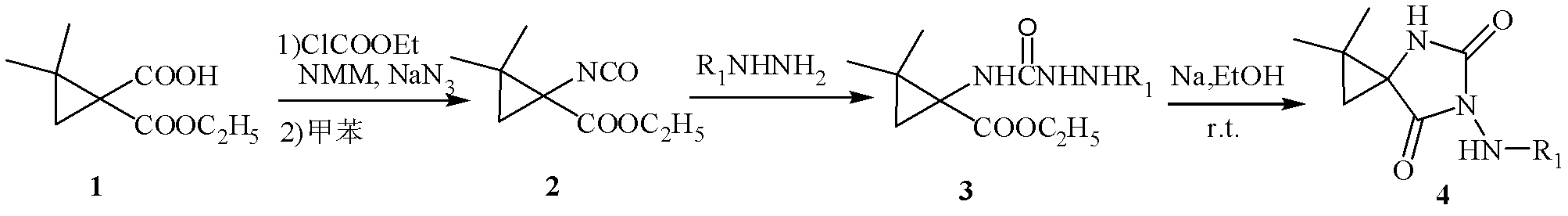
N-3-arylamine-5-cyclopropane spirohydantoin and preparation method and application thereof
InactiveCN103304483AEasy to prepareEasy to manufactureOrganic active ingredientsNervous disorderEthyl chloroformateCarboxylic salt
The invention discloses N-3-arylamine-5-cyclopropane spirohydantoin and a preparation method and application thereof. The structural general formula of the N-3-arylamine substituent-5-cyclopropane spirohydantoin is shown in the specification, wherein R1 is phenyl, substituted phenyl and heterocyclic arene. The preparation method comprises the following steps of: performing a reaction between 1-carboxyl-2,2-dimethylcyclopropane ethyl carboxylate and ethyl chloroformate to generate 1-acyl azide-2,2-dimethylcyclopropane ethyl carboxylate under the effect of NaN3; performing Curtius rearrangement on the 1-acyl azide-2,2-dimethylcyclopropane ethyl carboxylate to generate corresponding isocyanate; performing a reaction between the isocyanate and hydrazine to obtain N'-arylamine substituent ureidocyclopropane; and generating N-3-arylamine substituent-5-cyclopropane spirohydantoin from the N'-arylamine substituent ureidocyclopropane. The preparation method disclosed by the invention is simple, has higher yield, and can be used for easily preparing the N-3-arylamine substituent-5-cyclopropane spirohydantoin.
Owner:JIANGHAN UNIVERSITY
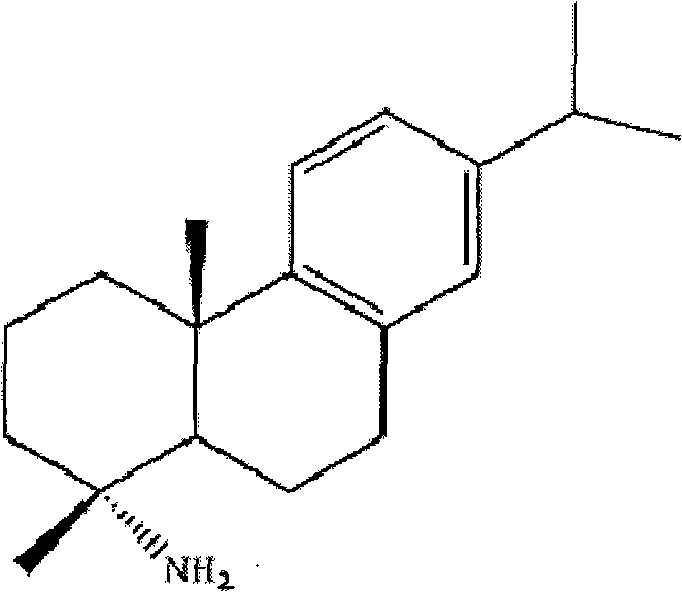
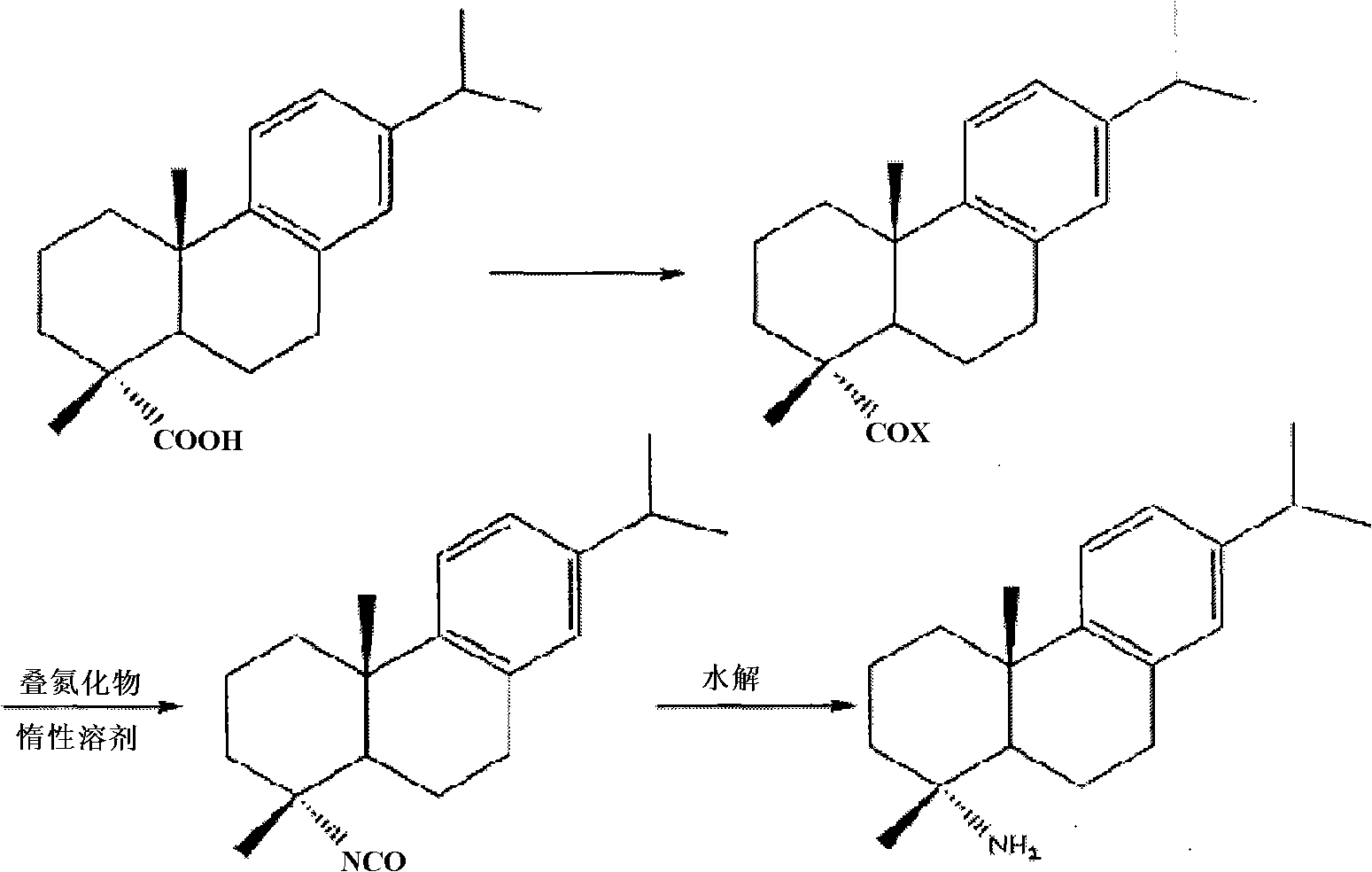
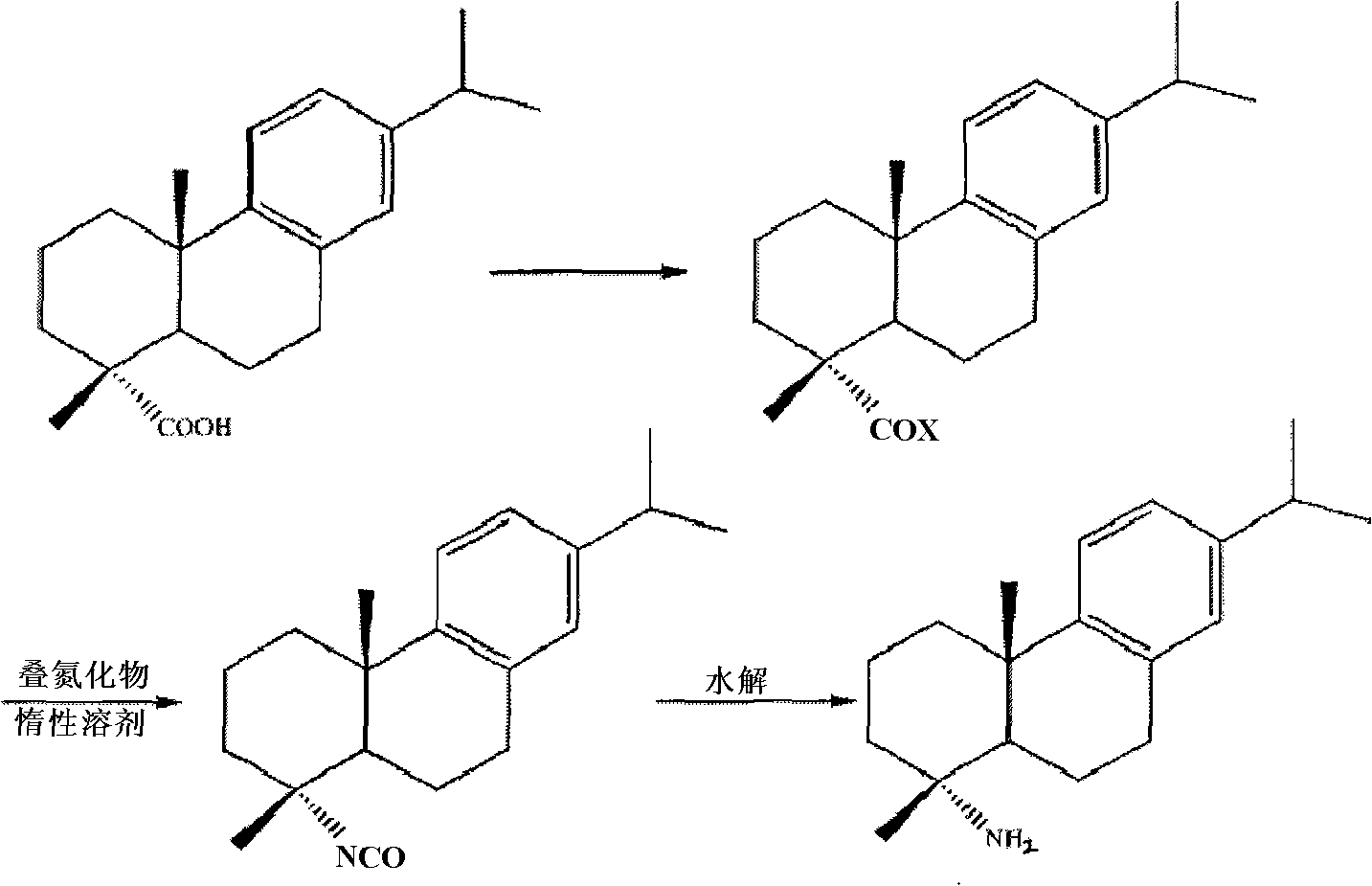
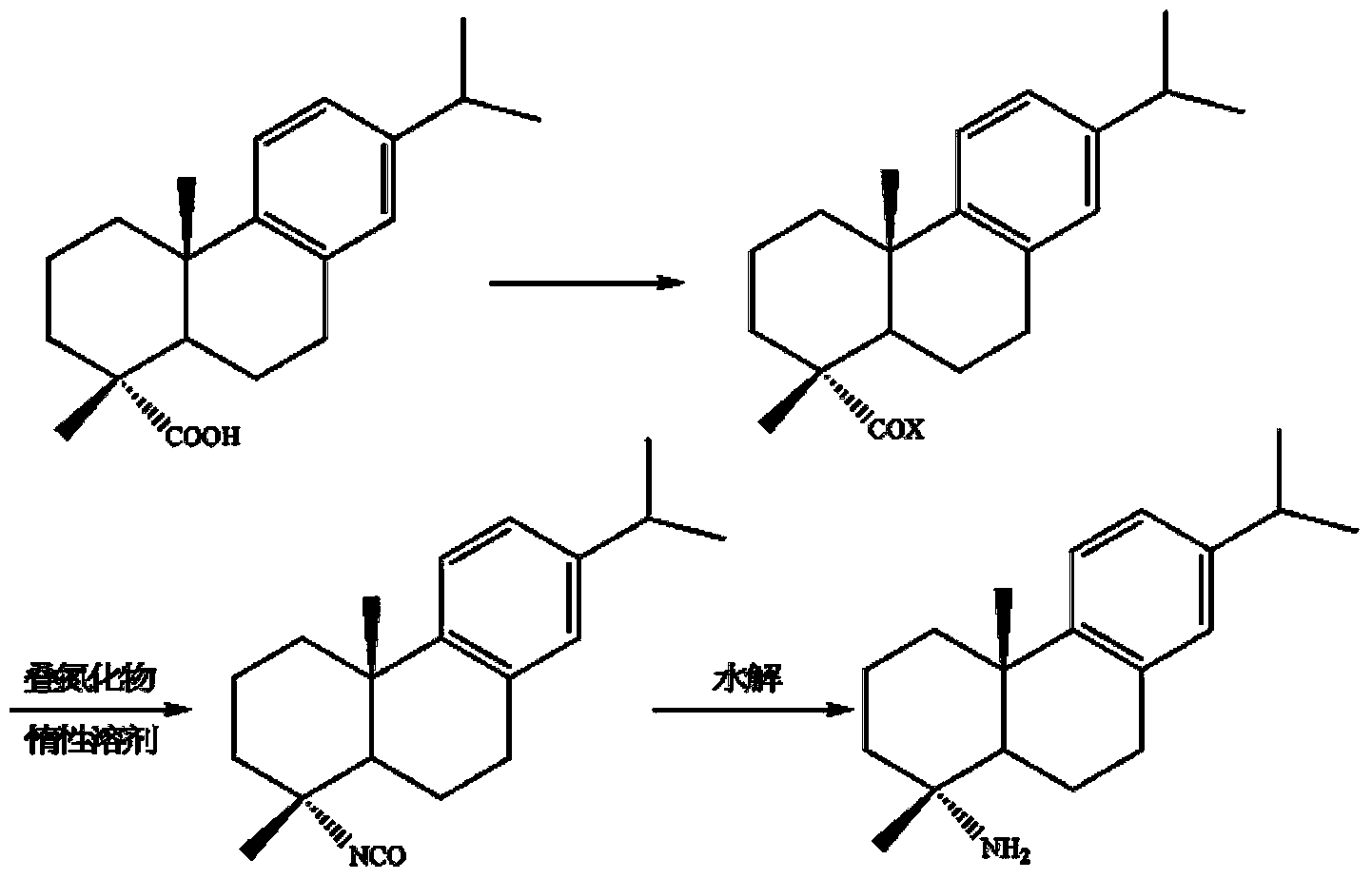
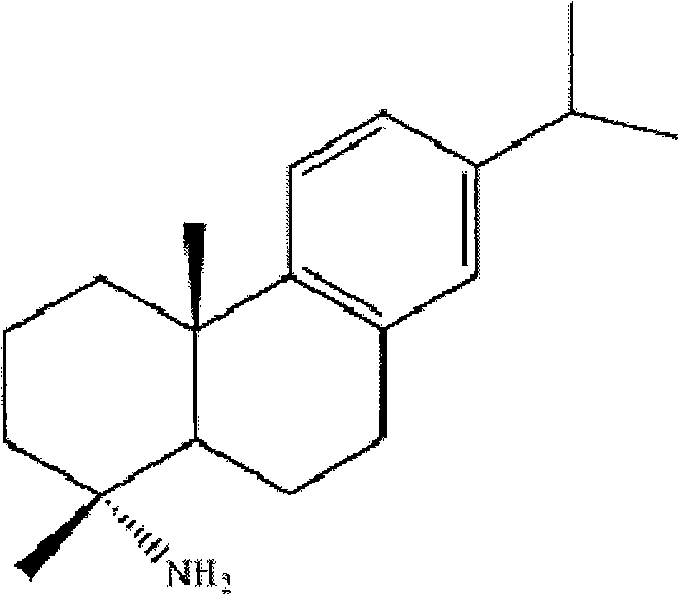
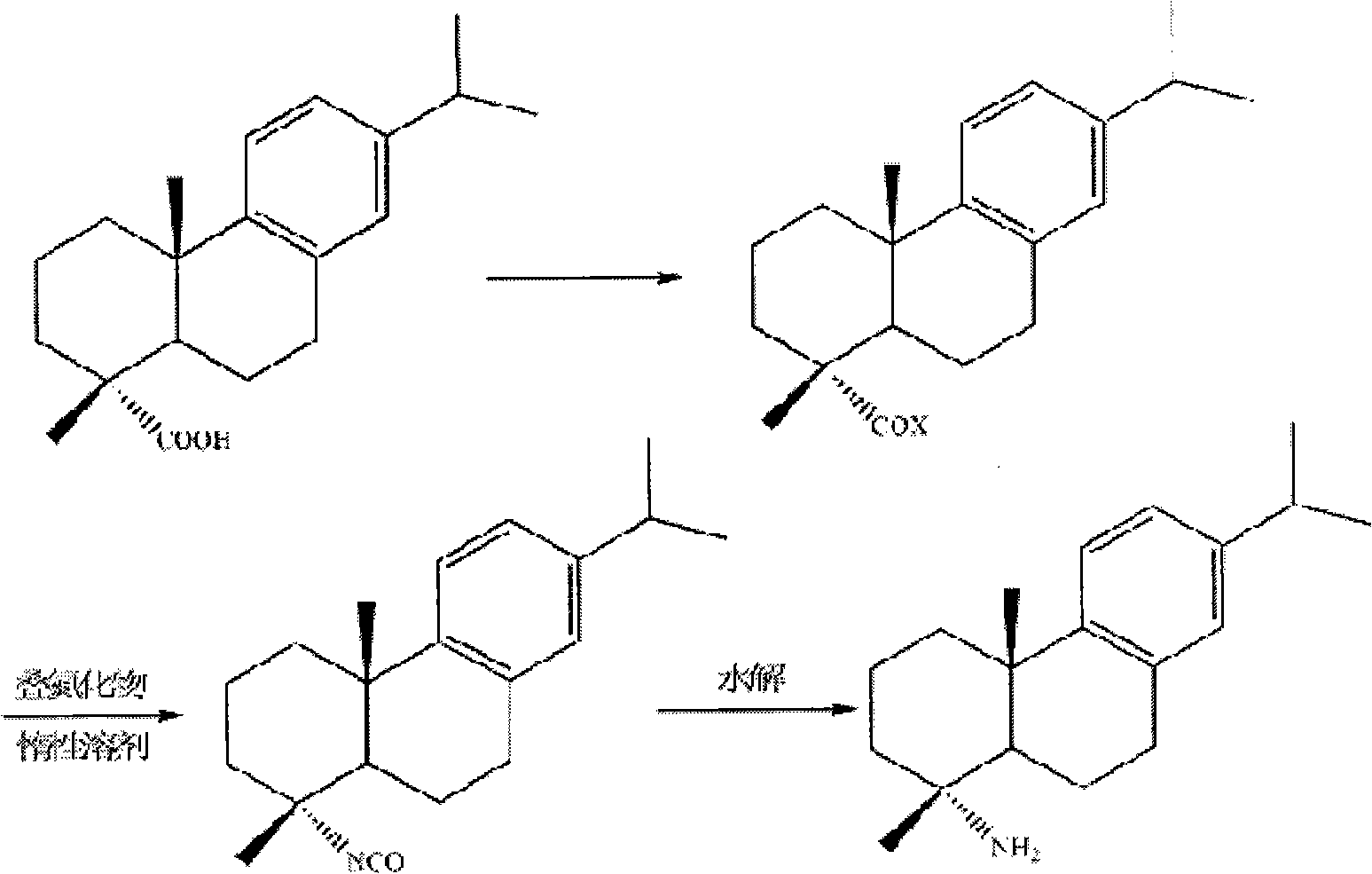
Preparation method for safely synthesizing dehydroabietic acid degraded amine
InactiveCN102050745AOrganic compound preparationAmino compound preparationOrganic solventAcyl halide
The invention discloses a method for safely preparing dehydroabietic acid degraded amine. The method comprises the following steps of: mixing an azide with an inert organic solvent; adding dehydroabietic acid acyl halide or active ester under the condition of heating; immediately rearranging a generated dehydroabietic acid acyl azide in situ to generate corresponding isocyanate; and then hydrolyzing to generate the dehydroabietic acid degraded amine. By means of the method, a process of enriching dehydroabietic acid acyl nitrine easy to explode is avoided, the reaction process is safe and controllable, and all reactions and purification measures conform to the requirement of industrial production.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Preparation method of chiral aryl cyclopropylamine derivative
InactiveCN108083997AReduce usageShort reaction pathOrganic compound preparationCarboxylic acid esters preparationAcyl groupPropylamine
The invention provides a preparation method of a chiral aryl cyclopropylamine derivative. The chiral aryl cyclopropylamine derivative is prepared by using benzene halide or benzene polyhalide as the initial raw material and subjecting the benzene halide or benzene polyhalide to Friedel-Crafts reaction, asymmetric reduction reaction, cyclization reaction, acylation reaction, hydrolysis reaction andCurtius rearrangement reaction, wherein the benzene halide or benzene polyhalide is preferably o-difluorobenzene, 2-chlorofluorobenzene or fluorobenzene. The preparation method has the advantages that carbonyl asymmetric reduction and a acylation reagent are used to build a cyclopropyl ester structure, and the use of chiral auxiliaries is avoided; the reaction route of the method is shortened ascompared with a reaction route in the prior art, and reaction yield is increased; acyl azide rearrangement is used to prepare primary amine, and the method is simple to operate, high in yield and suitable for large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Intramolecular C-H amination with sulfonyl azides
InactiveUS7956193B2Good yieldFunctional group formation/introductionPyrenesulfonyl azideNitrogen gas
Cobalt (II) complexes of porphyrins are effective catalysts for intramolecular nitrene insertion of C—H bonds with arylsulfonyl azides. The cobalt-catalyzed process can proceed efficiently under mild and neutral conditions in low catalyst loading without the need of other reagents or additives, generating nitrogen gas as the only byproduct. Using the simple tetraphenylporphyrin (TPP) as the ligand, the cobalt-catalyzed intramolecular amidation can be applied to primary, secondary, and tertiary C—H bonds and suitable for a broad range of arylsulfonyl azides, leading to the syntheses of various benzosultam derivatives in excellent yields
Owner:UNIV OF SOUTH FLORIDA
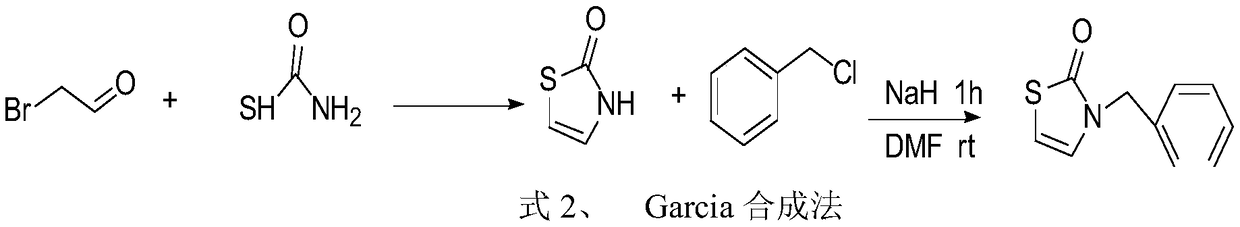
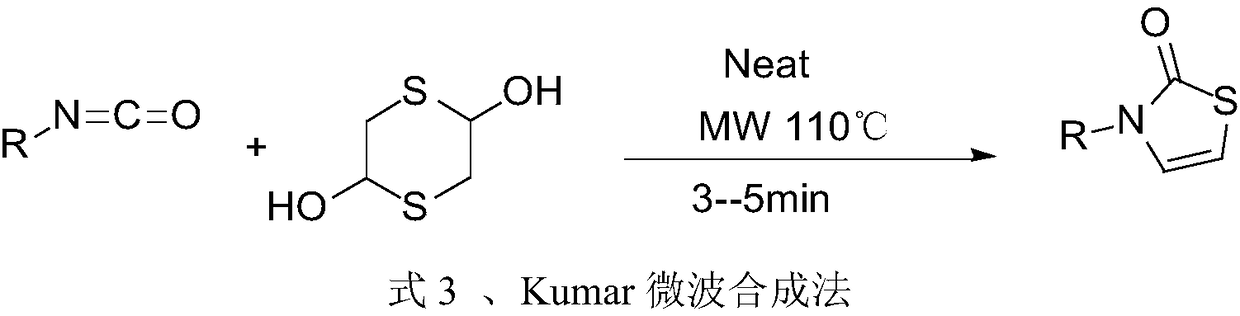
Preparation method of 3-substituted-thiazol-2(3H)-one compound
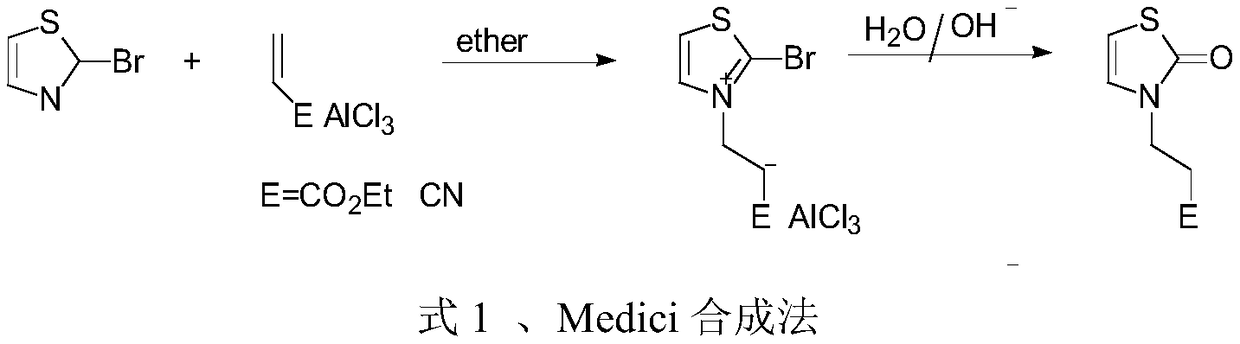
The invention discloses a preparation method of a 3-substituted-thiazol-2(3H)-one compound. The preparation method sequentially comprises the following steps: performing a reaction on an acyl azide compound and 2,5-dihydroxy-1,4-dithiane in a solvent at 80 DEG C plus or minus 5 DEG C for 18-21h; adding an aqueous sulfuric acid solution into an obtained reaction solution, reacting while stirring, and then filtering to remove a solid; performing a rotary evaporation on a filtrate to remove the solvent, then adding water, extracting by using ethyl acetate, washing an obtained organic layer by using a saturated salt solution, drying, then performing a rotary evaporation to remove the ethyl acetate, and performing silica gel column chromatography on an obtained concentrate to obtain the 3-substituted-thiazol-2(3H)-one compound. By the preparation method, the condition is mild, the yield is high, the posttreatment is convenient, the pollution is less, used raw materials are easy to obtain, and a simple and easily-implemented method is provided for efficiently synthesizing the 3-substituted-thiazol-2(3H)-one compound.
Owner:ZHEJIANG UNIV
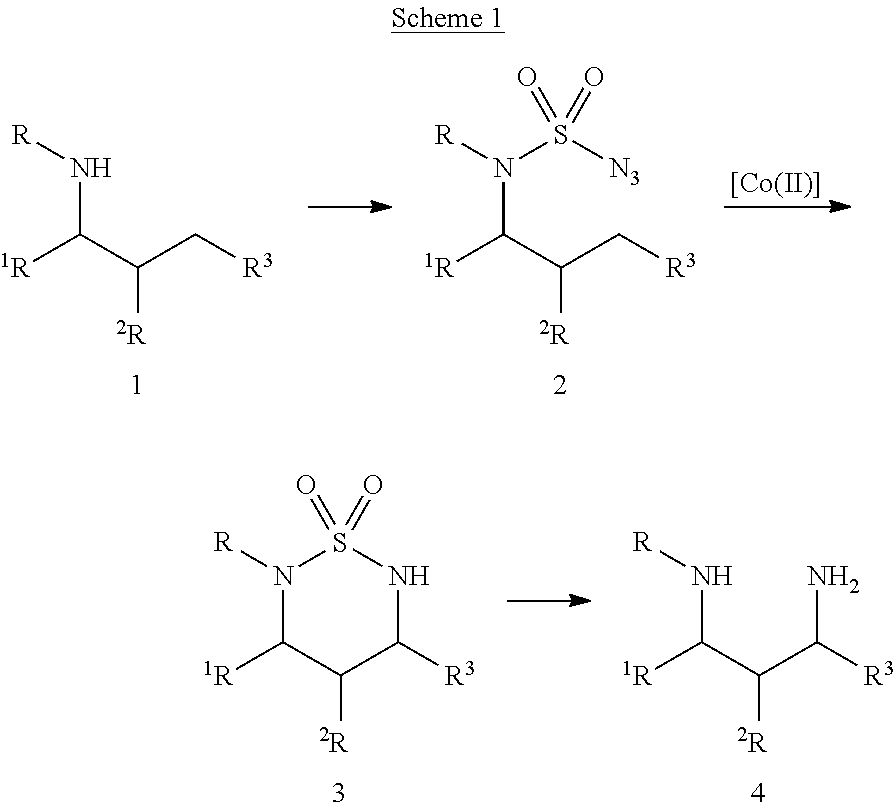
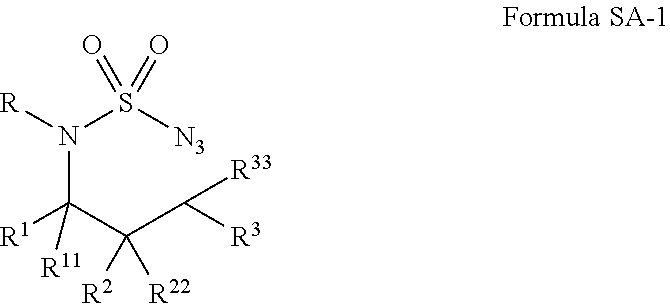
Diamine synthesis via catalytic c-h amination of azides
InactiveUS20120101271A1Good yieldHigh degree of functional group toleranceOrganic compound preparationSteroidsMetallolePorphyrin
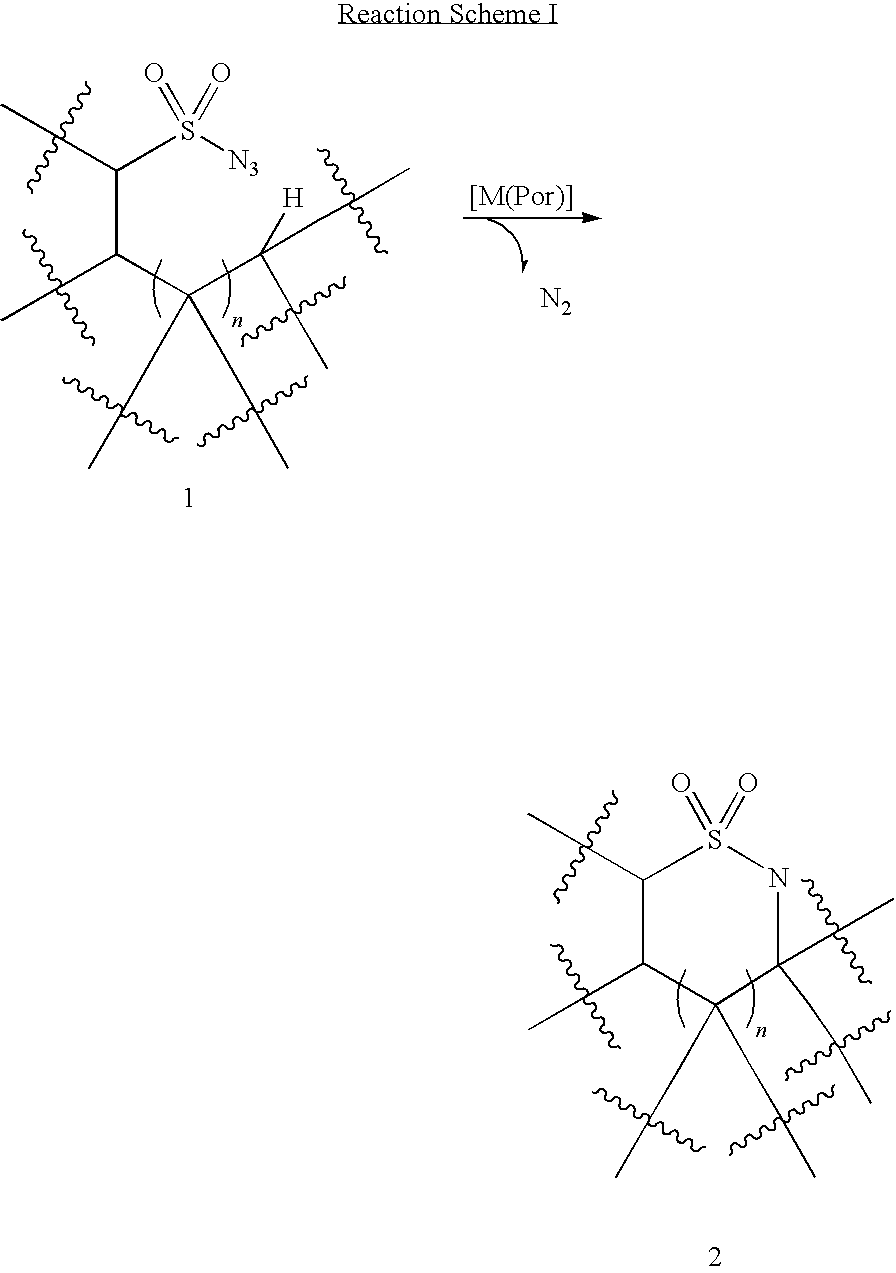
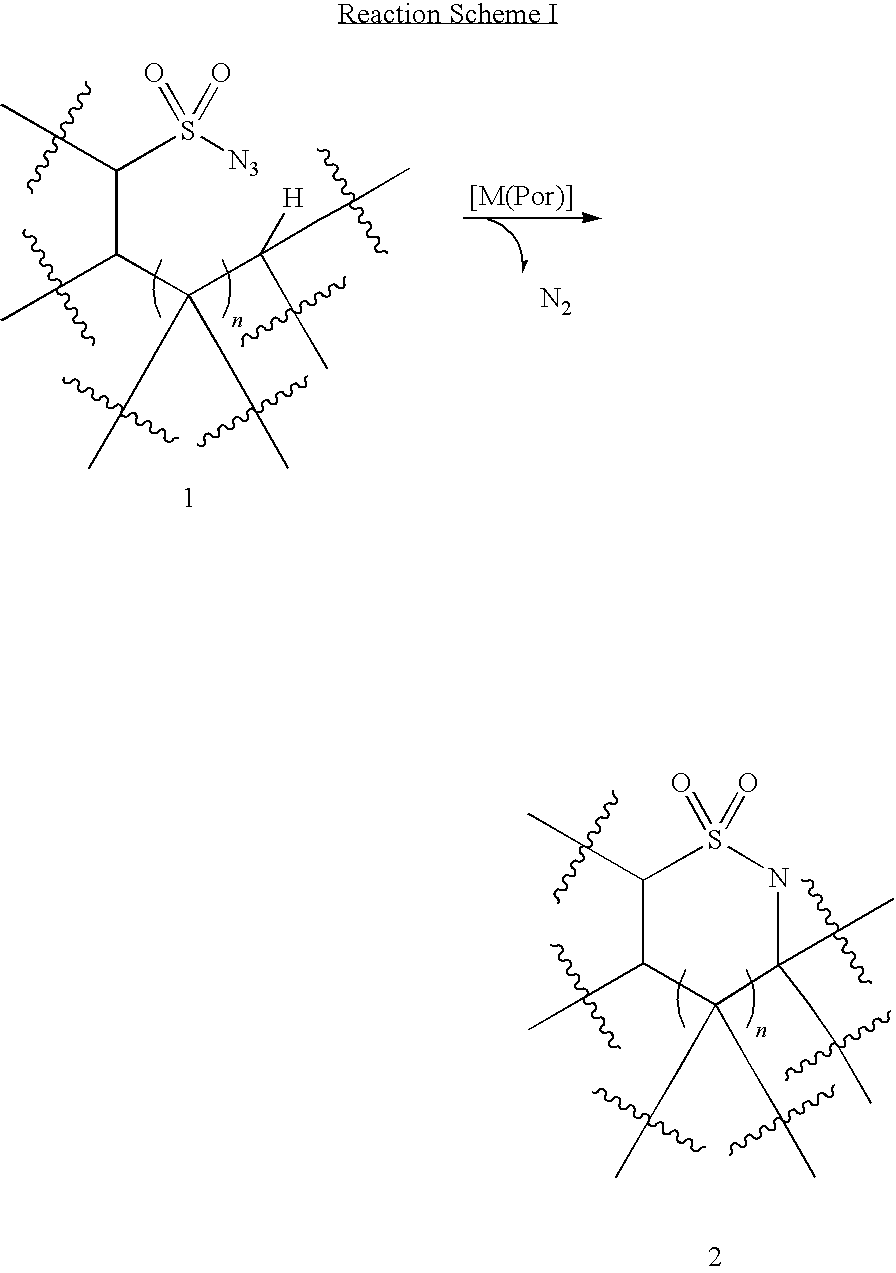
Selective intramolecular C—H amination via metalloradical activation of azides: synthesis of 1,3-diamines under neutral and nonoxidative conditions. One aspect of the present invention is the synthesis of 1,3-diamines by intramolecular C—H amination of sulfamoyl azides. More specifically, sulfamoyl azides may be selectively aminated via metalloradical activation of azides, preferably with Co(II) porphyrins. In a particularly preferred embodiment, the Co(II) porphyrin is a D2h-symmetric porphyrin.
Owner:UNIV OF SOUTH FLORIDA
Hydroxyl compound terminal modified functional group and method for modifying hydroxyl compound by using hydroxyl compound terminal modified functional group
PendingCN113831265AImprove the modification rateImprove stabilityCarbamic acid derivatives preparationOrganic compound preparationPolymer sciencePolyethylene terephthalate glycol
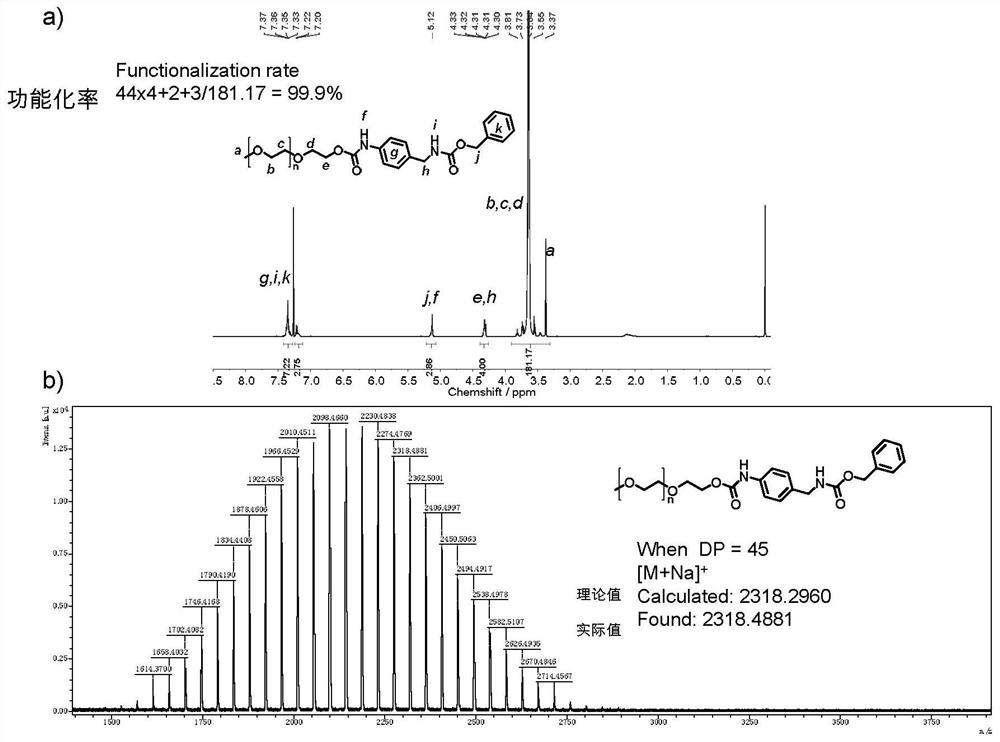
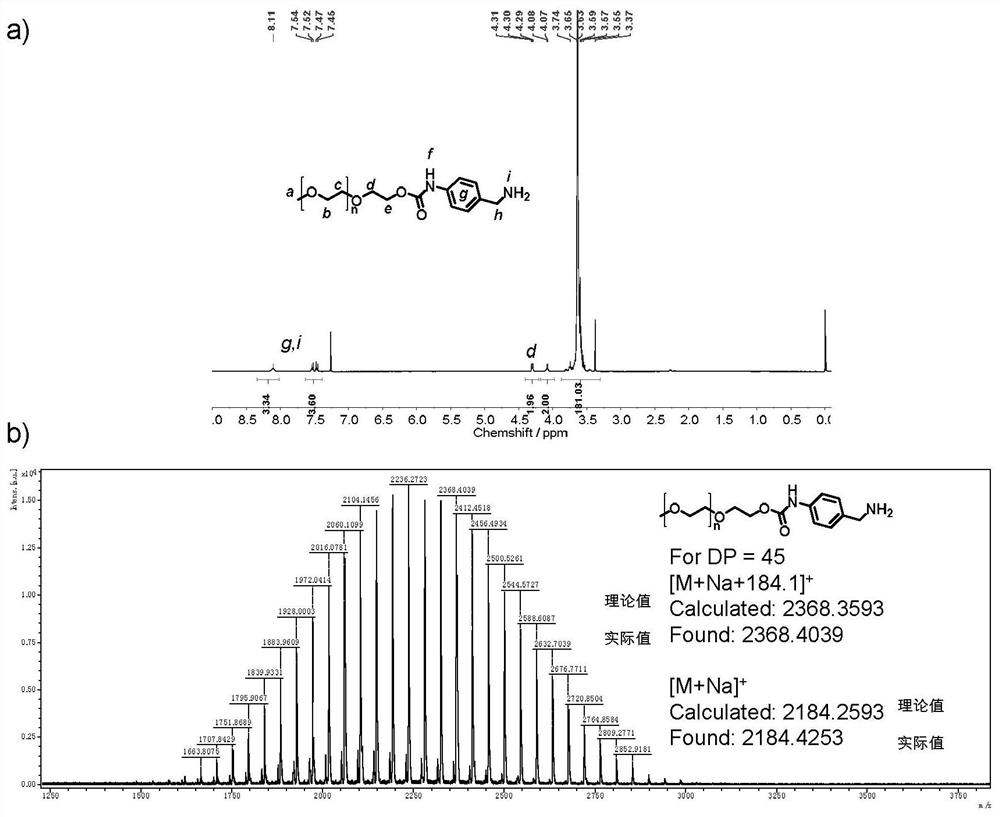
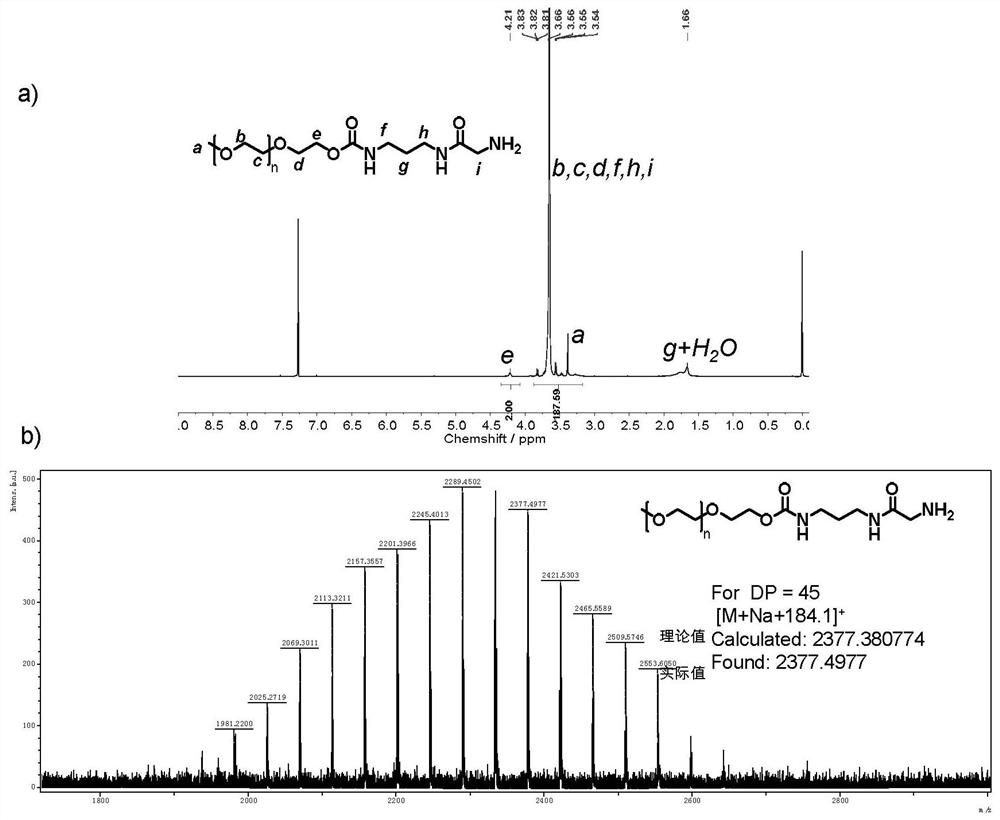
The invention provides a hydroxyl compound terminal modified functional group and a method for modifying a hydroxyl compound by using the hydroxyl compound terminal modified functional group. Specifically, the modified functional group is a functional group molecule containing acyl azide or a functional group molecule containing isocyanate converted from the functional group molecule. The modified functional group reacts with terminal hydroxyl groups of polyethylene glycol (PEG), polylactic acid (PLA), polycaprolactone (PCL), polycarbonate (PC) or polyethylene glycol terephthalate (PET) compounds with various topological structures, and a terminal functionalized product containing stable carbamate connection is obtained. Compared with hydroxyl esterification or etherification modification which is mostly researched at present, the method disclosed by the invention is thorough in reaction, short in reaction time and high in functionalization rate, the functionalization rate is greater than 99%, and the obtained functionalized product is high in stability.
Owner:UNIV OF SCI & TECH OF CHINA
Process for the preparation of 6-(7-((1-aminocyclopropyl)methoxy)-6-methoxyquinolin-4-yloxy)-n-methyl-1-naphthamide and synthetic intermediates thereof
ActiveUS20120010415A1High purityHigh yieldCarbamic acid derivatives preparationOrganic compound preparationCombinatorial chemistryMethyl group
A process for the preparation in high yields and purity of the compound 6-(7-4(1-aminocyclopropyl)methoxy)-6-methoxyquinolin-4-yloxy)-N-methyl-1-naphthamide of formula (I) and of the pharmaceutically acceptable salts thereof is described. The process has various advantages over those previously described, in particular it avoids the use of acyl azide intermediates and their Curtius rearrangement. Novel intermediates useful for the preparation of compound (I) are also described.
Owner:CLOVIS ONCOLOGY ITAL SRL
Thioester peptide synthesis method
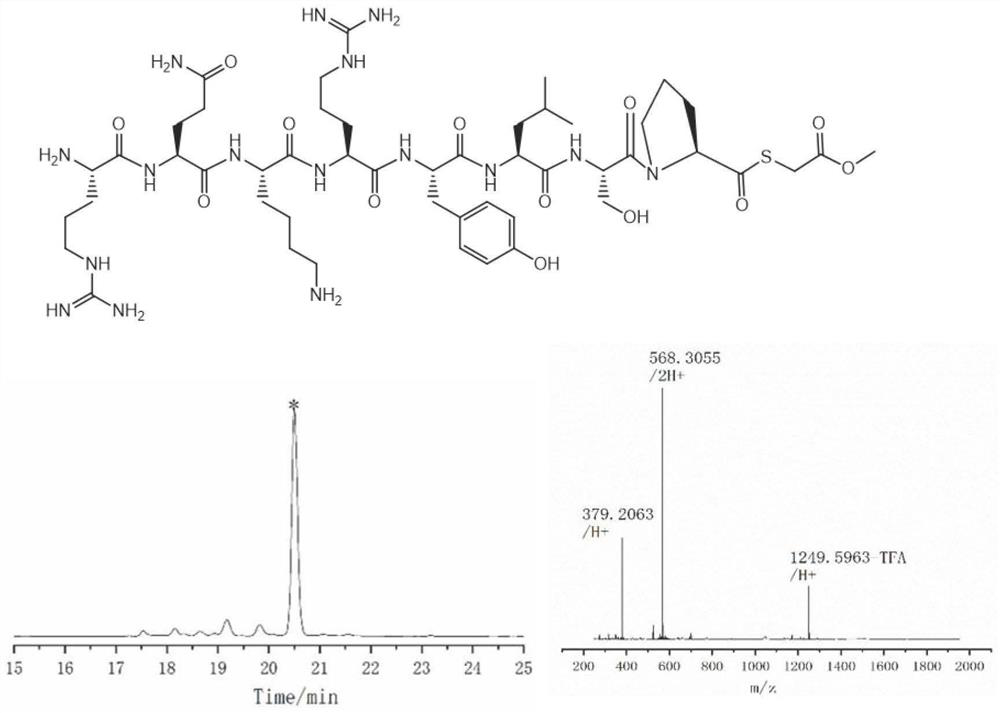
PendingCN114181276AThe synthesis process is simpleShorten production timePeptide preparation methodsFreeze-dryingAcyl group
The invention discloses a thioester peptide synthesis method, which specifically comprises the following steps: dissolving polypeptide hydrazide in a hydrochloric acid-containing mixed solvent consisting of dimethyl sulfoxide and water, and reacting with isoamyl nitrite in an ice-salt bath to generate acyl azide peptide; adding excessive methyl thioglycolate, and then adding ammonium bicarbonate prepared in advance to adjust the acidity of the solution to be neutral, so that the acyl azide peptide is converted into peptide thioester; pre-cooled trifluoroacetic acid and diethyl ether are sequentially added into a reaction system, so that thioester peptide is crystallized and separated. The thioester peptide is prepared through the low-cost, simple and rapid operation process, and the problem that high-cost high performance liquid chromatography and time-consuming freeze drying operation are needed in traditional thioester peptide synthesis is solved.
Owner:ANHUI UNIVERSITY
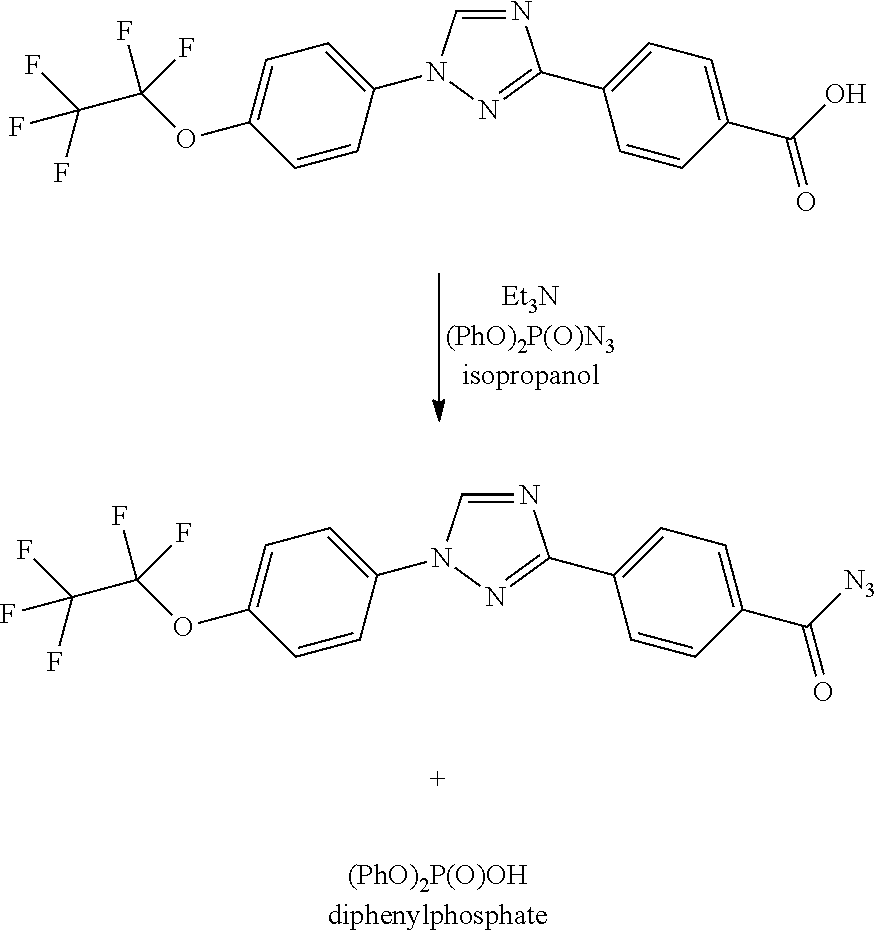
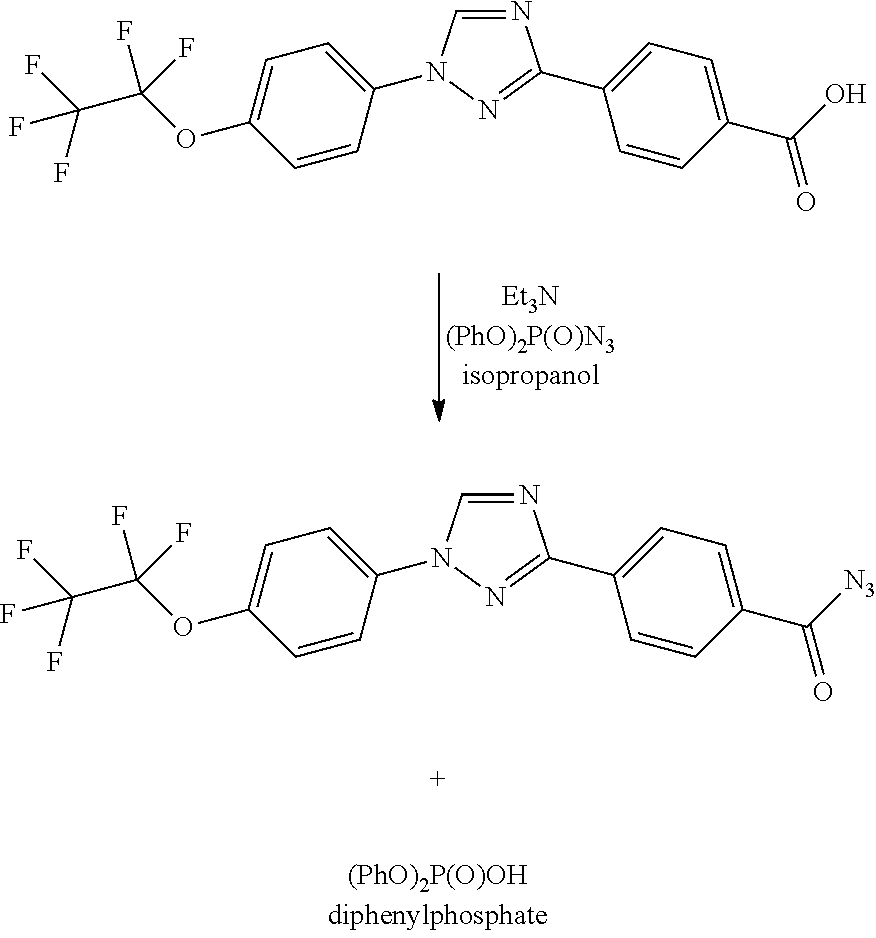
Process for preparation of 4-(1-(4-(perfluoroethoxy)phenyl)-1h-1,2,4-triazol-3-yl)benzoyl azide
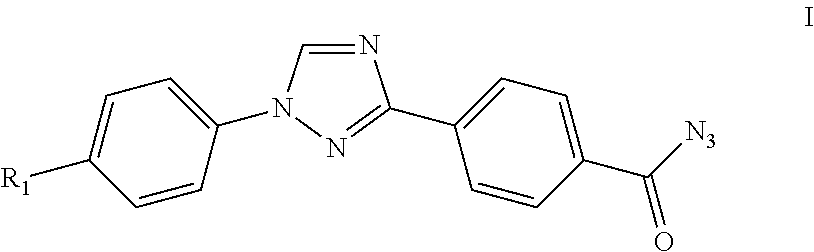
By either forming a triaryl acid halide or a triaryl mixed anhydride and subsequently treating with aqueous sodium azide, triaryl acyl azides are prepared in high yield using inexpensive reagents in a process in which by-products are easily removed from the triaryl acyl azide.
Owner:CORTEVA AGRISCIENCE LLC
A kind of method for synthesizing multi-substituted pyrimidine derivatives by a two-step method
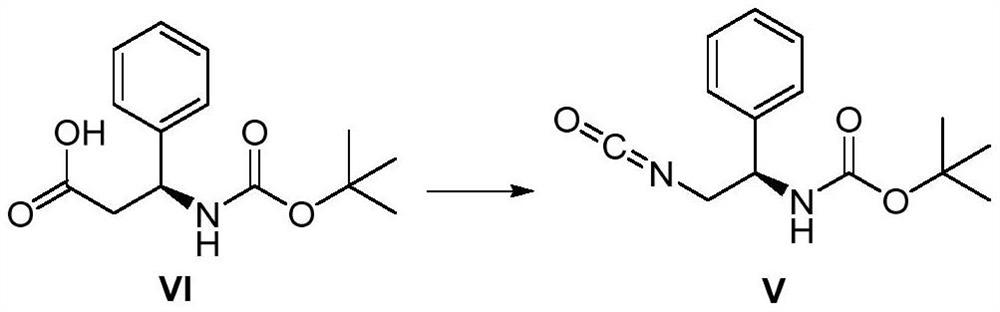
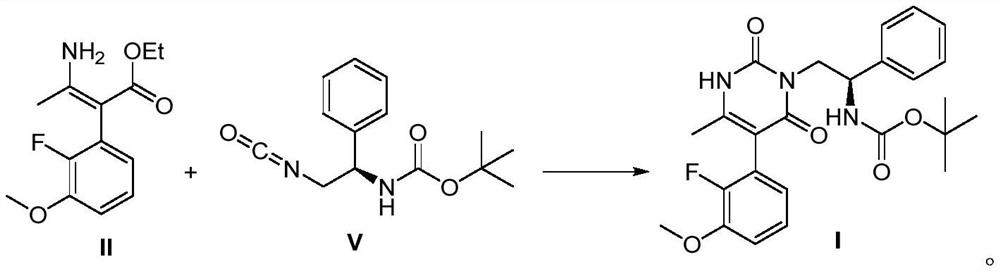
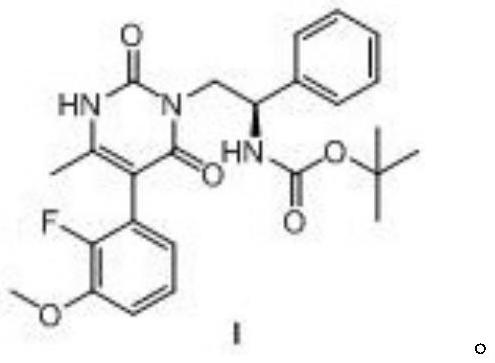
The invention discloses a method for synthesizing multi-substituted pyrimidine derivatives in a two-step method, comprising the following steps: (1) derivatizing N-Boc-beta-phenylalanine as shown in formula VI into an acyl azide, and then Curtius rearrangement obtains (R)-(2-isocyanato-1-phenylethyl) tert-butyl carbamate as shown in formula V; (2) (R)-( 2-isocyanato-1-phenylethyl) tert-butyl carbamate and (Z)-3-amino-2-(2-fluoro-3-methoxyphenyl)- Ethyl 2-butenoate obtains the polysubstituted pyrimidine derivatives as shown in formula I through cyclization reaction; The present invention has the advantages that the starting compound is simple and easy to get, the synthetic route is simple, easy to operate, and the yield of the reaction is relatively high. High; the method reduces the production cost of the target compound and is suitable for large-scale industrial production.
Owner:安徽诺全药业有限公司
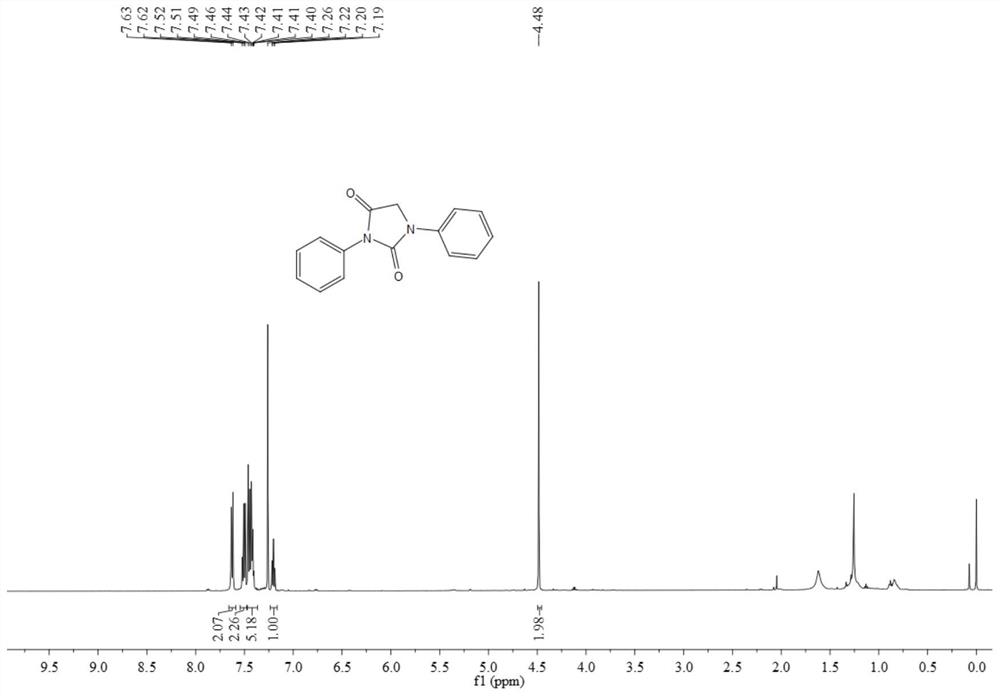
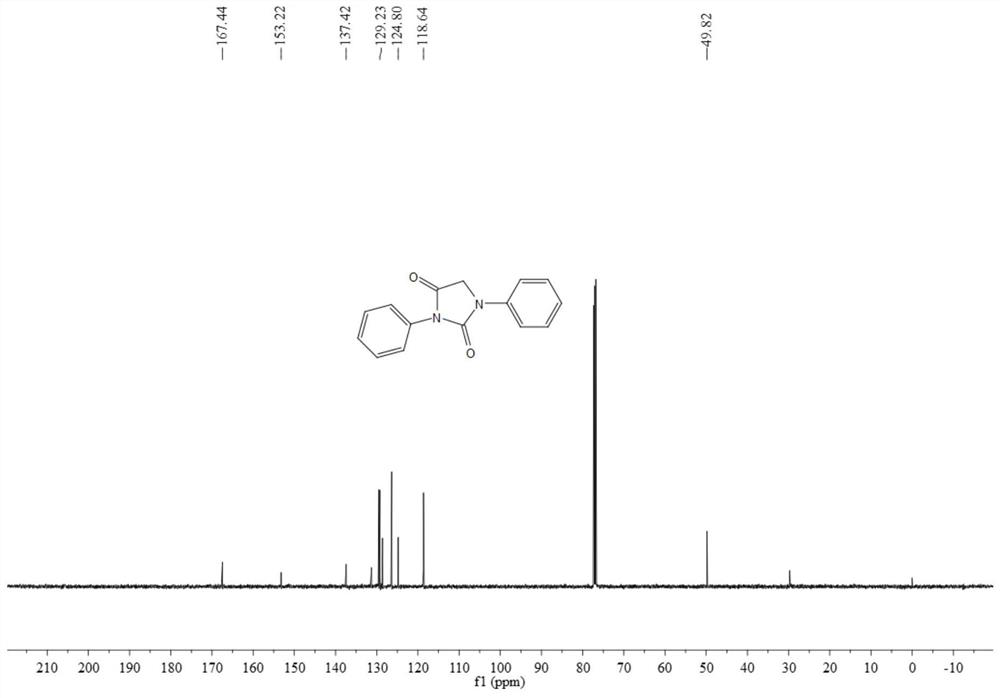
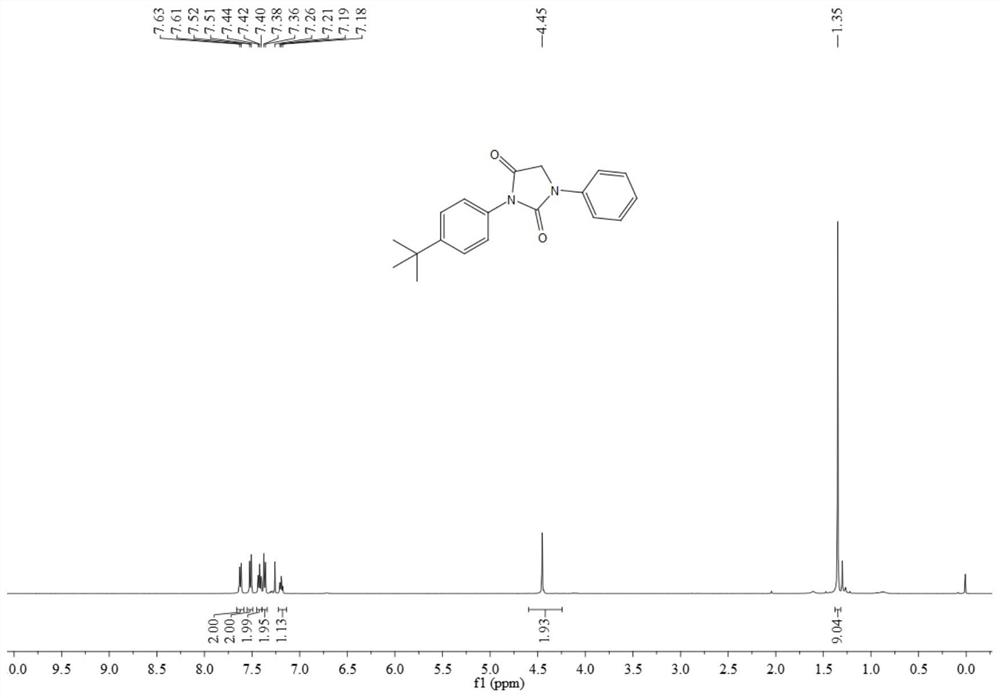
Synthesis method of N-substituted hydantoin compound
The invention discloses a synthesis method of an N-substituted hydantoin compound, which comprises the following steps: by using an acyl azide compound as shown in formula I and a glycine ethyl estercompound as shown in formula II as raw materials, carrying out heating reaction in the presence of an additive to obtain the N-substituted hydantoin compound as shown in formula III, wherein the reaction equation is shown as the specification; in the equation, R1 and R2 are independently selected from alkyl, substituted alkyl, aryl, substituted aryl or aromatic heteroradical, the R2 is selected from alkyl or aryl, and R3 is selected from a hydrogen atom and a hydrocarbyl group. The synthesis method can efficiently synthesize the functionalized N-substituted hydantoin compound, has the advantages of few synthesis steps, mild conditions, safe operation, nontoxic, cheap and easily available raw materials, good compatibility with functional groups and high atom economy, can obtain the N-substituted hydantoin compound with a novel structure and a nitrogen-containing heterocyclic skeleton, and has the yield of 92% and the purity of 99% , and the industrial synthesis is easy.
Owner:WUYI UNIV
Solid-phase synthesis method of peptide
PendingCN113735940AEmission reductionLow costLuteinising hormone-releasing hormonePeptide preparation methodsAcyl groupEngineering
The invention relates to the field of polypeptide synthesis, in particular to a solid-phase synthesis method of polypeptide. The method comprises the following steps: 1) activating hydroxyl resin into X-CO-O-Resin by an activating agent, wherein X is an activating group; 2) reacting the X-CO-O-Resin with NH2NH2 to synthesize NH2NH-CO-O-Resin, or reacting the X-CO-O-Resin with Fmoc-NHNH2 to synthesize Fmoc-NHNH-CO-O-Resin, and then removing Fmoc to obtain NH2NH-CO-O-Resin; 3) coupling the NH2NH-CO-O-Resin with amino acid or a peptide fragment according to a peptide sequence to obtain peptide hydrazide resin; 4) cracking the peptide hydrazide resin by a cracking reagent to obtain peptide hydrazide; 5) converting the peptide hydrazide into an acyl azide or hydrazine terminal structure which can be used for coupling in water, thereby preparing some polypeptides with special modification at carbon terminals. The method is particularly suitable for relin type polypeptide with ethylamino or semicarbazide at the carbon terminals. The relin polypeptide is prepared by a peptide hydrazide method, so that the cost can be greatly reduced, the operation steps are reduced, and the emission of waste water, waste gas and solid wastes is reduced. The synthesis process is safe and stable, has no adverse side reaction, and is suitable for industrial large-scale production.
Owner:SHENZHEN JYMED TECH
Protein preparation method
ActiveCN102199214BEffective peptide linkagePeptide preparation methodsHybrid peptidesCrystallographyNeutral ph
A protein preparation method provided by the invention comprises the following steps to connect a first peptide with hydrazide group on C terminus and a second peptide with cysteine on N terminus: mixing the first peptide and the second peptide in a solution containing NaNO2 and adjusting the mixed solution pH to an acidic pH so as to converting the hydrazide group of the first peptide into acyl azide group to obtain a first reaction mixture; adding a solution containing a reducing agent into the first reaction mixture and adjusting the pH to a neutral pH, wherein the reducing agent contains mercapto group, so as to convert the acyl azide group of the first peptide into thioester group, and maintaining the neutral pH so as to conducting a connection reaction between the thioester on the Cterminus of the first peptide and the N terminus of the second peptide to produce proteins. A method for preparing polypeptide thioester compounds is also provided. According to the protein preparation method provided by the invention, proteins can be effectively synthesized.
Owner:TSINGHUA UNIV
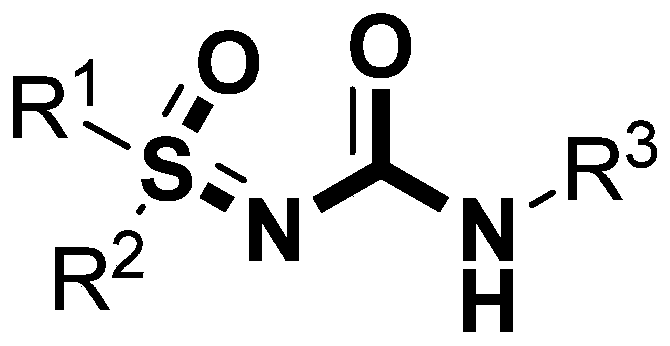
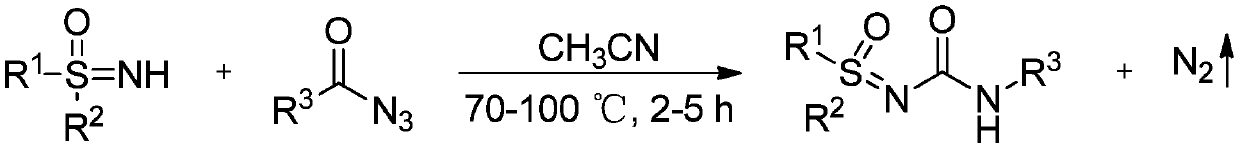
A kind of high-efficiency preparation method of sulfoxide sulfonylurea series compound
InactiveCN106946756BHigh reactivityImprove compatibilityOrganic chemistryReaction temperatureNitrogen gas
The invention discloses a high-efficiency preparation method of sulfoxide sulfonylurea series compounds. The method adopts sulfoxide sulfimide and aralkyl acyl azide as reaction substrates, so that it can be prepared in the presence of no metal elements. The heating reaction is carried out under the conditions, the reaction temperature is 70-100° C., and the reaction time is 2 to 5 hours, and the sulfoxide sulfinyl urea series compounds are efficiently prepared. Through the method of the invention, the compound containing sulfinyl urea can be efficiently prepared. The method has high chemical selectivity, wide substrate applicability, simple operation, short reaction time, extremely high yield, and the only by-product is nitrogen, which is beneficial to separation and purification, and the product has high purity, and is applicable to large-scale preparation.
Owner:JIANGXI NORMAL UNIV
Curable fluoroelastomer composition
Compositions comprising fluoroelastomers having copolymerized units of a cyano-containing cure site monomer are cured (i.e. crosslinked) with a fluoropolymer having pendant sulfonyl azide groups. The latter fluoropolymer comprises copolymerized units of a fluoroalkanesulfonyl azide of formula CF2═CF—(O)p—Rf—(CH2)n—SO2N3, wherein p=0 or 1; n=0-4; and Rf is a C1-C16 perfluoroalkyl or perfluoroalkoxy group. The crosslinks are tetrazole rings formed by the reaction of the pendant sulfonyl azide groups on the fluoropolymer with pendant cyano groups on the fluoroelastomer.
Owner:DUPONT POLYMERS INC
Preparation method for safely synthesizing dehydroabietic acid degraded amine
InactiveCN102050745BOrganic compound preparationAmino compound preparationOrganic solventAcyl halide
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Process for preparation of 4-(1-(4-(perfluoroethoxy)phenyl)-1H-1,2,4-triazol-3-yl)benzoyl azide
By either forming a triaryl acid halide or a triaryl mixed anhydride and subsequently treating with aqueous sodium azide, triaryl acyl azides are prepared in high yield using inexpensive reagents in a process in which by-products are easily removed from the triaryl acyl azide.
Owner:CORTEVA AGRISCIENCE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com