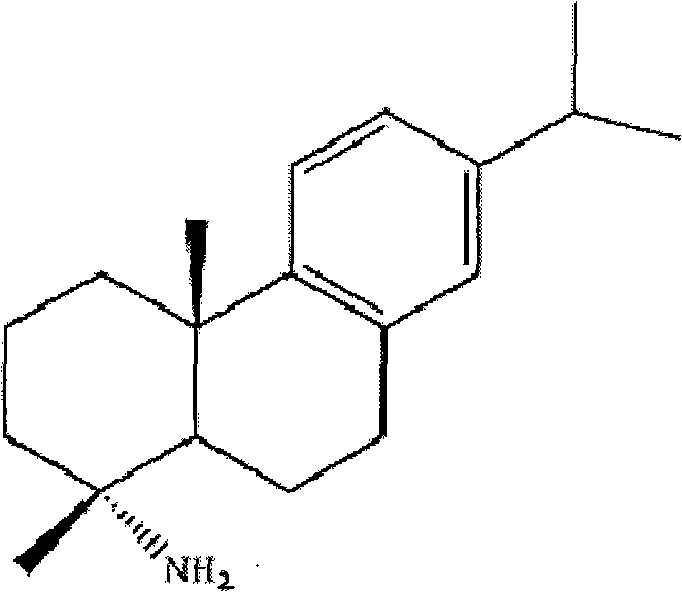
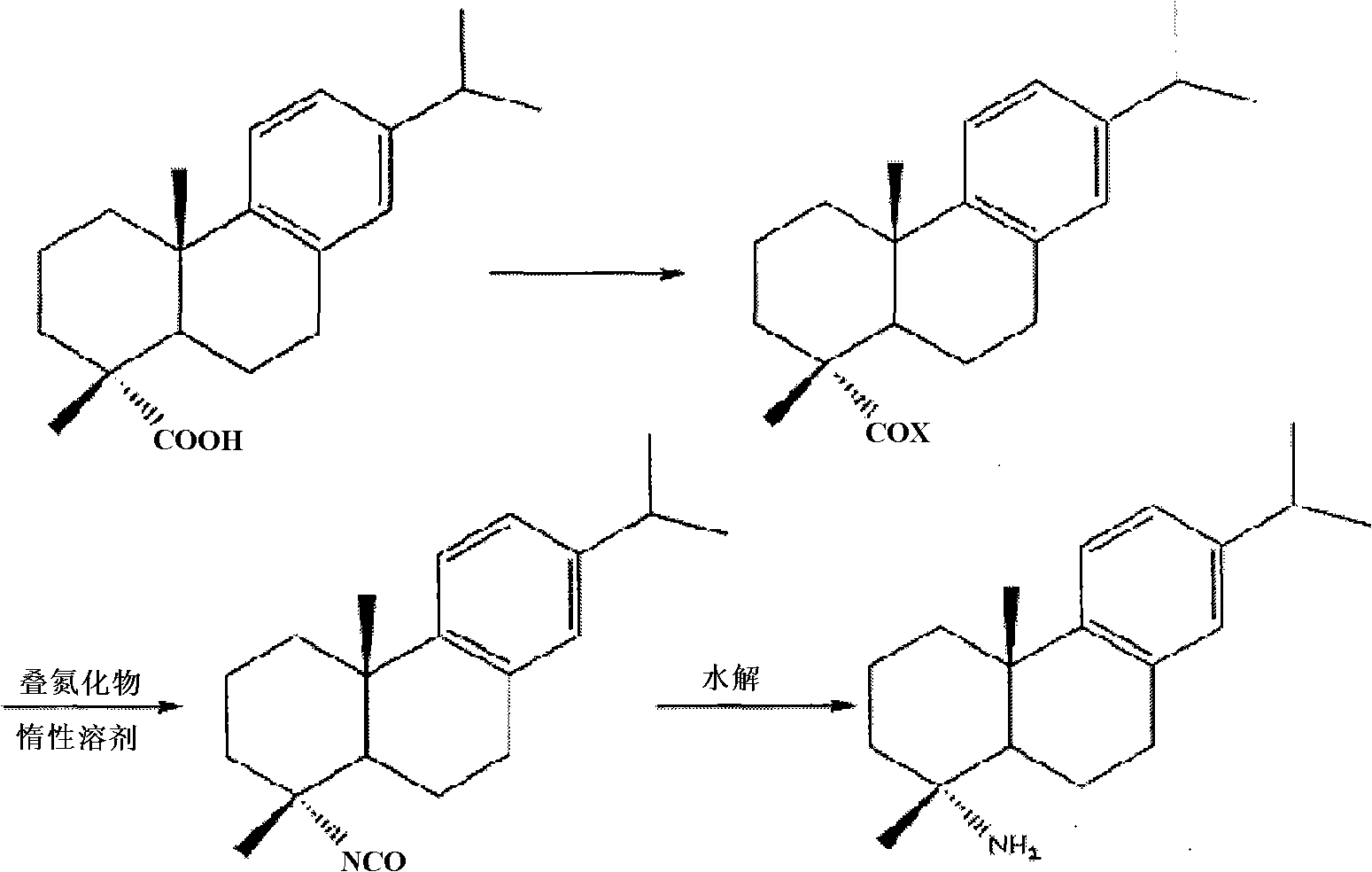
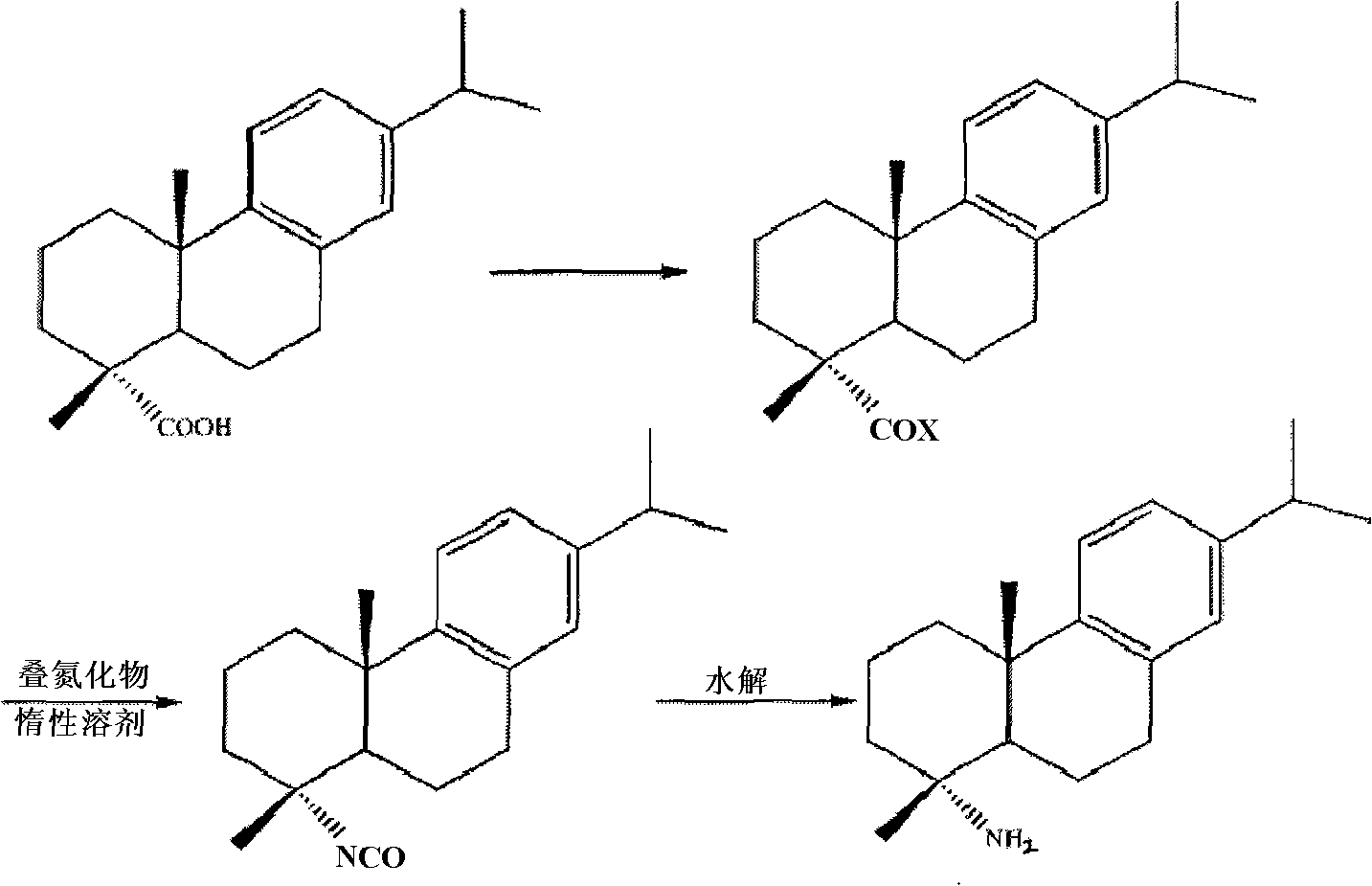
Preparation method for safely synthesizing dehydroabietic acid degraded amine
A dehydroabietic acid conversion technology, which is applied in the field of preparation of chiral compounds dehydroabietic acid degraded amines, can solve the problems of reduced yield, inability to prepare in large quantities, easy explosion and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] In a reflux condenser, a drying tube, a thermometer, a constant pressure dropping funnel, and a 500ml three-neck flask equipped with electromagnetic stirring, add 200ml of anhydrous toluene, start stirring, and dissolve 40g (0.133mol) of dehydroabietic acid in toluene Heating to 80°C, adding 40ml of freshly steamed thionyl chloride dropwise, and reacting for 5 hours, distilling off the solvent under reduced pressure, then adding 100ml of anhydrous toluene, removing the solvent under reduced pressure again to take away the residual thionyl chloride and Acid gas was obtained to obtain a yellow viscous liquid, which was dissolved in 100ml of anhydrous toluene and set aside.
[0054]In a 300ml three-neck flask equipped with a reflux condenser, a drying tube, a thermometer, a constant pressure dropping funnel, and electromagnetic stirring, add 100ml of anhydrous toluene and 9.8g (0.15mol) of sodium azide, and heat to 70°C under stirring , stop heating, slowly add the above-m...
Embodiment 2
[0059] In a 500ml there-necked flask equipped with a reflux condenser, a drying tube, a thermometer, a constant pressure dropping funnel, and electromagnetic stirring, add 200ml of anhydrous chloroform, start stirring, and dissolve 40g (0.133mol) of dehydroabietic acid In chloroform, heat to 60°C, add dropwise 40ml of freshly steamed thionyl chloride, react for 5 hours, evaporate the solvent under reduced pressure, and then add 100ml of anhydrous toluene, remove the solvent under reduced pressure again to take away the residual Thionyl chloride and acid gas were used to obtain a yellow viscous liquid, which was dissolved in 100ml of anhydrous chloroform and set aside.
[0060] In a 300ml three-neck flask equipped with a reflux condenser, a drying tube, a thermometer, a constant pressure dropping funnel, and electromagnetic stirring, add 100ml of anhydrous chloroform, 9.8g (0.15mol) of sodium azide, and heat to 50°C, stop heating, slowly add the chloroform solution of the above...
Embodiment 3
[0065] In a 500ml there-necked flask equipped with a reflux condenser, a drying tube, a thermometer, a constant pressure dropping funnel, and electromagnetic stirring, add 200ml of anhydrous xylene, start stirring, and dissolve 40g (0.133mol) of dehydroabietic acid in In xylene, heat to 80°C, add 40ml of freshly steamed thionyl chloride dropwise, react for 5 hours, evaporate the solvent under reduced pressure, and then add 100ml of anhydrous xylene, remove the solvent under reduced pressure again to take away the residual chlorine Dissolve sulfoxide and acid gas to obtain a yellow viscous liquid, which is dissolved in 100ml of anhydrous xylene and set aside.
[0066] In a 300ml three-neck flask equipped with a reflux condenser, a drying tube, a thermometer, a constant pressure dropping funnel, and electromagnetic stirring, add 100ml of anhydrous xylene and 9.8g (0.15mol) of sodium azide, and heat to 100 ℃, stop heating, slowly add the xylene solution of the above-mentioned deh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com