Patents
Literature
61 results about "Chrysanthemic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
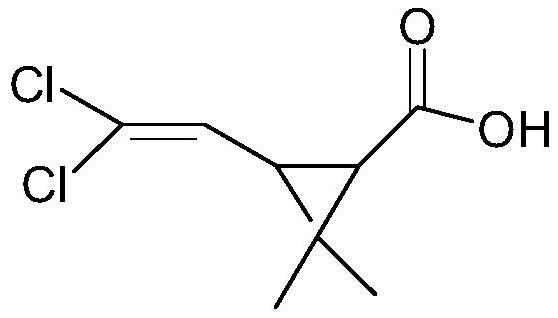
Chrysanthemic acid is an organic compound that is related to a variety of natural and synthetic insecticides. It is related to the pyrethrin I and II, as well as the pyrethroids. One of the four stereoisomers, (1R,3R)- or (+)-trans-chrysanthemic acid (pictured), is the acid part of the ester pyrethrin I, which occurs naturally in the seed cases of Chrysanthemum cinerariaefolium. Many synthetic pyrethroids, for example the allethrins, are esters of all four stereoisomers.
Process for preparing cis-halochrysanthemic acid
InactiveCN1390825AIncrease dosageReduce dosagePreparation from carboxylic acid esters/lactonesHexamethylphosphoramideTert butyl
A process for preparing cis-halochrysanthemic acid includes such steps as the addition reaction of raw material (sodium tert-butyl alkoxide), reaction for removing hydrogen chloride and hydrolysis, reflux with excess sodium hydroxide in the mixed solvent of methanol or ethanol and water to obtain cyclocompound, and acidation. Its advantages are simple process, high output rate, low cost and less pollution.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Method for preparing dexiotropous antifoim dichlor chrysanthemic acid in high optical purity through method of induced crystallization
InactiveCN1480445AHigh optical purityHigh selectivityCarboxylic compound separation/purificationOrganic solventChrysanthemic acid
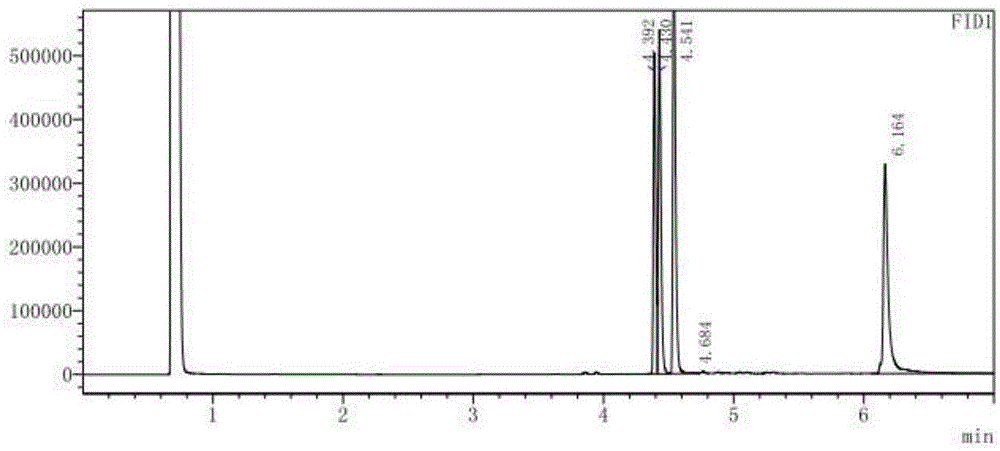
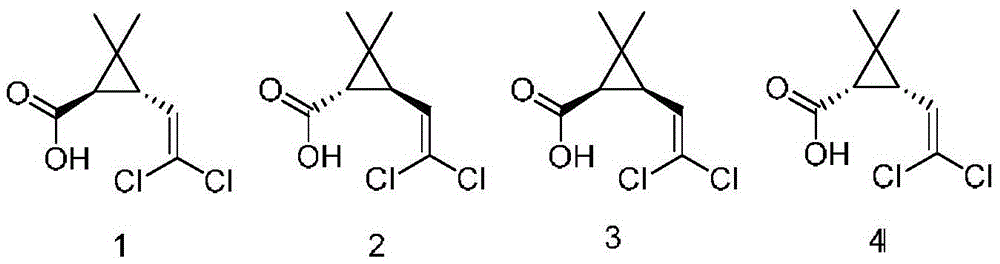
A process for preparing high-optical-purity R(+)-trans-dichlorochrysanthemic acid by induced crystallizing method includes dissolving chiral dichlorochrysanthemic acid in hot solvent to become supersaturated solution, adding the inducing crystal seeds, slow natural crystallizing, drying and testing its 4 isomers by gas-phase chromatography. Its advantage is high optical purity increased by 5-10%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
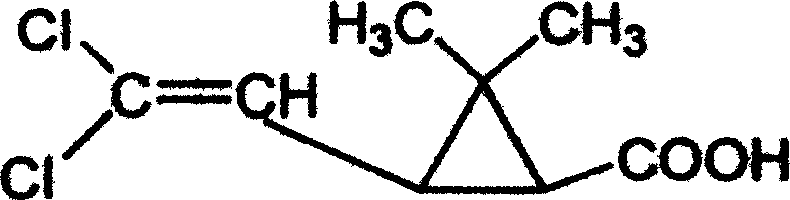
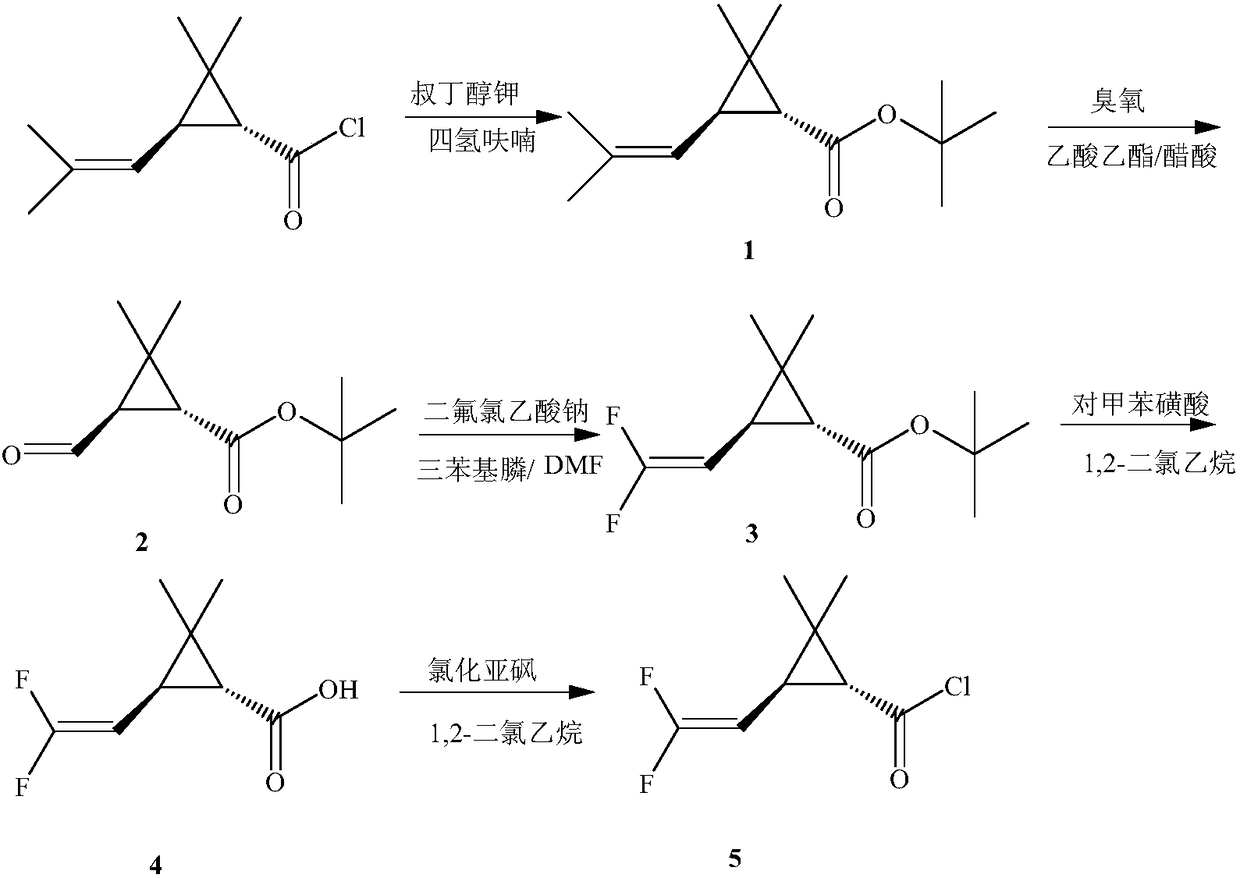
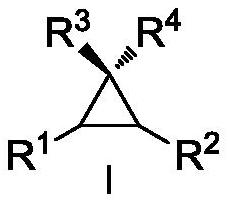
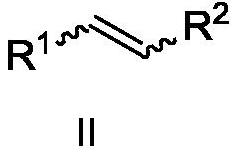
Preparation method of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane
ActiveCN113999160AMild conditionsImprove production safetyOrganic chemistryHypochloritePtru catalyst
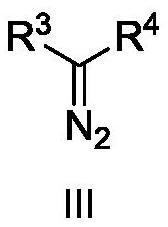
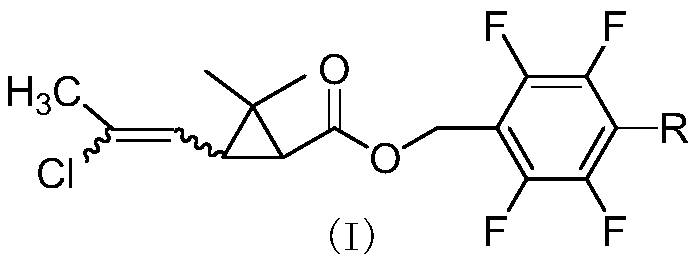
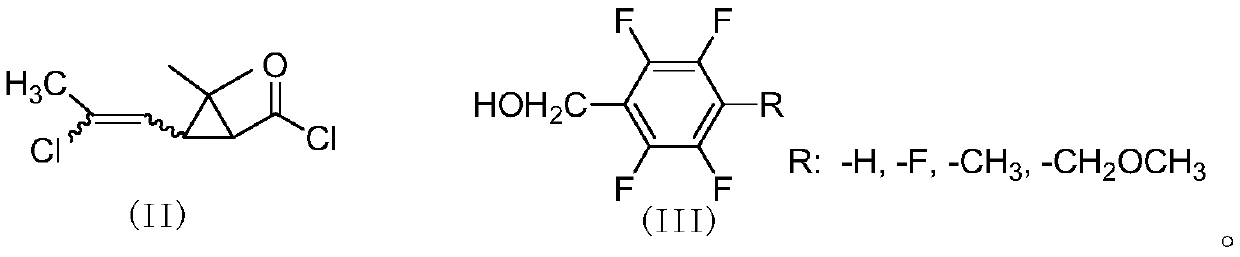
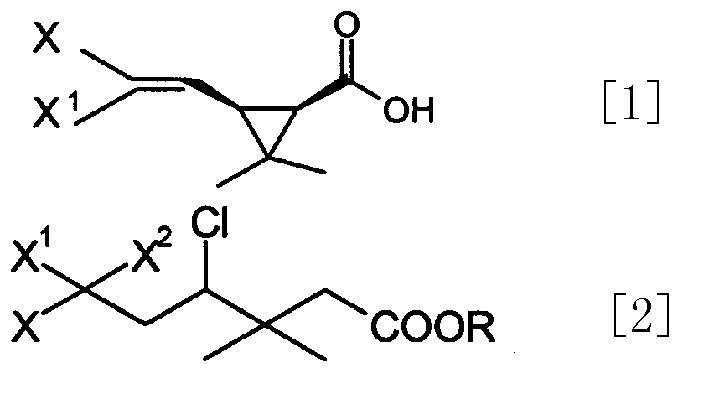
In order to solve the problems of high raw material cost, high safety risk in the reaction process and low product purity of an existing preparation method of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane, the invention provides a novel preparation method which comprises the following steps: reacting dichlorochrysanthemic acid serving as a raw material with alkali metal hydroboron and lewis acid in a reaction solvent, and converting the dichlorochrysanthemic acid into a compound as shown in a formula V; performing hydrolysis reaction on the compound in the formula V in a sulfuric acid solution to obtain a compound in a formula IV; carrying out halogenation reaction on the compound as shown in the formula IV and a halogenation reagent in a reaction solvent, and then conducting conversion in ammonia water to form a compound as shown in a formula III; reacting the compound shown in the formula III with alkali metal hydroxide and hypochlorite in water to obtain a compound shown in a formula II; and adding the compound in the formula II into a reaction solvent, and carrying out self-cyclization reaction under the action of organic alkali to obtain a compound in the formula I. The method is mild in condition, high in safety and easy for industrial production; the cost is effectively reduced; and the use of highly toxic, flammable, explosive and expensive reducing reagents, high pressure equipment, and flammable noble metal catalysts is avoided.
Owner:江苏省药物研究所有限公司
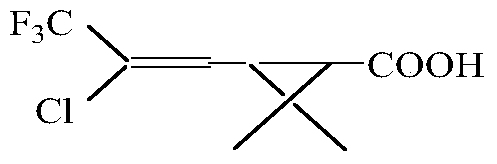
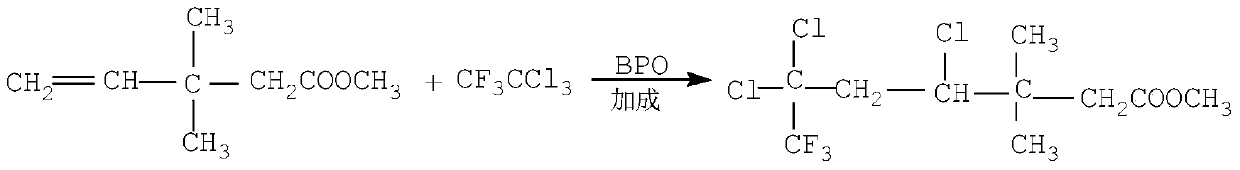
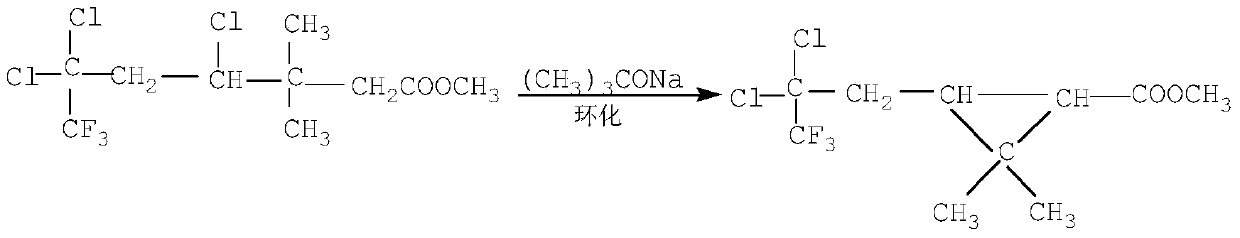
Continuous production method for trifluoro monochloro chrysanthemic acid
InactiveCN105503582ABest production methodQuality improvementOxygen-containing compound preparationOrganic compound preparation4-pentenoic acidChrysanthemic acid
The invention discloses a continuous production method for trifluoro monochloro chrysanthemic acid. The method includes the steps of (1) performing an addition reaction to prepare 3,3-dimethyl-4,6,6-trichloro-7,7,7-trifluoro heptylic acid ester with 3,3-dimethyl-4-pentenoic acid methyl ester and trifluorotrichloroethane as initial raw materials; (2) performing a cyclization reaction with sodium tert-butoxide to generate cis(trans)-3-(2,2-dichloro-3,3,3-trifluoropropyl)-2,2-dimethyl cyclocarboxylate; and (3) performing a saponification reaction to prepare 3-(2-chloro-3,3,3-trifluoro-propylene-1-yl)-2,2-dimethylcyclopropane carboxylic acid, namely, the trifluoro monochloro chrysanthemic acid. In the invention, continuous control is employed in the production method, so that the method is simple in process, is high in equipment utilization, is high in yield and stable in quality of products, is low in production cost and has a wide application prospect.
Owner:LIANYUNGANG CCA CHEM CO LTD
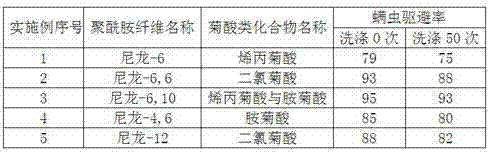
Anti-mite polyamide fiber and preparation method thereof
The invention discloses an anti-mite polyamide fiber and a preparation method thereof. The anti-mite polyamide fiber is prepared from the following raw materials in parts by weight: 100-250 parts of polyamide fiber, 1-5 parts of chrysanthemic acid compound, 200-500 parts of solvent, 0.10-10 parts of dehydrating agent and 0.1-0.5 part of catalyst. In the invention, chrysanthemic acid with anti-mite function reacts with the polyamide fiber, so that carboxyl in the chrysanthemic acid molecule and amino in the polyamide molecule are subject to a condensation reaction to further obtain the polyamide fiber with anti-mite function. The chrysanthemic acid is bonded with polyamide macromolecule to improve the stability and is not easy to loss in usage, the washing fastness is increased, and meanwhile, the safety is improved.
Owner:FUJIAN JINJIANG TECH
Preparation technology of DV-chrysanthemyl chloride with cis-trans ratio of 80:20-25:75
InactiveCN102718648AOrganic compound preparationCarboxylic compound preparationSodium methoxideAqueous sodium hydroxide
The invention discloses a preparation technology of DV-chrysanthemyl chloride with a cis-trans ratio of 80:20-25:75, characterized by using tetrachloroate or trichloroate to prepare DV-chrysanthemic acid with different cis-trans ratios, then directly using the DV-chrysanthemic acid with different cis-trans ratios in the following reaction to prepare DV-chrysanthemyl chloride, wherein the content of the obtained DV-chrysanthemyl chloride is no less than 98%. In the process of using the technology to prepare the DV-chrysanthemic acid with different cis-trans ratios from tetrachloroate or trichloroate, cheap sodium hydroxide solution is utilized to replace sodium methoxide, the intermediate products have no need of refining method and can be directly used in the following reaction to prepare DV-chrysanthemyl chloride, and the content of the obtained DV-chrysanthemyl chloride is no less than 98%.
Owner:NANTONG TENDENCI CHEM
Process for preparing optical active chrysanthemic acid
InactiveCN1373116AImproved trans/cis ratioHigh optical purityOrganic chemistryArticle feedersChrysanthemic acidMedicinal chemistry
The invention discloses a preparation method of optically active chrysanthemum acid, which is characterized in that the ratio of the trans isomer is not less than 70% and the optical purity is 2%e.e.-less than 10%e.e. through the optical analysis of an optically active organic amine acid.
Owner:SUMITOMO CHEM CO LTD
Preparation method of chrysanthemic acid with high optical purity
InactiveCN101830795AHigh split selectivityLess solventCarboxylic compound separation/purificationChrysanthemic acidDouble salt
The invention discloses a preparation method of chrysanthemic acid with high optical purity. The preparation method is characterized by comprising the following steps of: (1) forming a double salt of trans-dextrotatory chrysanthemic acid phenylglycine butyl ester from trans-dl chrysanthemic acid and a resolving agent of D type phenylglycine butyl ester or hydrochloride of the resolving agent of D type phenylglycine butyl ester in a solvent system; (2) acidizing the double salt obtained from the step (1), standing and dimixing, wherein the upper layer is the trans-dextrotatory chrysanthemic acid with high optical purity and the lower layer is an acid solution of the resolving agent of D type phenylglycine butyl ester; and (3) obtaining a trans-dextrotatory chrysanthemic acid product by separation, neutralizing the acid solution of the D type phenylglycine butyl ester with alkali and recovering the resolving agent. The preparation method has the advantages of easy operation, high resolving speed, short operation period, high selectivity and low energy consumption.
Owner:SHANDONG UNIV OF TECH
Method for preparing dextral cis-permethrinic acid
InactiveCN112301064AEasy to recycleImprove single selectivity of hydrolysisFermentationChrysanthemic acidAqueous solution
The invention discloses a method for preparing dextral cis-permethrinic acid. The method comprises the step of selectively hydrolyzing racemic permethrinic acid ester in an aqueous solution in the presence of biocatalyst esterase to obtain dextral cis-permethrinic acid with an ee value being more than 99 percent. According to the method, the hydrolysis single selectivity of racemic permethrinic acid ester is improved by selecting proper esterase, the ee value of the finally obtained dextral cis-permethrin is greater than 99%, the dextral cis-permethrinic acid can be directly used for producting and manufacturing various sanitary or agricultural pyrethroid products, can avoid the steps of crystallization and purification of low ee value intermediates and the like, and enables the productionprocess to be simplified.
Owner:JIANGSU YANGNONG CHEM +1
Preparation method for 3-(2,2-dichloroethyenyl)-2,2-dimethylcyclopropanecarboxylic acid
ActiveCN105272848ASolve productivityAddress process complexityPreparation from carboxylic acid saltsOrganic compound preparationChrysanthemic acidDouble salt
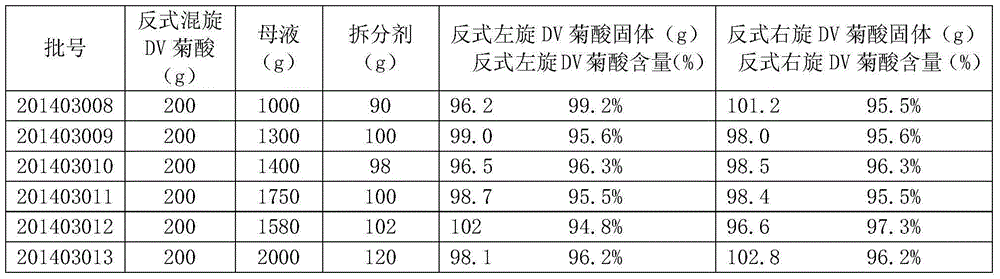
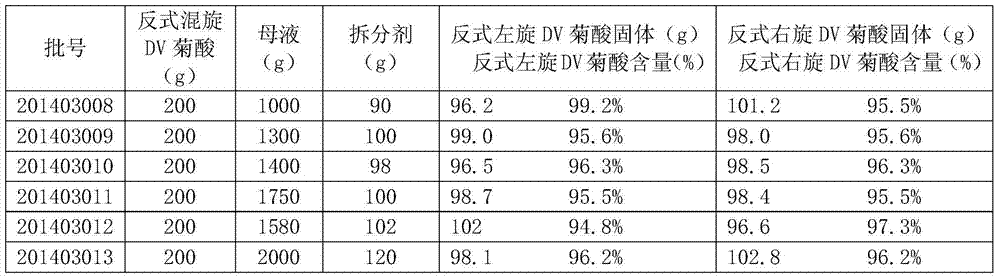
The invention provides a preparation method for 3-(2,2-dichloroethyenyl)-2,2-dimethylcyclopropanecarboxylic acid. The preparation method comprises the following steps: adding 200 g of trans-(+ / -)-DV-chrysanthemic acid into 1000 to 2000 g of mother liquor under stirring and adjusting a pH value to 6.5 to 8.2 by using alkali so as to produce a trans-(+ / -)-DV-chrysanthemate solution; dissolving 90 to 110 g of a resolving agent in water under stirring and adjusting a pH value to 2 to 5 by using acid so as to obtain an aqueous solution of the resolving agent; adding the aqueous solution of the resolving agent into the trans-(+ / -)-DV-chrysanthemate solution drop by drop within 1 to 3 h under stirring to produce a solution including solid double salt; adding 200 to 400 g of a separating agent into the solution including the solid double salt under stirring and carrying out stirring to separate a solid double salt particle and a water phase; and adding acid under stirring to adjust the pH value of separated water phase liquid to 2 to 4 so as to form a solid and an aqueous solution, wherein the solid is 3-(2,2-dichloroethyenyl)-2,2-dimethylcyclopropanecarboxylic acid. The preparation method provided by the invention overcomes the problems of long production flow, complex process and high cost in conventional resolution methods for preparation of 3-(2,2-dichloroethyenyl)-2,2-dimethylcyclopropanecarboxylic acid.
Owner:正泓(盘锦)精细化工科技有限公司
Ethyl chrysanthemate esterase and coding gene and specific engineering baterium for expression and uses of the enzyme
Owner:XIAHAN BIOENG SHANGHAI
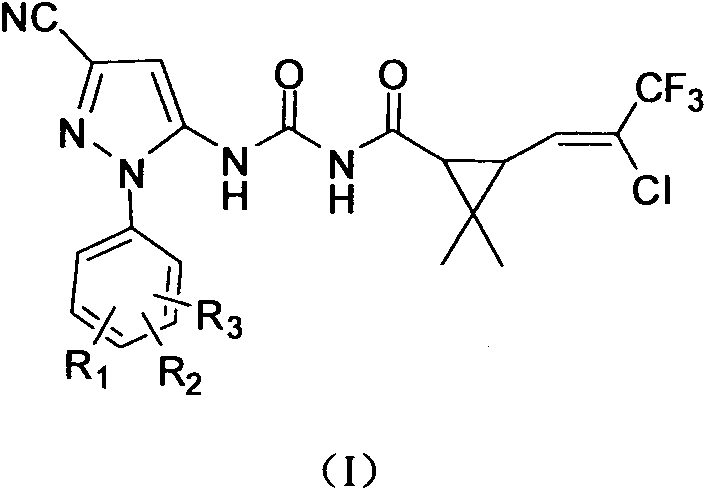
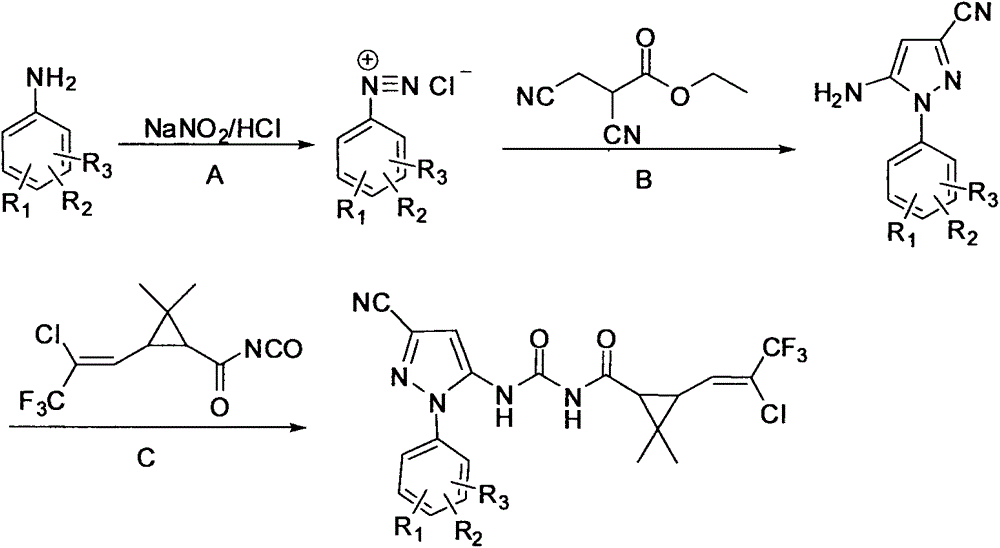
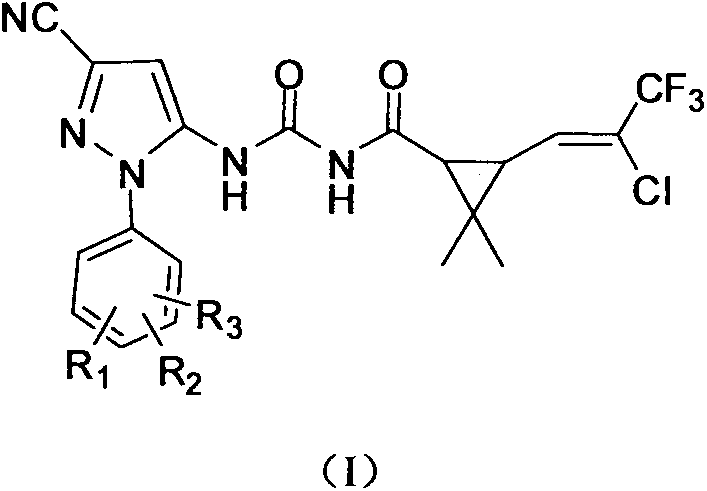
Pyrazole compound containing chrysanthemic acid structure and preparation method and purpose of pyrazole compound
ActiveCN104829538AReduce dosageImprove insecticidal effectBiocideOrganic chemistryChrysanthemic acidStructural formula
The invention discloses a pyrazole compound containing chrysanthemic acid structure and a preparation method and purpose of the pyrazole compound with the general structural formula (I). The definition of each substituent group in the general structural formula (I) is showed in the description and claims. The pyrazole compound is good in insect killing effect, simple in production process and high in yield.
Owner:NANJING UNIV OF TECH
Method for synthesizing esbiothrin pesticide intermediate through enzyme technology
ActiveCN105567746ASolve the problem of low optical puritySolve pollutionFermentationPolyesterEsbiothrin
The invention discloses a method for synthesizing an esbiothrin pesticide intermediate through an enzyme technology. The method is characterized in that cis- and trans-chrysanthemic acid isomer mixed DV methyl chrysanthemate with the structure of a compound 5 is adopted as a substrate, the feeding concentration of the substrate is 1-50g / L, and a polyester pesticide intermediate is generated through a hydrolysis reaction in a buffering solution with the temperature of 25-50DEG C and the pH value of 6.0-8.0 under the action of hydrolase, wherein the feeding mass ratio of cis-isomer to trans-isomer in the mixed DV methyl chrysanthemate is 2:8-6:4, the hydrolase is protease or esterase, and the feeding mass of the hydrolase is 10-100% of the mass of the substrate. The method for preparing the polyester pesticide intermediate has the advantages of simple process, short reaction time, high conversion rate, high product purity, environmental protection, and suitableness for industrial production.
Owner:CODEXIS INC
Novel solid wood adhesive
InactiveCN104293271AImprove water resistanceImprove moisture resistanceNon-macromolecular adhesive additivesRubber derivative adhesivesAdhesive cementPolymer science
The invention discloses a novel solid wood adhesive. The novel solid wood adhesive is prepared from the following raw materials in parts by weight: 2.5-4.2 parts of bisphenol epoxy resin, 2-4 parts of polybutadiene diol, 1.5-2.7 parts of saccharose oleate, 2.5-3.7 parts of sec-octyl phenol polyoxyethylene ether, 1.2-2.3 parts of laurinol dibutyl tin, 0.5-0.8 part of dipolyglycerol, 1.2-1.8 parts of butylated hydroxyl anisole and 2.5-3.6 parts of ethyl chrysanthemate. Compared with the prior art, the novel solid wood adhesive disclosed by the invention can be used for lowering free formaldehyde which is smaller than 0.11, so that the E0 level is achieved, and thus, the novel solid wood adhesive is relatively environmentally friendly. The novel solid wood adhesive disclosed by the invention is long in storage time, easily available for raw materials and more suitable for large-scale production, and a bamboo wood product adopting the novel solid wood adhesive has good water resistance and moisture resistance. By adding a smell remover rosin resin, the strength and pre-pressing effects of the resin adhesive are increased, and the free formaldehyde is lowered.
Owner:王璐
Prepn of dibromochrysanthemic acid
InactiveCN1417191AReduce lossesReduce dosagePreparation from carboxylic acid esters/lactonesHalogenMedicinal chemistry
Using dichlorochrysanthemic acid as raw material, dibromochrysanthemic acid is prepared through the direct halogen exchange reaction of AlBr3 with dichlorochrysanthemic acid, with obtaining tribromochrysanthemic acid as side product. Through an added HBr eliminating reaction, the tribromochrysanthemic acid as side product is converted into dibromochrysanthemic acid. The dibromochrysanthemic acid product with purity of 97% is obtained in the total yield of 91%. During the reaction, the product and the raw material have their configuration maintained.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Wear-resistant sealing material and preparation method thereof
InactiveCN104804348AImprove corrosion resistanceImprove high temperature resistanceOther chemical processesBenzoic acidActive agent
The invention discloses a wear-resistant sealing material and a preparation method thereof. The wear-resistant sealing material comprises the following components in parts by weight: 60-65 parts of acrylate rubber, 21-23 parts of carbon black, 5-7 parts of benzotriazole, 1-3 parts of stearic acid, 3-5 parts of an active agent magnesium oxide, 2-4 parts of a fire retardant tetrachlorophthalic anhydride, 1-3 parts of tung oil, 1-2 parts of an anti-aging agent 4010NA, 0.5-0.8 part of graphite powder, 0.3-0.5 part of talcum powder, 1-3 parts of dioctyl adipate, 3-5 parts of zinc oxide, 0.5-0.8 part of copper powder, 1-2 parts of ethyl chrysanthemate, 1-3 parts of an accelerant TMTD, 7-9 parts of high styrene resin and 1-2 parts of benzoic acid.
Owner:SUZHOU TONGMING MACHINERY
Preparation method of dextro trans-chrysanthemic acid
InactiveCN108486171AEasy to recycleImprove single selectivity of hydrolysisFermentationChrysanthemic acidHydrolysis
The invention discloses a preparation method of dextro trans-chrysanthemic acid. The method includes: in ammonia water or an aqueous solution of organic amine with a carbon atom number of less than 6,subjecting racemic chrysanthemic acid ester to selective hydrolysis in the presence of biocatalyst esterase to obtain dextro trans-chrysanthemic acid with an ee value of greater than 99%. The methodprovided by the invention selects a specific buffer system to change the biotic environment of esterase, improves the hydrolysis single selectivity of racemic chrysanthemic acid ester, the finally obtained dextro trans-chrysanthemic acid has an ee value of greater than 99%, and can be directly used for the production and manufacturing of various sanitary pyrethroids, crystallization purification of the low ee value intermediate and other steps can be avoided, and the production process is simplified.
Owner:JIANGSU YANGNONG CHEM +1
Process of inductive crystallization for preparing enriched trans-racemic dichloro-chrysanthemic acid
InactiveCN1502600AReduce contentIncrease contentCarboxylic compound separation/purificationChemical synthesisOrganic solvent
The present invention relates to a method for preparing enriched trans-racemic-dichlorochrysanthemic acid by induced crystallization, and is characterized by that it uses racemic dichlorochrysanthemic acid as raw material, and dissolves it in organic solvent and makes it form supersaturated solution, then adds small quantity of inductive crystal seed and makes them implement slow and natural crystal precipitation at room temperature. The adoption of said invention can separate four isomers of racemic-dichlorochrysanthemic acid, can obviously increase content of trans-isomer which can be reached to about 95%, and can obviously raise purity of trans-dichlorochrysanthemic acid.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Pyrethroid compound
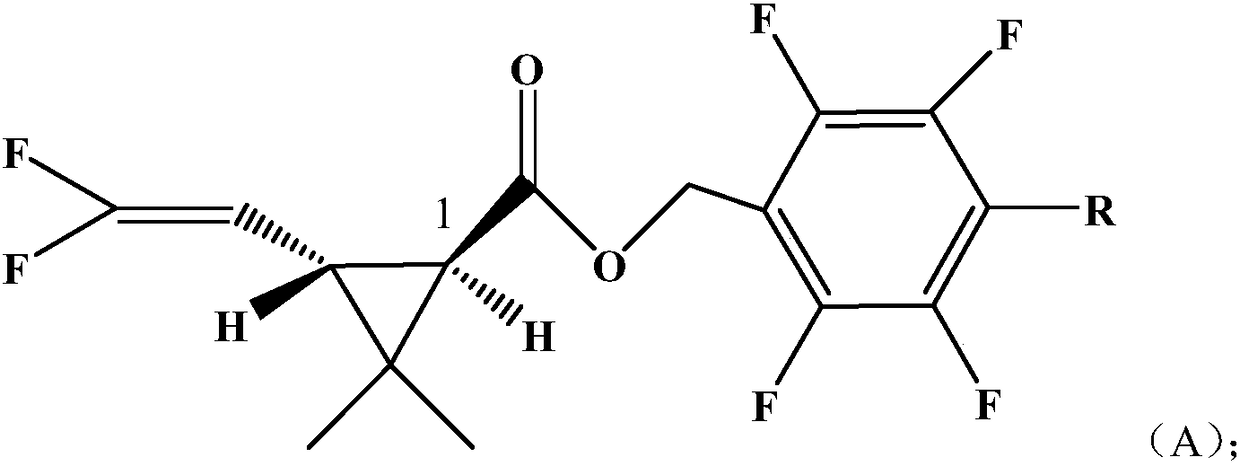
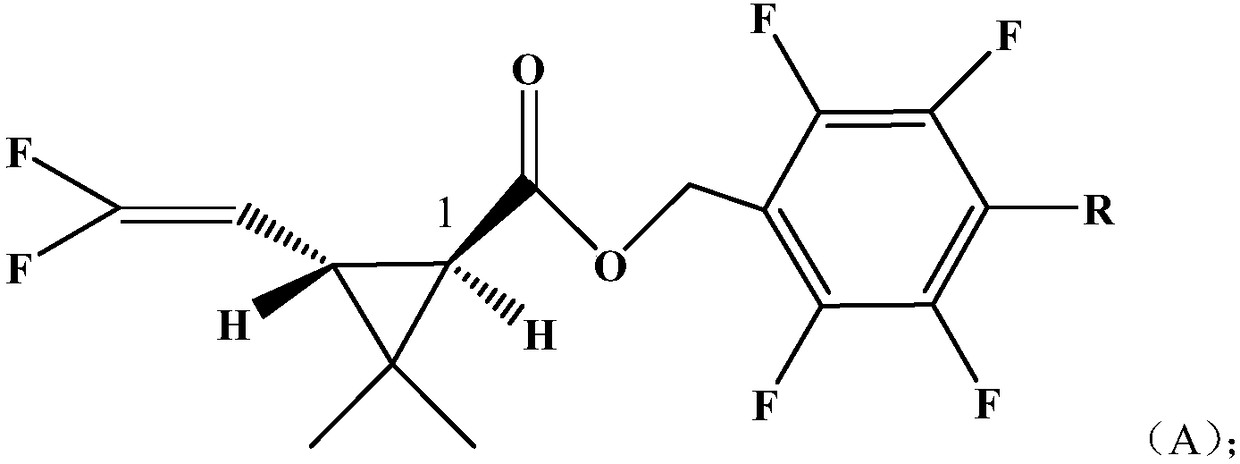
InactiveCN108250078AHigh activityBiocidePreparation from carboxylic acid halidesChrysanthemic acidStructural formula
The invention discloses a pyrethroid compound. The structural formula of the compound is represented by formula A; the chrysanthemic acid part of the compound is a 1R, trans single optical isomer, wherein R is a H atom or a methyl group. The compound in the invention has a better insecticidal effect on certain target pests than compounds in the prior art.
Owner:JIANGSU YANGNONG CHEM
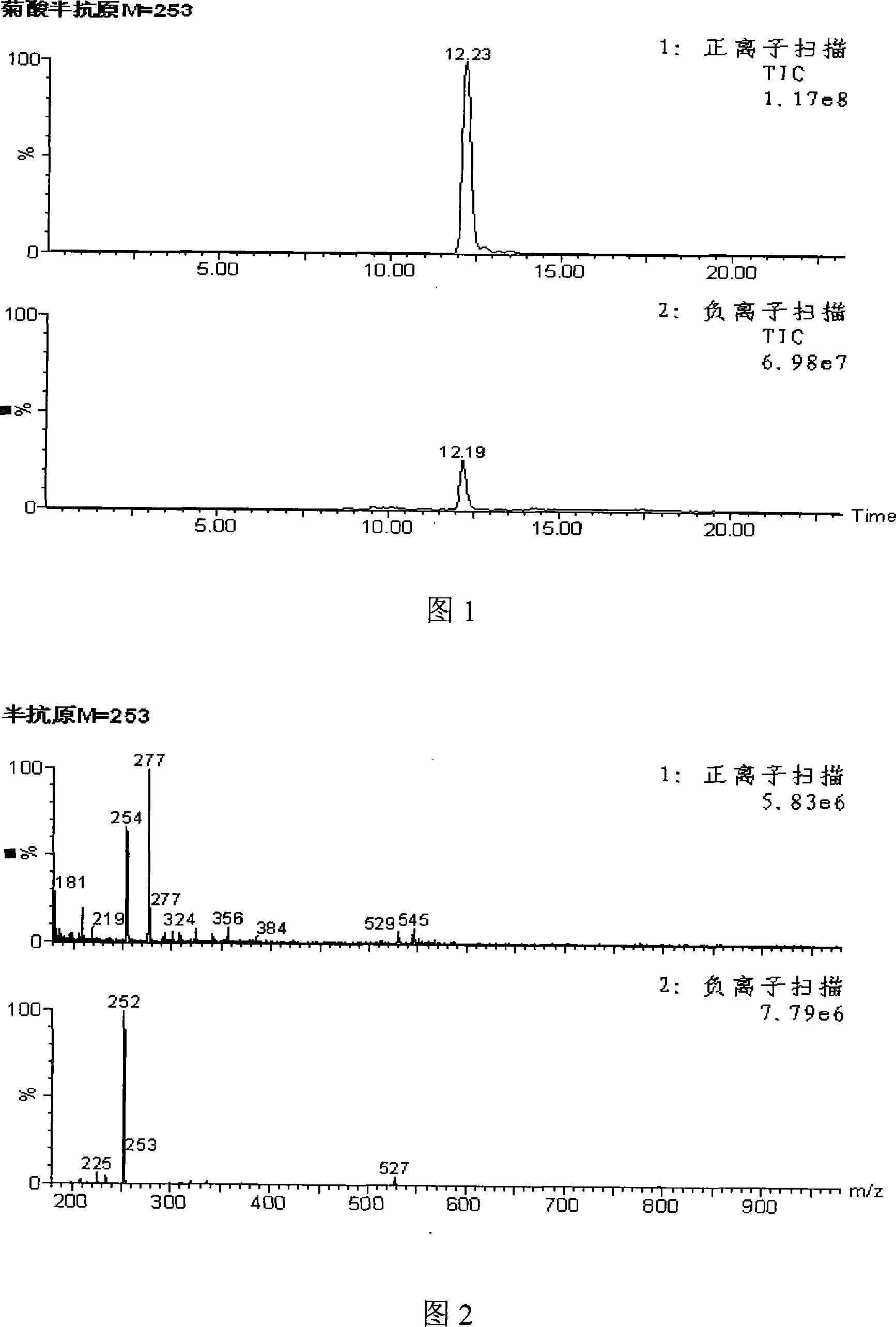
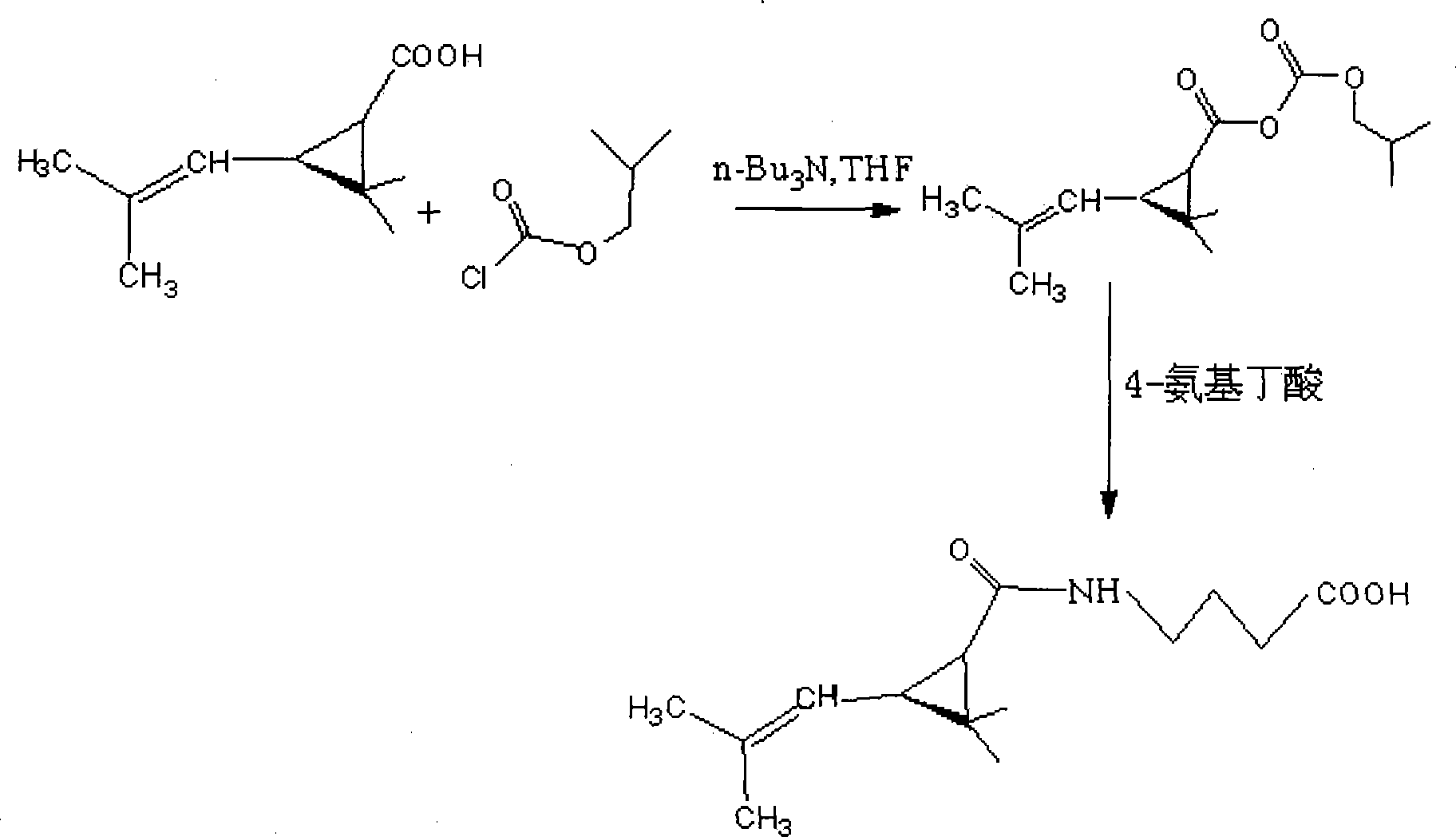
Method for synthesizing 4-reanal condensation chrysanthemic acid hapten
ActiveCN101219965AThe synthesis steps are simpleHigh purityOrganic compound preparationComponent separationSynthesis methodsChrysanthemic acid
Owner:山东美正生物科技有限公司
Asymmetric cyclopropanation method of copper-catalyzed olefin and application of asymmetric cyclopropanation method
InactiveCN112824372AHigh activityLigand preparation is simpleOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystCyclopropanation
The invention discloses an asymmetric cyclopropanation method of copper-catalyzed olefin and application thereof. The copper catalyst adopted by the method is generated in situ from a metal copper precursor and a chiral P, N, N-ligand in a reaction medium. The method has the characteristics of cheap catalyst, simple ligand preparation, high activity, high selectivity, mild reaction conditions, simple operation and the like, can realize continuous operation, and is suitable for large-scale industrial production. The method is also suitable for asymmetric synthesis of chiral first chrysanthemic acid which is an important intermediate of pyrethroid pesticides, the yield can reach 80%, the enantioselectivity can reach 85%, and the method can be applied to industrial preparation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Methyl cloro chrysanthemic acid polyfluoro benzyl alcohol pyrethroid compound as well as preparation method and application thereof
InactiveCN109776327AHigh killing activityEasy to synthesizeBiocideOrganic compound preparationAlcoholOrganic base
The invention belongs to the field of pyrethroid compounds, and particularly relates to a methyl cloro chrysanthemic acid polyfluoro benzyl alcohol pyrethroid compound as well as a preparation methodand application thereof. The methyl cloro chrysanthemic acid polyfluoro benzyl alcohol pyrethroid compound is obtained by an esterification reaction of methyl cloro chrysanthemoyl chloride and polyfluoro benzyl alcohol in a solvent in the presence of an organic base. The compound provided by the methyl cloro chrysanthemic acid polyfluoro benzyl alcohol pyrethroid compound has very high killing activity on sanitary pests such as mosquitoes, housefly and german cockroaches; the methyl cloro chrysanthemic acid polyfluoro benzyl alcohol pyrethroid compound is easy to synthesize, low in productioncost and easy to industrialize, and the synthesis complexity is obviously reduced; in addition, due to the asymmetric structure of the chrysanthemic acid part and polyfluoro chrysanthemic acid alcohol, the problems of resistance and environmental residue of pests can be effectively reduced.
Owner:HUANGSHAN UNIV
Despinning method of left-handed trans-inulin alkyl ester
InactiveCN108276288AAchieve recyclingReduce dosageOrganic compound preparationOrganic chemistry methodsSolventHydrolysis
The invention discloses a despinning method of left-handed trans-inulin alkyl ester. The despinning method comprises the following steps: performing despinning reaction on the left-handed trans-inulinalkyl ester, peroxide and a bromine-containing compound in a solvent at 40 to 50 DEG C for 1 to 2 h, adding water for washing, and desolventizing an oil layer to obtain the despun inulin alkyl ester.The despinning method aims to directly despin the low-pharmaceutical-effect left-handed trans-inulin alkyl ester which is a byproduct obtained by enzyme hydrolysis by a one-step method to obtain despun chrysanthemic acid, so as to realize comprehensive cyclic utilization of raw materials, reduce the production cost and reduce the wastewater amount; through selection of a proper chemical initiatorand a catalyst, the despinning yield is increased; the content of trans-(+) inulin alkyl ester is 45 to 48 percent; the content of trans-(-) inulin alkyl ester is 45 to 48 percent; the content of cis(+ / -)inulin alkyl ester is 4 to 6 percent. Therefore, the effect of despinning the left-handed trans-inulin alkyl ester is achieved; moreover, reaction processes are reduced, the production cost is reduced, and the treatment capacity of wastewater is reduced.
Owner:JIANGSU YANGNONG CHEM +1
Method for separating and detecting components in permethrin
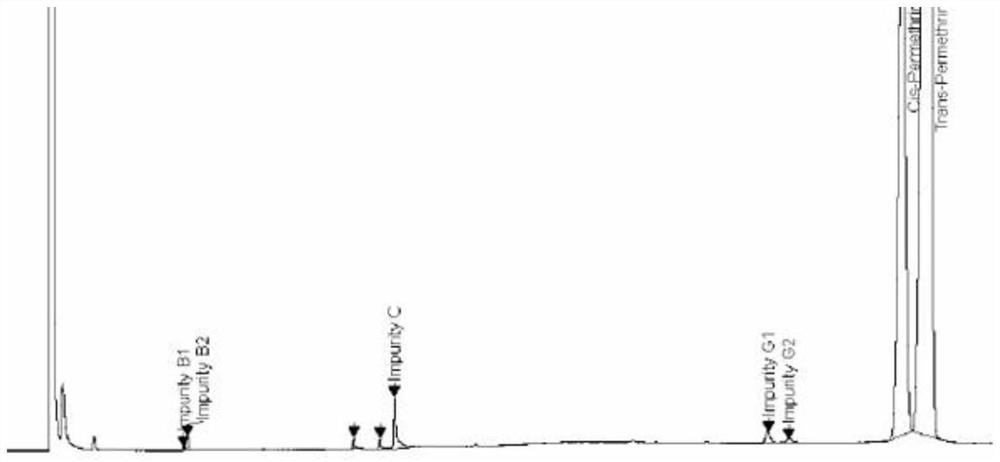
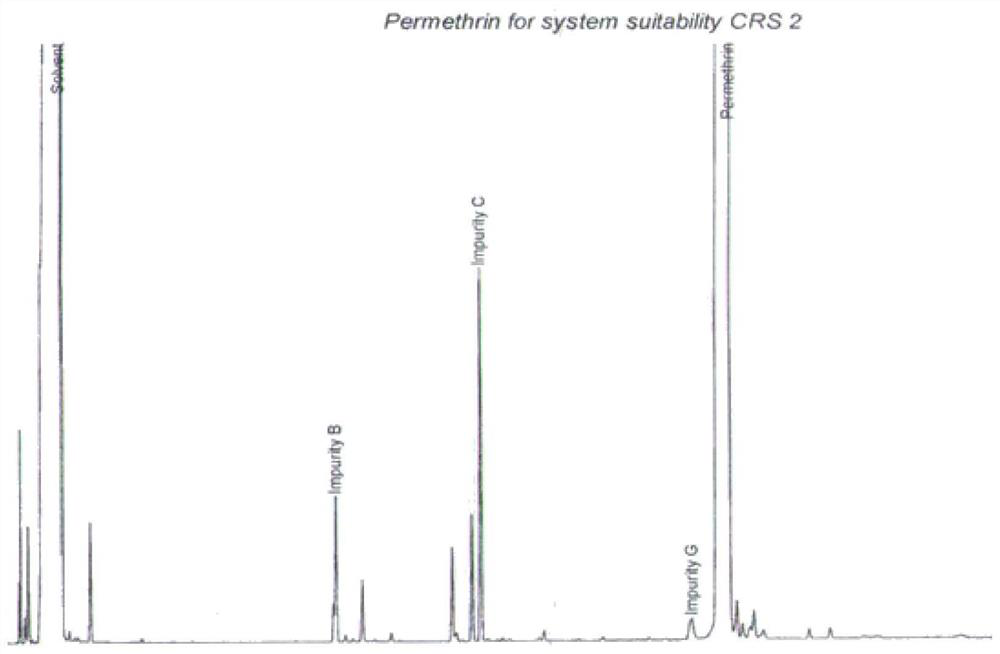
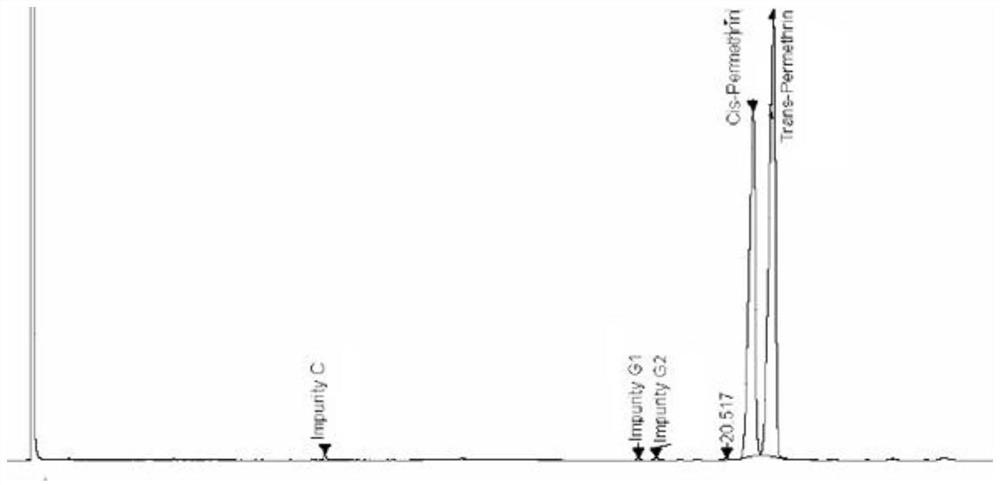
ActiveCN112834640AEfficient separationApplicable controlComponent separationAgainst vector-borne diseasesVapor phase chromatographyCarboxylic acid
The invention relates to a method for separating and detecting components in permethrin. The permethrin comprises the following components: cis-permethrin, trans-permethrin, an impurity B, an impurity C and an impurity G, the impurity B is methyl dichlorochrysantheate, the impurity C is m-phenoxybenzyl alcohol, the impurity G is 3-(phenoxy phenyl) methyl-(1RS, 2RS)-2-(chloroethynyl)-3, 3-dimethyl cyclopropyl-1-carboxylic acid ester. According to the method, a gas chromatographic method is adopted for separation and detection, a capillary chromatographic column with 100% concentration of dimethyl polysiloxane as a stationary liquid is used, and the heating procedure of the capillary chromatographic column is as follows: the initial column temperature is 125 DEG C and maintained for 2 min, then the temperature is raised to 235 DEG C at the rate of 10 DEG C / min and maintained for 15 min, a detector is a hydrogen flame ionization detector, the temperature of a sample inlet is 230-270 DEG C, and the detection time is 30-50 min. The temperature of a detector is 250-290 DEG C, and the diluent is methanol. The method for separating and detecting the components in the permethrin can well separate the components in the permethrin, and has the advantages of high sensitivity, strong specificity, stable separation state and the like.
Owner:上海汉维生物医药科技有限公司
Adhesive for laying of geothermal wood floor
InactiveCN104629631AExtended service lifeHigh strengthNon-macromolecular adhesive additivesOrganic non-macromolecular adhesiveAdhesiveEthylic acid
The invention discloses an adhesive for laying of geothermal wood floor. The adhesive is prepared by using the following raw materials in parts by weight: 2.5-5 parts of dioctyl sodium succinate salt, 1.5-2.8 parts of crylic acid, 1.8-4.2 parts of hydrazine hydrate, 3.2-5.8 parts of ammonium acetate, 1.6-4.8 parts of ethyl chrysanthemumate, 0.8-2.2 parts of 2-xylenol, 2.3-4.2 parts of barium hydroxide and 0.5-1.8 parts of a polyurethane copolymer. Compared with the existing products, the adhesive for laying of geothermal wood floor disclosed by the invention has the advantages of longer service life, higher strength after being dried and no shrinkage and no cracks, has the functions of absorbing and reducing noise, and further has the properties of fire prevention, air permeability, temperature and humidity conditioning due to use of ammonium acetate and ethyl chrysanthemumate, and has higher performance price ratio and great market competition with a reduction of 30 percent of the cost compared with other materials having the same effect.
Owner:QINGDAO DESHENGTAI CONSTR INSTALLATION ENG
Racemization method of trans-levochrysanthemic acid
ActiveCN114539045AImprove general performanceMild reaction conditionsOrganic compound preparationOrganic chemistry methodsChrysanthemic acidOrganic synthesis
The invention discloses a universal racemization method of trans-levochrysanthemic acid, and belongs to the field of organic synthesis. The preparation method comprises the following steps: esterifying trans-levorotatory acid, carrying out racemization reaction on the esterified trans-levorotatory acid and lewis acid in a solvent at 70-120 DEG C for 4-6 hours, adding water, continuously reacting for 2 hours, washing an oil layer with water, desolventizing, acidifying and filtering to obtain the trans-racemic chrysanthemic acid, and the structure of the trans-racemic chrysanthemic acid is shown as the following I. The invention aims to provide the preparation method of the trans-racemic chrysanthemic acid with low drug effect or ineffective trans-levorotatory chrysanthemic acid. The trans-racemic chrysanthemic acid is obtained through esterification, racemization and other two-step reaction, recycling of ineffective chrysanthemic acid bodies is achieved, and the purposes of saving raw materials, reducing resource waste, reducing three wastes and saving the production cost of chiral chrysanthemic acid are achieved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for recycling lambda-cyhalothric acid from lambda-cyhalothric acid residual liquid
InactiveCN112110791AAffectGood insecticidal effectBiocideOxygen-containing compound preparationChrysanthemic acidSodium hydroxide
The invention discloses a method for recycling lambda-cyhalothric acid from lambda-cyhalothric acid residual liquid. According to the technical scheme, the method comprises the following preparation steps that S1, the recycled lambda-cyhalothric acid residual liquid is placed into a storage tank in a centralized mode; S2, the lambda-cyhalothric acid residual liquid in the storage tank is filtered;S3, the filtered lambda-cyhalothric acid residual liquid is poured into a reaction kettle; S4, water is added into the reaction kettle; S5, a stirring device is inserted to thereaction kettle, and stirring and cleaning are carried out; S6, secondary filtration on the stirred lambda-cyhalothric acid residual liquid is carried out; S7, the filtered lambda-cyhalothric acid residual liquid is pouredinto a heating device and constant-temperature heating is carried out; S8, sodium hydroxide is added into the catalyzed lambda-cyhalothric acid residual liquid; and S9, the target product lambda-cyhalothric acid is obtained by saponification of the lambda-cyhalothric acid residual liquid. Thestorage tank in the S1 is made of glass. The method has the beneficial effects that recycling is achieved,the filterability is high, and the insecticidal effect of extracted lambda-cyhalothric acid is remarkable.
Owner:泰兴市新宏阳化工有限公司
A kind of preparation method of trans DV chrysanthemic acid
ActiveCN105272848BPreparation from carboxylic acid saltsOrganic compound preparationChrysanthemic acidSolid particle
The present invention proposes a preparation method of trans dextrorotatory DV chrysanthemic acid, comprising: adding 200 g of trans dextrorotatory DV chrysanthemic acid into 1000-2000 g of mother liquor under stirring, adjusting the pH value to 6.5-8.2 with alkali, Generate a trans-swirling DV chrysanthemum salt solution; dissolve 90-110g of the resolving agent in water under stirring, add acid to adjust the pH value to 2-5, and obtain an aqueous solution of the resolving agent; dissolve the aqueous solution of the resolving agent under stirring Add dropwise to the trans-swirling DV chrysanthemum salt solution for 1 to 3 hours to generate a solution containing solid double salt; add 200 to 400 g of separating agent to the solution containing solid double salt under stirring, and stir so that Separating the solid double salt particles and the water phase; adding acid under stirring to adjust the pH value of the separated water phase to 2-4 to form a solid and an aqueous solution, and the solid is trans-DV chrysanthemic acid. The invention solves the problems of long production process, complex process and high cost for preparing trans-DV chrysanthemic acid by the existing resolution method.
Owner:正泓(盘锦)精细化工科技有限公司
Preparation method of trans-D-chrysanthemic acid
InactiveCN101723826BHigh split selectivityLess solventOrganic compound preparationCarboxylic compound preparationOrganic solventChrysanthemic acid
A preparation method of trans-D-chrysanthemic acid relates to an improvement of the preparation method of trans-D-chrysanthemic acid. The invention provides a preparation method with easy operation of trans-D-chrysanthemic acid. The method of the invention comprises the following steps: (1) dissolving D-(-)phenylglycine ester.HCl in water, dissolving mixed trans-chrysanthemic acid in organic solvent; or after dissolving mixed trans-chrysanthemic acid in organic solvent, once adding D-(-)phenylglycine ester.HCl; mixing the two solutions, adjusting the pH value to 5-9 with weak base, wherein the molar ratio of mixed trans-chrysanthemic acid to D-(-)phenylglycine ester.HCl is 1.5-2.5:1; (2) precipitating D-(-)phenylglycine ester.trans-D-chrysanthemic acid double salt at 0-40 DEG C; (3) separating the double salt generated in the step (2), placing in an acidic aqueous solution with the pH value of 1-4, standing for standby; and separating to obtain the trans-D-chrysanthemic acid product, neutralizing the acidic aqueous solution of D-(-)phenylglycine ethyl ester with weak base to recycle D-(-)phenylglycine ester.HCl.
Owner:沈阳古德科技有限公司
Process for obtaining enantiomers of chrysanthemic acid
A new process is described, easy to perform on industrial scale, with high yield, for obtaining enantiomers of chrysanthemic acid starting from mixtures containing them. The process involves reacting said mixtures with enantiomers of 2- dimethylamino-1-phenyl-1 ,3-propanediol (DMPP) and 2-dimethylamino-1-[4- (methylthio)phenyl]propane-1 ,3-diol (MTDP) as chiral selectors. The invention includes salts of chrysanthemic acid with the aforesaid chiral selectors as process intermediates. The process allows operation in a single solvent rather than in solvent mixtures difficult to recover and reuse, and the final crystallization product does not incorporate molecules of solvent. The bases used are then recovered and reused in subsequent separations.
Owner:ENDURA AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane Preparation method of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane](https://images-eureka.patsnap.com/patent_img/0b2c7dd5-be71-4c61-9bde-affc4e0b190c/HDA0003366663220000011.png)
![Preparation method of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane Preparation method of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane](https://images-eureka.patsnap.com/patent_img/0b2c7dd5-be71-4c61-9bde-affc4e0b190c/HDA0003366663220000021.png)
![Preparation method of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane Preparation method of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane](https://images-eureka.patsnap.com/patent_img/0b2c7dd5-be71-4c61-9bde-affc4e0b190c/HDA0003366663220000022.png)