Method for synthesizing esbiothrin pesticide intermediate through enzyme technology
An enzymatic synthesis and pesticide technology, applied in the direction of fermentation, can solve the problems of difficult application, low product purity, low target protein content, etc., and achieve the effects of simple product separation, high product purity, and high reaction conversion rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
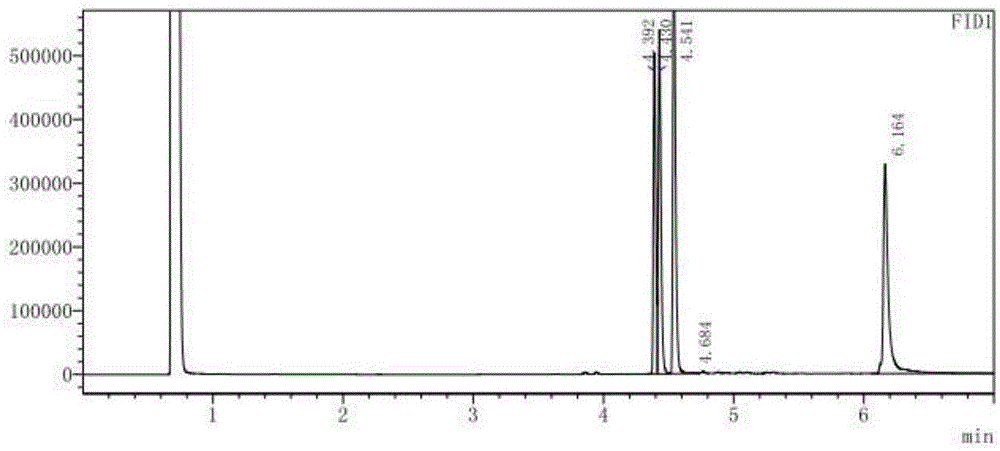
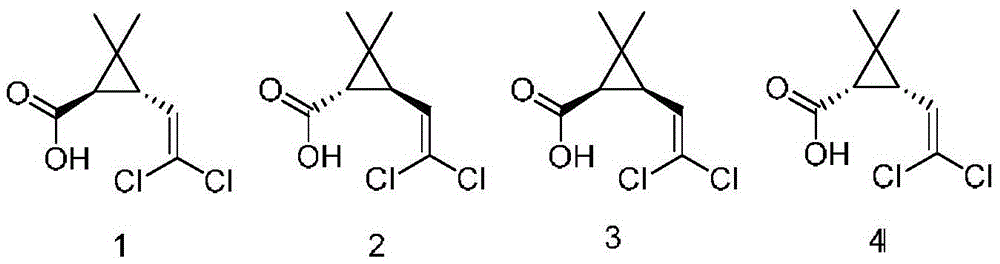
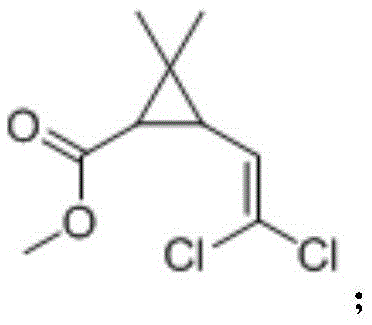
[0027] Take 1 g of mixed methyl esters of cis and trans DV methyl chrysanthemum acid isomers with a mass ratio of 4:6 (the theoretical yield of product 1 is 30%, the same below), add pH7.0, 0.1M phosphoric acid Salt buffer solution 19mL, acetonitrile 1mL, lipase LipasePS (purchased from AMANO / Amano Enzyme Company) 1g, after 2 hours of reaction at 30 ℃, an equal volume of ethyl acetate was extracted, detected by gas chromatography, the conversion rate was 4.5%, the four products The ratio (1:2:3:4) is 2:94.5:0:3.5.
Embodiment 2
[0029] Take 1 g of mixed methyl esters of cis and trans DV methyl chrysanthemum acid isomers, add pH 7.0, 19 mL of 0.1 M phosphate buffer solution, 1 mL of acetonitrile, 1 g of Aspergillus saitoi protease (purchased from sigma company), and react at 30 ° C After 2 hours, an equal volume of ethyl acetate was extracted and detected by gas chromatography. The conversion rate was 5.3%, and the ratio (1:2:3:4) of the four products was 94.4:0.7:0:4.9.
Embodiment 3
[0031] Take 1 g of mixed methyl esters of cis and trans DV methyl chrysanthemum isomers, add pH 7.0, 19 mL of 0.1 M phosphate buffer solution, 1 mL of acetonitrile, and esterase CES-E2 (purchased from AMANO / Amano Enzyme Co., Ltd. The same below) 1g, reacted at 30°C for 2 hours, extracted with equal volume of ethyl acetate, detected by gas chromatography, the conversion rate was 24.8%, and the ratio of the four products (1:2:3:4) was 93.1:4:0: 2.9.
[0032] Can find out from embodiment 1,2,3, select the Aspergillussaitoi protease produced by sigma company or the esterase CES-E2 produced by AMANO / Amano Enzyme Company as the hydrolase, the yield of its product 1 will be obviously higher than that selected from Yield obtained by the lipase LipasePS of AMANO / Amano Enzyme Co., Ltd. as a hydrolase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com