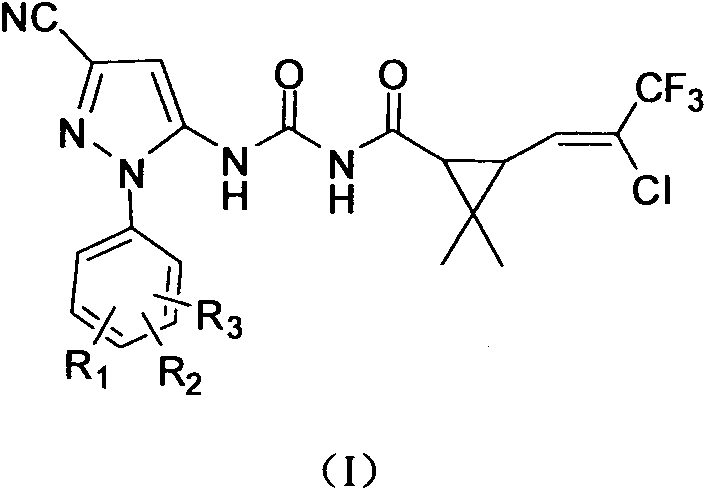
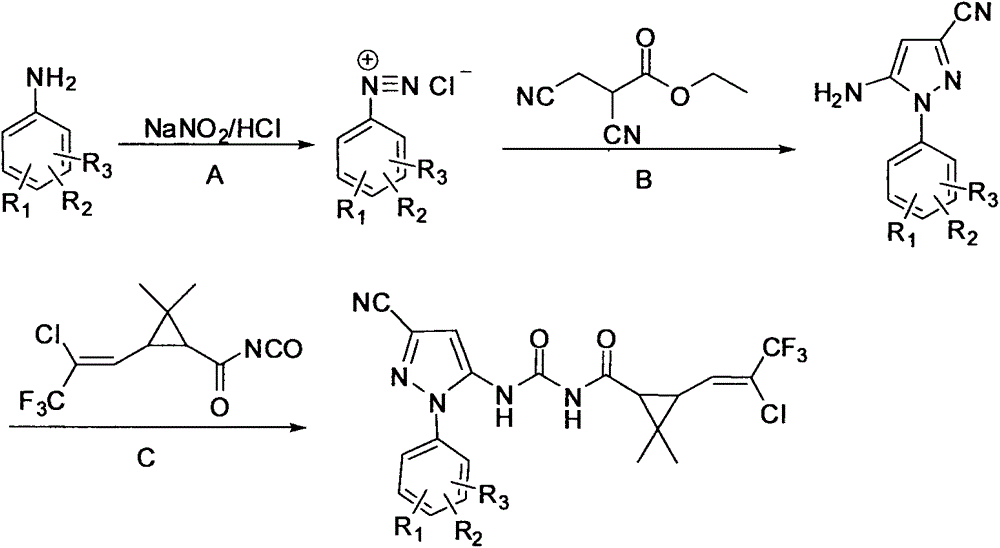
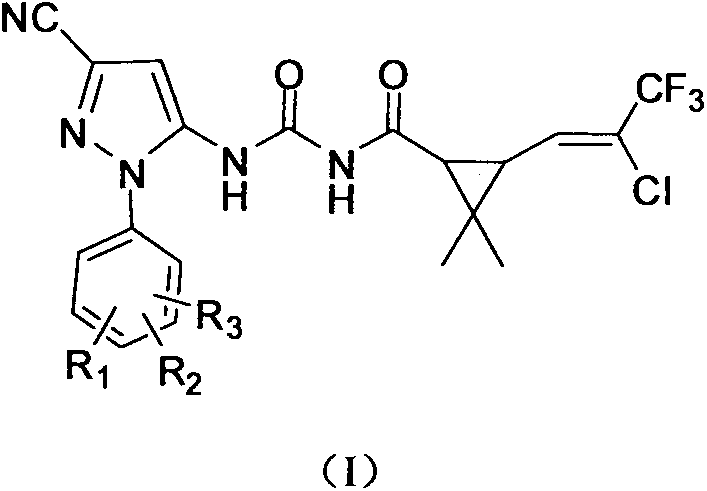
Pyrazole compound containing chrysanthemic acid structure and preparation method and purpose of pyrazole compound
A compound, technology of pyrazoles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This example illustrates the preparation of 5-amino-1-(2-fluorophenyl)-3-cyano-1H-pyrazole
[0032] Add 0.01 mol of 2-fluoroaniline aniline, 5 ml of ethanol and 3.0 ml (0.035 mol) of concentrated hydrochloric acid into a four-necked flask under ice-salt bath conditions. Slowly drop 0.018mol sodium nitrite and 10ml aqueous solution into the reaction flask, and react for 0.5h after the addition to obtain a yellow diazonium salt solution.
[0033] Add 0.01mol ethyl 2,3-dicyanopropionate into the three-necked flask, slowly drop the above diazonium salt solution into the flask, and react for 2 hours. Add ammonia water to adjust the pH to 9-10, and react at room temperature for 2 hours. After the reaction was completed, it was extracted with 40ml of ethyl acetate, the organic phase was washed with water (2×30mL), washed with saturated sodium chloride solution (1×40mL), dried over anhydrous sodium sulfate, and part of the solvent was removed by rotary evaporation, and 2.13g o...
Embodiment 2
[0035] This example illustrates that N-(5-(3-cyano-1-(2-fluorophenyl)-1-H-pyrazole)carbamoyl)-3-(2-chloro-3,3,3-tri Preparation of fluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxamide
[0036] Add 0.01mol 5-amino-1-(2-fluorophenyl)-3-cyano-1H-pyrazole and 40ml toluene into a four-neck flask, heat to 90°C, add 3-(2-chloro-3 dropwise, 3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropaneformyl isocyanate 0.01mol and 5ml toluene solution, reflux reaction for 5h. After the reaction, cooling and crystallization, filtration, and recrystallization of the filter cake with ethanol to obtain 3.77 g of the product. Yield 80.2%. Product melting point: 155-156°C; 1 H NMR (DMSO-d 6 , δ): 1.12 (s, 3H, -CH 3 ), 1.25(s, 3H, -CH 3 ), 2.28 (s, 2H, cyclopropane-CH), 6.87 (s, 1H, pyrazole-CH), 7.10 (m, 1H, C=CH), 7.48-7.51 (d, J=6.3Hz, 2H, Ph- H), 7.67-7.69 (d, J=6Hz, 2H, Ph-H), 10.81 (s, 1H, CO-NH), 11.36 (s, 1H, CO-NH-CO).
Embodiment 3
[0038] This example illustrates the preparation of 5-amino-1-phenyl-3-cyano-1H-pyrazole
[0039] Add 0.01 mol of aniline, 5 ml of ethanol and 3.0 ml (0.035 mol) of concentrated hydrochloric acid into a four-necked flask under ice-salt bath conditions. Slowly drop 0.018mol sodium nitrite and 10ml aqueous solution into the reaction flask, and react for 0.5h after the addition to obtain a yellow diazonium salt solution. .
[0040] Add 0.01mol ethyl 2,3-dicyanopropionate into the three-necked flask, slowly drop the above diazonium salt solution into the flask, and react for 2 hours. Add ammonia water to adjust the pH to 9-10, and react at room temperature for 2 hours. After the reaction was completed, it was extracted with 40ml of ethyl acetate, the organic phase was washed with water (2×30mL), washed with saturated sodium chloride solution (1×40mL), dried over anhydrous sodium sulfate, and part of the solvent was removed by rotary evaporation, and 2.89g of the product was obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com