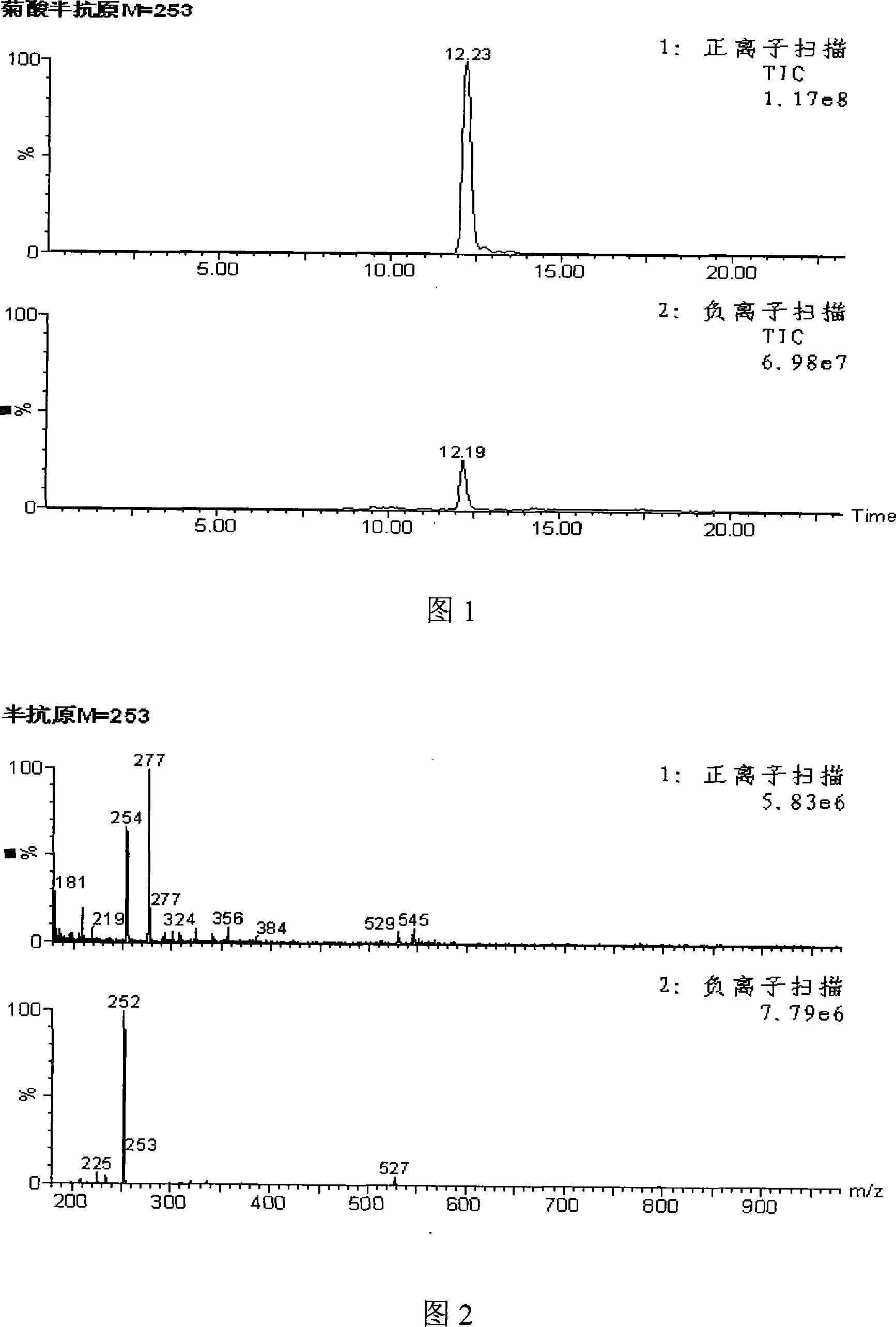
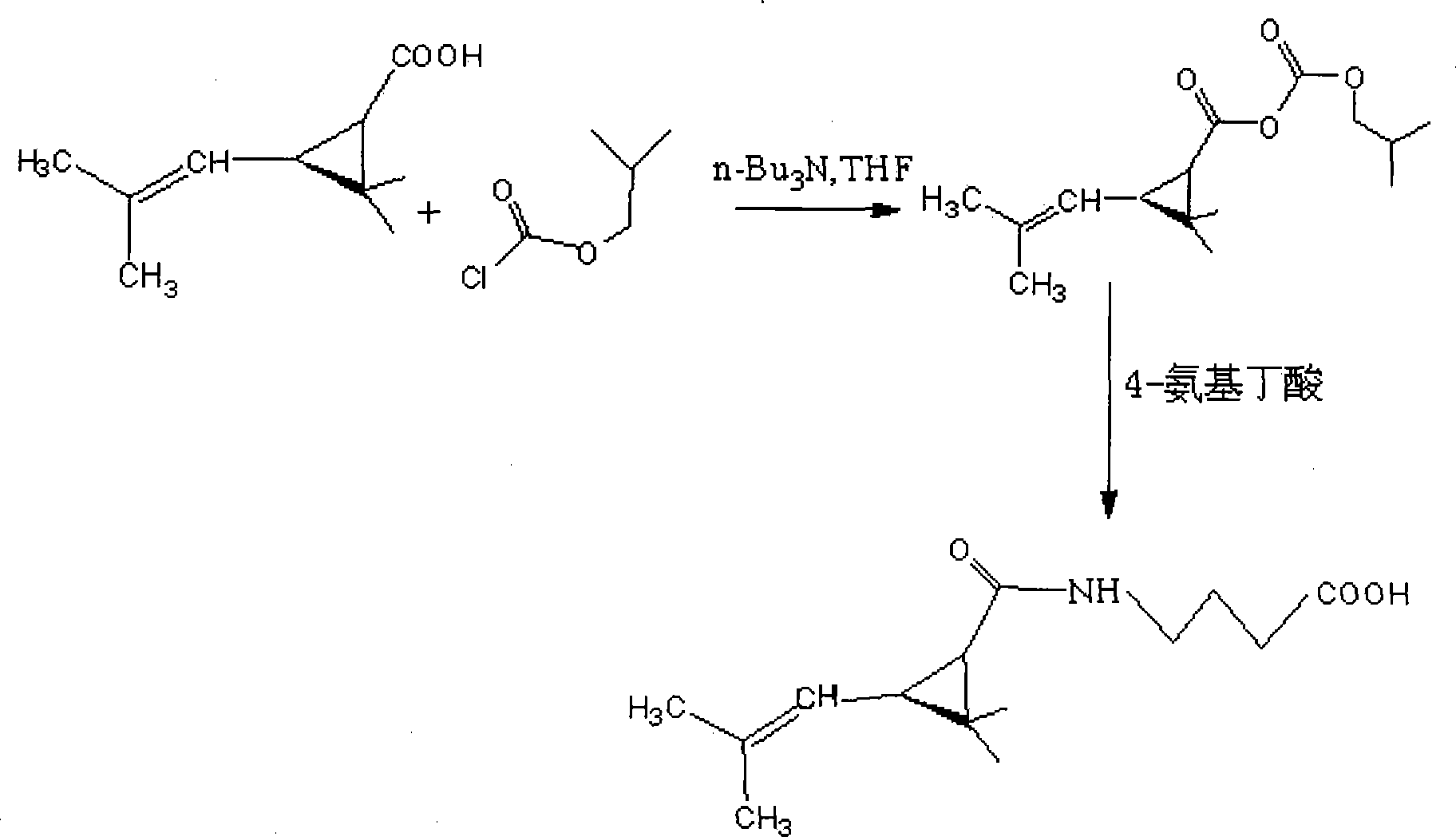
Method for synthesizing 4-reanal condensation chrysanthemic acid hapten
A technology of aminobutyric acid and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc., can solve the problems of expensive instruments, difficult to achieve on-site detection, cumbersome and other problems, and achieve simple synthesis steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] (1) Dissolve 1.68g (0.01mol) chrysanthemic acid in 3mL DMF (N,N-dimethylformamide) in a small reaction flask equipped with a stirrer, then add a small amount of n-butylamine, and stir for 30min Add 1.3658g of isobutyl chloroformate (0.01mol), lower the temperature to 0°C, and react for 1h, which is called reaction solution A; in addition, weigh 1.03g of 4-aminobutyric acid (0.01mol) and dissolve it in 5mL of carbonic acid with a pH of 9.6 In the salt buffer solution, it is called solution B; then slowly drop the reaction solution A into the carbonate buffer solution B dissolved in 4-aminobutyric acid at 0°C, and react for 1.5 hours, called the reaction solution C .
[0015] (2) Thin-layer chromatography detection end point: draw a horizontal line at 1 cm from the lower end of the silica gel sheet, draw 1 μL of the reaction solution C and point it on the line, dry it after sample application, put the thin plate into the chromatography cylinder, and the chromatography liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com