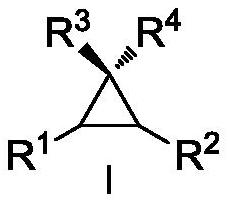
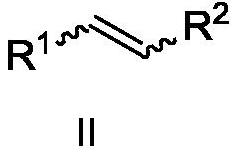
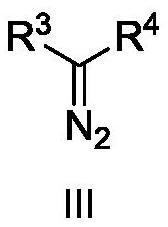
Asymmetric cyclopropanation method of copper-catalyzed olefin and application of asymmetric cyclopropanation method
A technology for cyclopropanation and catalysis of olefins, which is applied in catalytic reactions, asymmetric synthesis, organic chemical methods, etc., can solve problems such as low selectivity, narrow reaction substrate range, and difficulty in industrial application, achieving high selectivity and easy operation. Simple and cheap catalyst effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Copper-catalyzed asymmetric cyclopropanation of alkenes: under the protection of nitrogen, the metallic copper precursor CuOTf 0.5C 6 h 6 (5mol%) and chiral P,N,N-ligand L-1-1 (5.5mol%) were placed in a 25mL Scheurunk tube, added 1mL dichloroethane, and stirred at room temperature for 2h in situ coordination to obtain hand copper catalyst. Dissolve the substrate cis-β-methylstyrene II-1 in 1 mL of dichloroethane, add it to the above stirred chiral copper catalyst solution, add the newly activated Molecular sieves, after stirring at 60°C for 0.5 hours, slowly add the diazo compound III-1 with a syringe pump, the dropwise addition is completed in 8 hours, and the reaction is continued for 10 hours. After completion of the reaction, filter, concentrate under reduced pressure to almost no solvent, separate by silica gel column chromatography, concentrate under reduced pressure, and dry in vacuo to obtain the cyclopropanated product I-1 with a yield of 95%, dr>19:1, and 9...
Embodiment 2
[0036] L-1-2 reacts as a ligand to generate product I-1
[0037] The ligand L-1-1 in Example 1 is replaced by the ligand L-1-2, and the rest are the same as in Example 1. The reaction yielded compound I-1 in 87% yield, dr=16:1, 75% ee.
[0038] The structural formula of L-1-2 is as follows:
[0039]
Embodiment 3
[0041] L-1-3 reacts as a ligand to generate product I-1
[0042] The ligand L-1-1 in Example 1 is replaced by the ligand L-1-3, and the rest are the same as in Example 1. The reaction gave compound I-1 in 50% yield, dr=16:1, 70% ee.
[0043] The structural formula of L-1-3 is as follows:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com