Racemization method of trans-levochrysanthemic acid
A technology of levochrysanthemic acid and ethyl levochrysanthemum acid, which is applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems that racemization cannot be applied, and achieve high yield of racemate and smooth reaction process Mild Conditions and Versatile Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
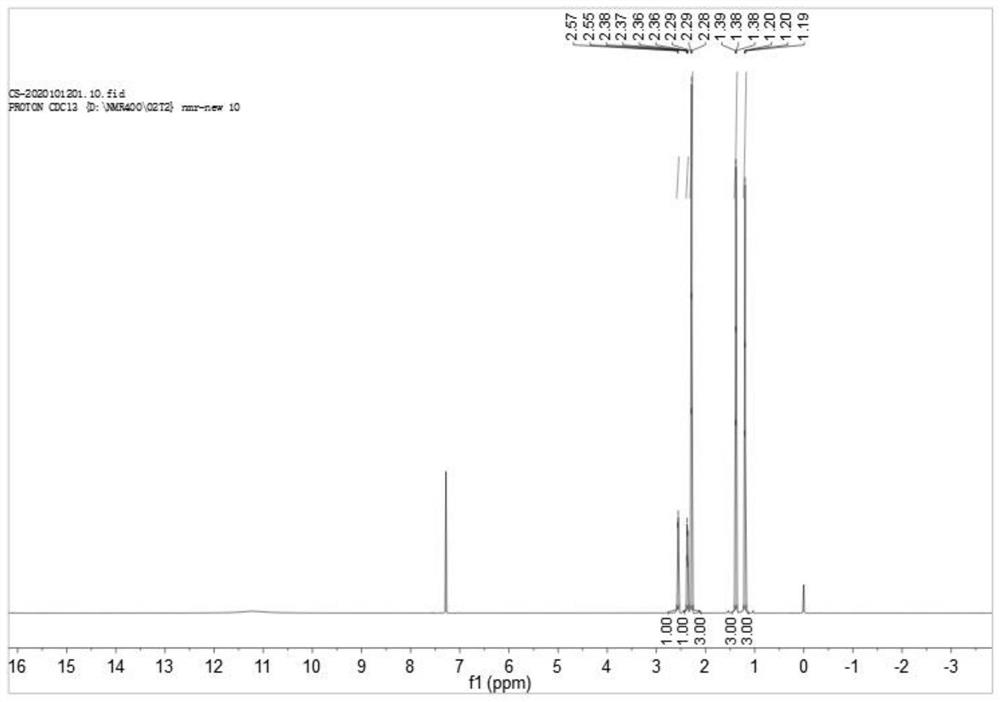
[0026] 17.0 g (0.1 mol) of trans-L-3-acetyl-2,2-dimethylcyclopropane ethyl ester prepared by esterification, 50 g of toluene, and 2.67 g (0.02 mol) of aluminum trichloride were heated under reflux. React for 5 hours, add 50 g of water, continue to reflux for 2 hours, drop to room temperature, stand for liquid separation, after the organic layer is concentrated under reduced pressure to remove the solvent, add 50 g of 2mol / L hydrochloric acid, stir at room temperature for 1 hour, and separate out trans-racemic 3-acetyl 12.5 g of base-2,2-dimethylcyclopropanecarboxylic acid, yield 80%. Toluene is added to the aqueous solution for extraction, and the organic layer is concentrated to recover trans-L-3-acetyl-2,2-dimethylcyclopropanecarboxylic acid, which can be recycled and applied mechanically. 1 H NMR (400MHz, Chloroform-d) δ 2.56 (d, J=5.8Hz, 1H), 2.37 (dd, J=5.5, 1.7Hz, 1H), 2.28 (d, J=1.4Hz, 3H), 1.38 (d, J=1.6 Hz, 3H), 1.20 (d, J=1.7 Hz, 3H). The detected product is trans-...
Embodiment 2
[0028] Heat 17.0 g (0.1 mol) of ethyl trans-L-3-acetyl-2,2-dimethylcyclopropane carboxylate prepared by esterification, 50 g of dichloroethane, and 2.73 g (0.02 mol) of zinc chloride React under reflux for 4 hours, add 50 g of water, continue to reflux for 2 hours, drop to room temperature, stand for liquid separation, after the organic layer is concentrated under reduced pressure to remove the solvent, 50 g of 2mol / L hydrochloric acid is added, and stirred at room temperature for 1 hour to precipitate trans-racemic 3 -Acetyl-2,2-dimethylcyclopropanecarboxylic acid 13.2g, yield 84.6%. Toluene is added to the aqueous solution for extraction, and the organic layer is concentrated to recover trans-L-3-acetyl-2,2-dimethylcyclopropanecarboxylic acid, which can be recycled and applied mechanically.
Embodiment 3
[0030] Heat 17.0 g (0.1 mol) of ethyl trans-L-3-acetyl-2,2-dimethylcyclopropane carboxylate prepared by esterification, 50 g of dioxane, and 5.9 g (0.02 mol) of iron bromide. React under reflux for 4 hours, add 50 g of water, continue to reflux for 2 hours, drop to room temperature, stand for liquid separation, after the organic layer is concentrated under reduced pressure to remove the solvent, 50 g of 2mol / L hydrochloric acid is added, and stirred at room temperature for 1 hour to precipitate trans-racemic 3 -Acetyl-2,2-dimethylcyclopropanecarboxylic acid 12.2g, yield 78.2%. Toluene is added to the aqueous solution for extraction, and the trans-L-3-acetyl-2,2-dimethylcyclopropane carboxylic acid is concentrated and recovered, which can be recycled and applied mechanically.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com