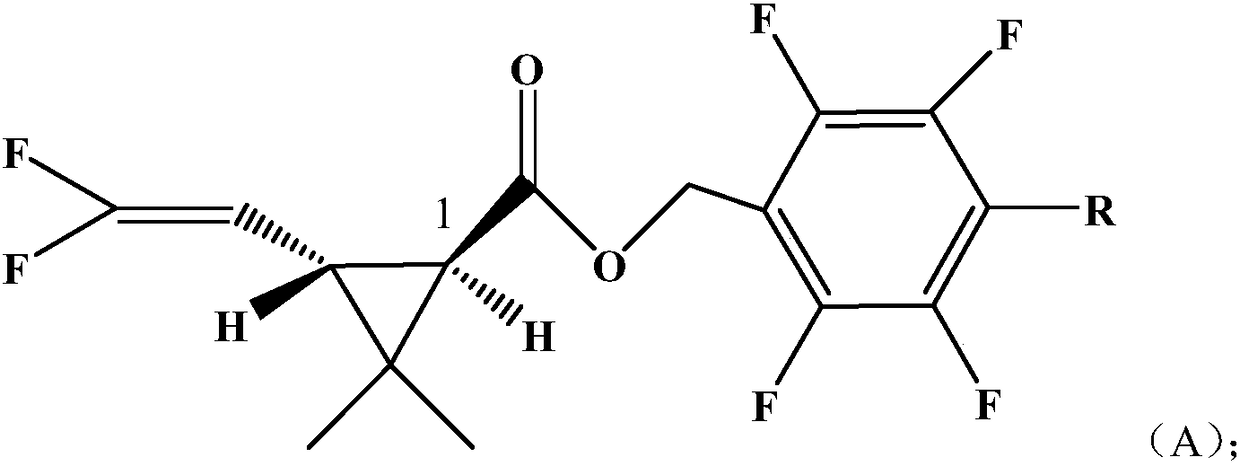
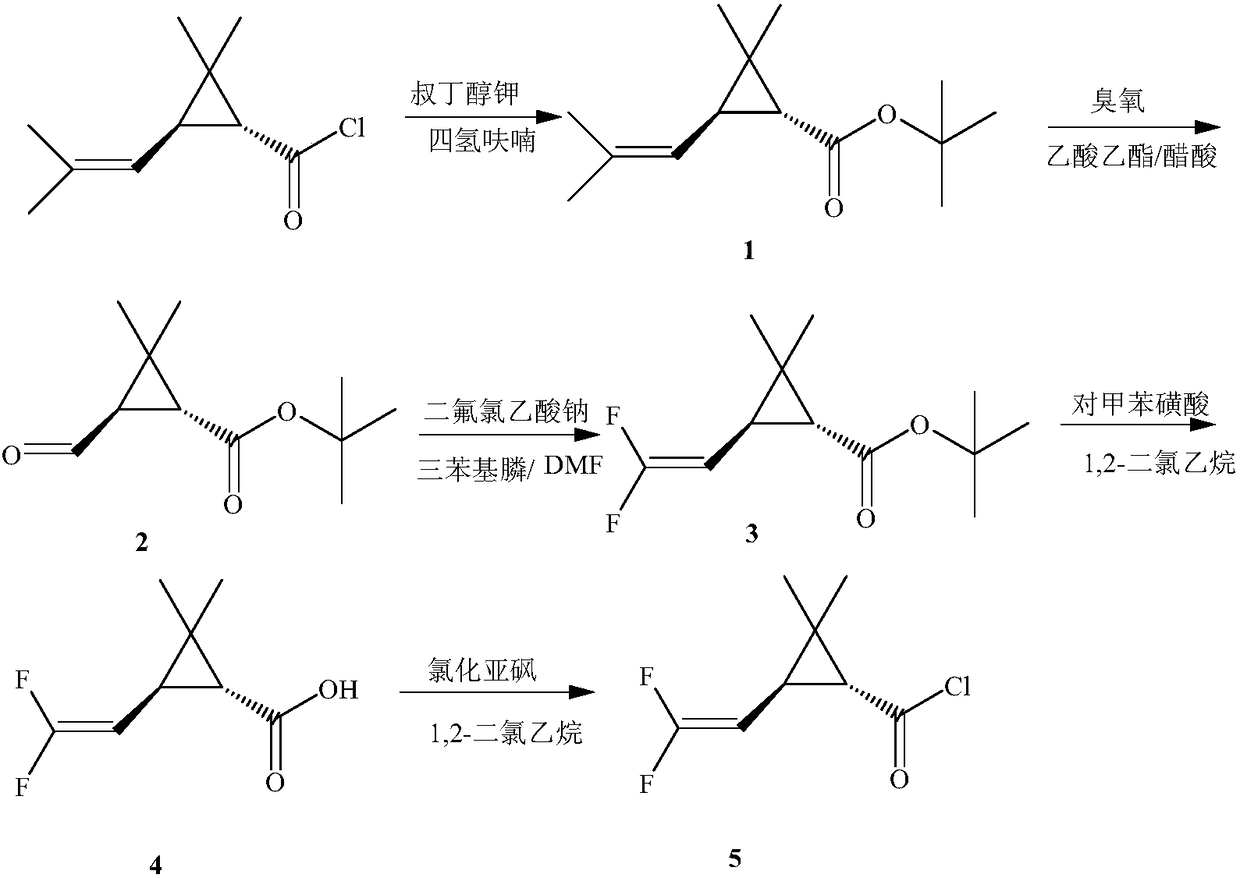
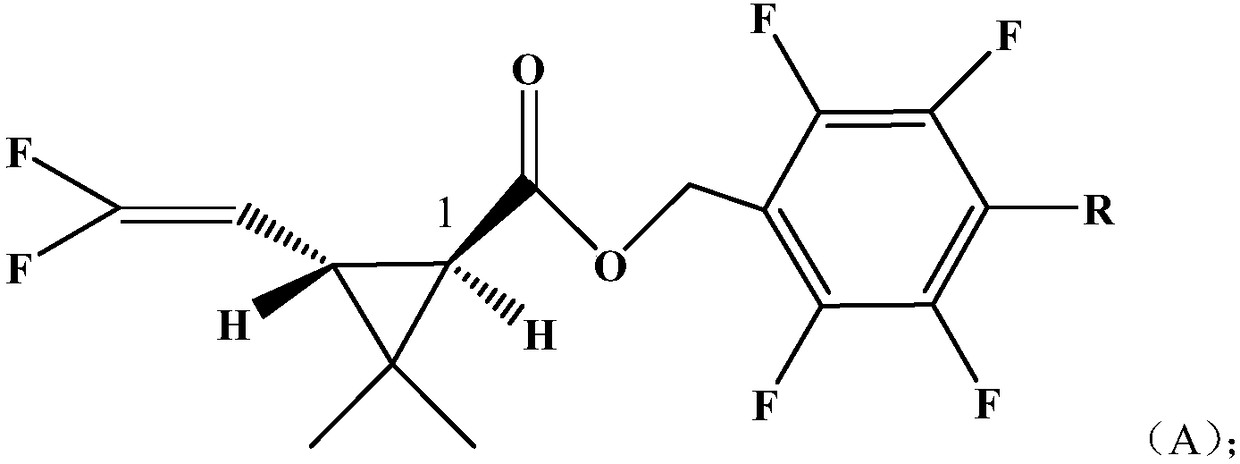
Pyrethroid compound
A technology for pyrethroids and compounds, which is applied in the field of pyrethroid compounds, and can solve problems such as the difficulty of costing new pyrethroid compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Compound A1 of the present invention: 2,3,5,6-tetrafluorobenzyl-2,2-dimethyl-1R-trans-3-(2,2-difluorovinyl)cyclopropanecarboxy Synthesis of esters
[0044] In a 200ml four-neck bottle, put 10.0g (0.05mol) of 2,3,5,6-tetrafluorobenzyl alcohol, 5.0g of pyridine, dissolve in 60ml of toluene, stir after throwing in, add dropwise at 0~5℃ 9.73 g (0.05 mol) of 2,2-dimethyl-1R-trans-3-(2,2-difluorovinyl)cyclopropanecarboxylic acid chloride was added dropwise to 20°C for 4 hours. Wash with 400ml of 5% hydrochloric acid, and then with 400ml of 5% NaHCO3, separate the oil layer and heat it to 85°C under a negative pressure of 10mmHg to remove the solvent toluene to obtain the compound 2,3,5,6-tetrafluorobenzyl-2,2 -Dimethyl-1R-trans-3-(2,2-difluorovinyl)cyclopropanecarboxylate, column chromatography treatment, weight 15.1g light brown oily liquid, content is 97.7%, yield 86%. The molecular formula of the compound: C 15 h 12 f 6 o 2Molecular weight: 338.24 Optical...
Embodiment 2
[0045] Example 2: Compound A2 of the present invention 2,3,5,6-tetrafluoro-4-methylbenzyl-2,2-dimethyl-1R-trans-3-(2,2-difluorovinyl ) Synthesis of cyclopropane carboxylate
[0046] In a 200ml four-necked bottle, put 10.7g (0.05mol) of 2,3,5,6-tetrafluoro-p-methylbenzyl alcohol, 5.0g of pyridine, dissolve in 60ml of toluene, and stir after the addition, at 0 to 5°C 9.73 g (0.05 mol) of 2,2-dimethyl-1R-trans-3-(2,2-difluorovinyl)cyclopropanecarboxylic acid chloride was added dropwise, and the temperature was raised to 18° C. to react for 4 hours. Wash with 400ml of 5% hydrochloric acid, then wash with 400ml of 5% NaHCO3, separate the oil layer and heat it to 85°C under a negative pressure of 10mmHg to remove the solvent toluene to obtain the compound 2,3,5,6-tetrafluoro-4-methylbenzyl Base-2,2-dimethyl-1R-trans-3-(2,2-difluorovinyl)cyclopropanecarboxylate, treated by column chromatography, the yield was 15.3g light brown oily liquid, and the content was 97.9%, yield 85%. Mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com