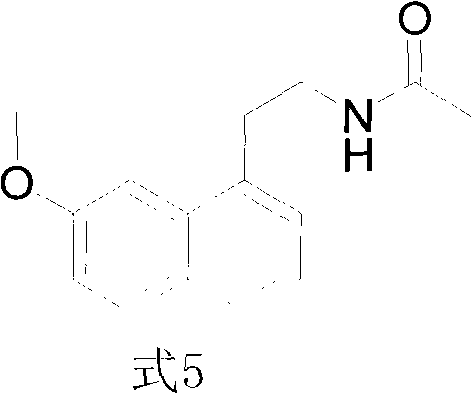
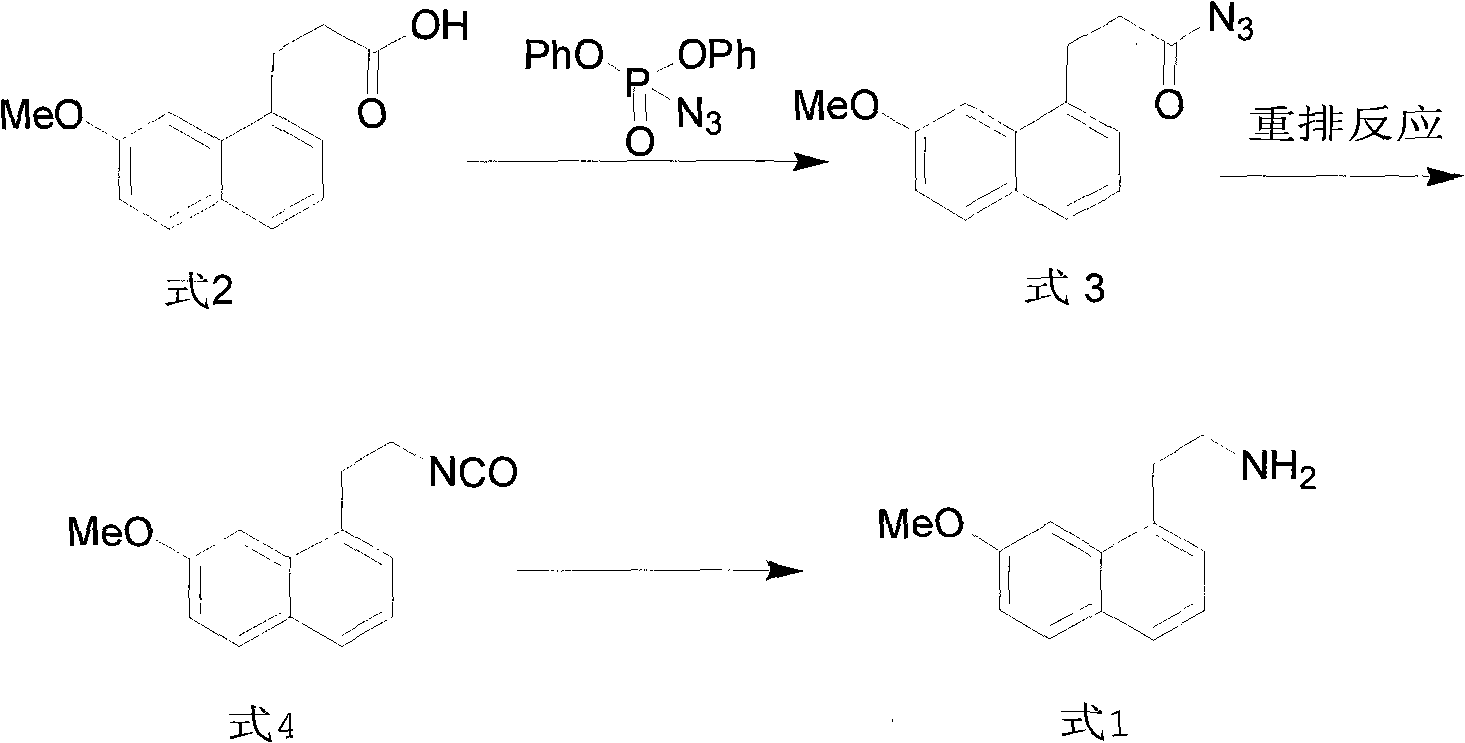
Synthesis method of valdoxan intermediate 2-(7-methoxy-1-naphthyl)ethylamine
A technology of methoxynaphthalene and compounds, which is applied in the field of preparation of agomelatine intermediates, can solve the problems of poor reproducibility, easy spontaneous combustion, and incompleteness of the reaction, and achieve short reaction time, low cost, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 2.3g of 3-(7-methoxynaphthalene-1-yl)propionic acid, 20ml of toluene, and 1.5ml of triethylamine into a 50ml reaction flask, cool down to -10°C, and then dilute 3.03g of phosphoric azide Add diphenyl ester dropwise to the cooled reaction solution, keep it warm for 6 hours, then slowly raise the temperature to 80°C and heat it for 6 hours, then cool down to below 0°C, then dropwise add 0.88g of sodium hydroxide solution Aqueous solution, stirring reaction at room temperature, adding 10ml of water to the reaction solution after completion, separating the layers, extracting the aqueous layer with toluene (10ml) for 3 times, combining the organic layers, drying over anhydrous sodium sulfate, and evaporating the solvent to obtain the target product, light yellow The oil was 1.87g, the yield was 93%, and the HPLC (normalized method) purity was 95%.
Embodiment 2
[0034] Add 2.3g 3-(7-methoxynaphthalene-1-yl)propionic acid, 20ml toluene, 1.53g diisopropylethylamine to the 50ml reaction flask, cool down to 0°C, then dilute the diluted 3.03g Add diphenylphosphoryl azide dropwise to the cooled reaction solution, keep it warm for 1.5 hours, then slowly raise the temperature to 60°C and heat it for 6 hours, then cool down to below 0°C, then dropwise add 1.12g of hydrogen into the cooling solution An aqueous solution of potassium oxide was stirred at room temperature until the reaction was completed. Add 10ml of water to the reaction solution, separate the layers, extract the aqueous layer 3 times with toluene (10ml), combine the organic layers, dry over anhydrous sodium sulfate and evaporate the solvent to obtain the target product, 1.85g of light yellow oil, yield 92 %, HPLC (normalized method) purity 94%.
Embodiment 3
[0036] Add 2.3g 3-(7-methoxynaphthalene-1-yl)propionic acid, 20m dichloromethane, 1.53g triethylamine to the 50ml reaction bottle, cool down to 30°C, then dilute 3.03g azide Add diphenyl phosphate dropwise to the cooled reaction liquid, keep it warm for 0.5 hours, then slowly raise the temperature to 60°C and heat it for 2 hours, then cool down to below 0°C, then dropwise add 0.88g of sodium hydroxide into the cooling liquid aqueous solution, and the reaction was stirred at room temperature until completion. Add 10ml of water to the reaction solution, separate the layers, extract the aqueous layer 3 times with dichloromethane (10ml), combine the organic layers, dry over anhydrous sodium sulfate, and evaporate the solvent to obtain the target product, 1.68g of light yellow oil, collected Yield 85%, HPLC (normalized method) purity 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com