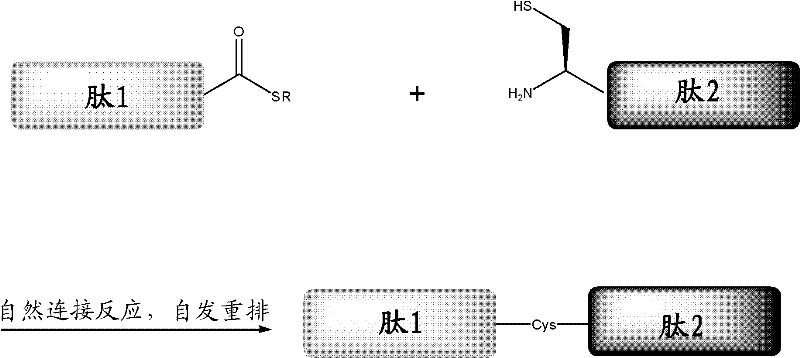
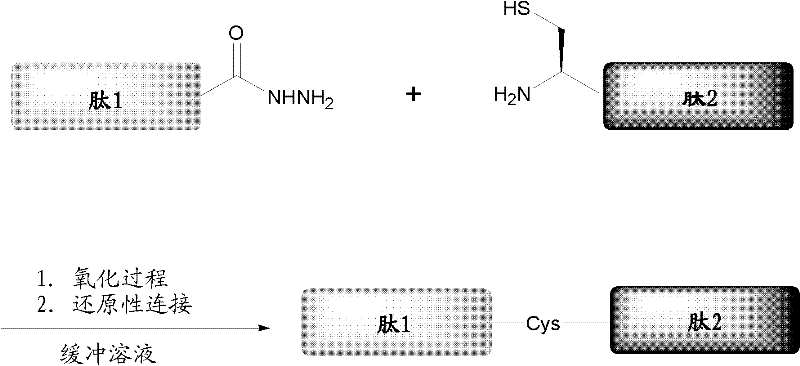
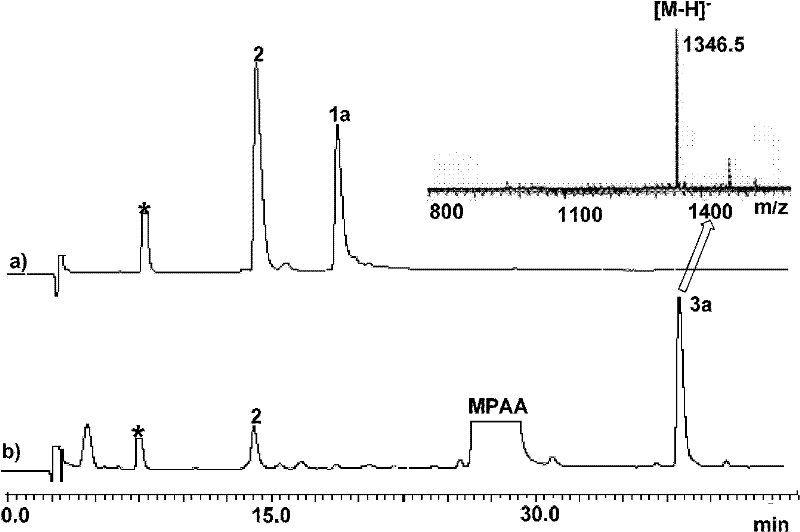
Protein preparation method
A protein and hydrazide technology, applied in the field of peptide connection, can solve the problems of inefficient synthesis, unstable HF, and high corrosiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1, synthetic hydrazide resin
[0076] 1. Swell Wang resin (1.5 g) with DCM (14 mL), put it into a round-bottomed flask, add N-methylmorpholine (0.16 mL) and p-nitrophenoxychloride in turn in an ice-salt bath with stirring (0.29 g), after the system was stirred overnight at room temperature, the resin was transferred to a sand core funnel, the resin was washed with a large amount of DCM / DMF / MeOH / DCM, and the resin was dried overnight with a vacuum dryer.
[0077] 2. Put the Wang resin of p-nitrophenoxycarbonylation (purchased from Tianjin Nankai Hecheng Technology Co., Ltd.) into a round bottom flask, add the configured precooled mixed solution (composition: hydrazine hydrate: DMF: DCM = 0.16mL: 30mL: 22mL), stirred overnight at room temperature. The resin was transferred to a sand core funnel, the resin was washed with plenty of DCM / DMF / MeOH / DCM, and the resin was dried overnight with a vacuum desiccator. That is, the hydrazide resin used for synthesizing p...
Embodiment 2
[0078] Embodiment 2, Fmoc method solid-phase synthesis polypeptide hydrazide
[0079] 1. Add the hydrazide resin to the solid phase reactor and swell it with 1:1 DMF / DCM for 3 hours, and now configure the mixed solution (3.6 equivalents of HBTU: 4 equivalents of HOBt: 8 equivalents of DIEA: 0.1 equivalents of DMAP: 4 equivalents of the protected target peptide The first amino acid at the C-terminal) was added to the resin to react for 8 hours, and the resin was washed successively with a large amount of DMF, DCM, and DMF. After the masking reagent (acetic anhydride: DIEA: DMF = 1:1:8) soaked the resin for 10 minutes, the resin was washed again with a large amount of DMF, DCM, and DMF. Add 20% piperidine in DMF to soak the resin for 5 minutes and 10 minutes. After washing with a large amount of DMF, DCM, and DMF, add the currently prepared mixed solution (3.6 equivalents of HBTU: 4 equivalents of HOBt: 8 equivalents of DIEA: 4 equivalents of the second amino acid at the C-term...
Embodiment 3
[0081] Example 3, synthesis of H-Leu-Tyr-Arg-Ala-Tyr-Cys-Lys-Tyr-Met-His-OH by polypeptide hydrazide method
[0082] 1. Weigh the polypeptide hydrazide H-Leu-Tyr-Arg-Ala-Tyr-NHNH 2 (1.5mg), H-Cys-Lys-Tyr-Met-His-OH (1.95mg) dissolved in 0.65mL PBS (6.0M guanidine hydrochloride, 0.2M phosphate, pH 3.0), plus 50uL benzamide solution (40mM) .
[0083] 2. Take 0.25mL of the above solution and put it into a 1.5mL glass bottle, add a magnet, and add 25uL NaNO dropwise under stirring in an ice-salt bath (about -10°C) 2 (0.2M) aqueous solution, react at low temperature for about 20 minutes.
[0084] 3. After oxidation, add 0.25mL MPAA guanidine hydrochloride solution (0.2MMPAA, 6.0M guanidine hydrochloride, 0.2M phosphate, pH 5.0) dropwise, remove the ice-salt bath, take NaOH (2.0M) solution to carefully adjust the acidity of the reaction solution to neutral (acidity measured with a micro glass pH meter).
[0085] 4. After 2 hours of reaction, take 20uL of the reaction solution an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com