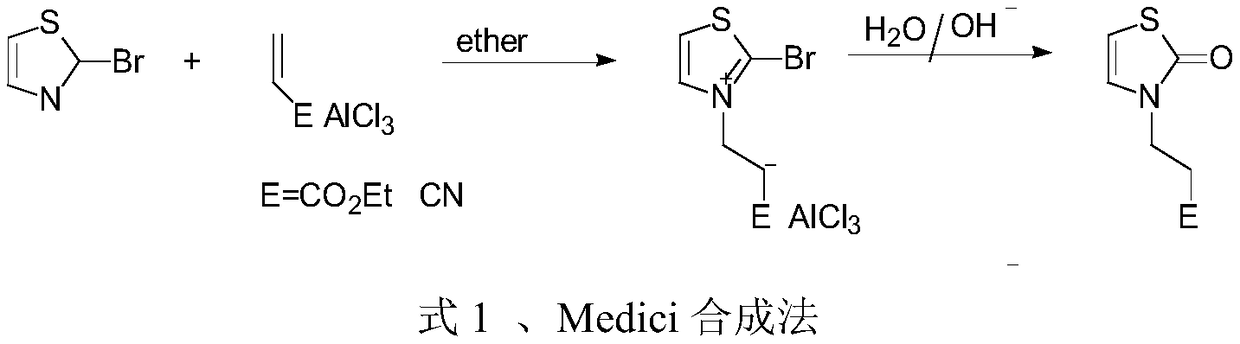
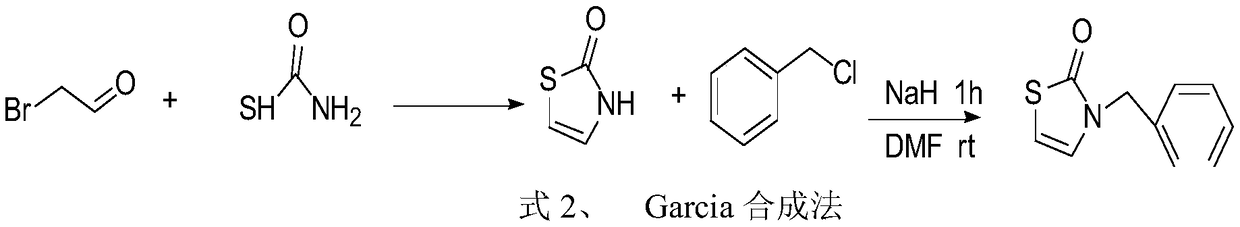
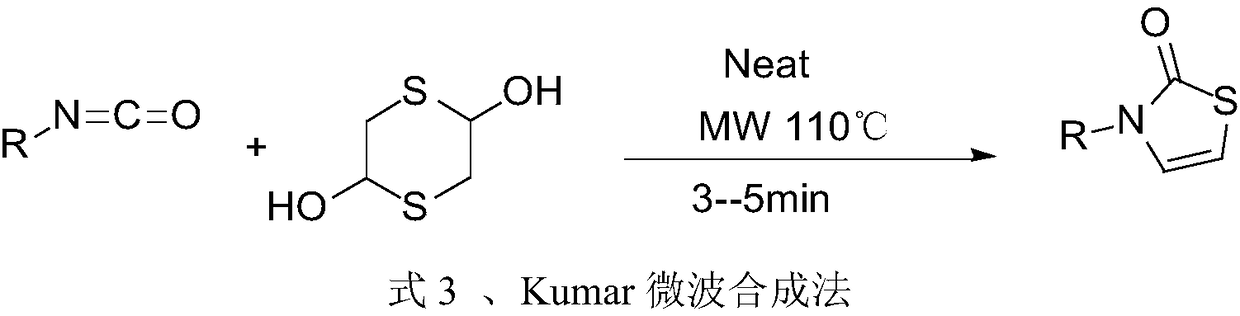
Preparation method of 3-substituted-thiazol-2(3H)-one compound
A technology of ketone compounds and compounds, which is applied in the field of preparation of 3-substituted-thiazol-2-one compounds, can solve the problems of poor applicability of different functional groups, use of precious metal reagents, and difficult acquisition of raw materials, etc. Easy handling and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0039] Example 1-1, 3-phenyl-thiazol-2(3H)-one (m1)
[0040] 1) Add 220.5 mg (1.5 mmol) of benzoyl azide and 125.4 mg (0.825 mmol) of 2,5-dihydroxy-1,4-dithiane into the tube (sealed tube), and then add 7.5 ml of solvent- -Acetonitrile, after the addition, stirred and reacted at 80°C for 21h, and detected the reaction by TLC (petroleum ether:ethyl acetate=5:1 volume ratio), at this time, the result of the TLC detection reaction was that the benzoyl azide disappeared.
[0041] 2), add mass concentration 40% sulfuric acid aqueous solution (the molar weight of sulfuric acid is 0.375mmol) to the resultant of step 1), stir at room temperature for one hour, TLC monitoring (petroleum ether: ethyl acetate=3:1 volume ratio) step 1) The obtained 4-hydroxy-3-phenylthiazolidin-2-one disappeared, indicating that the reaction had ended.
[0042] That is, sulfuric acid: benzoyl azide = 25 mol%.
[0043] After the reaction was finished, the solid was removed by filtration, the filtrate was ...
Embodiment 1-2
[0050] Embodiment 1-2, replace acetonitrile with dioxane, the volume is constant, all the other are the same as embodiment 1-1. 218 mg of 3-phenylthiazol-2(3H)-one was obtained as light yellow oily product, and the yield was 80%.
Embodiment 2
[0062] Example 2, 3-(4-fluoro-phenyl)thiazol-2(3H)-one (m2)
[0063] Use p-fluorobenzoyl azide instead of benzoyl azide, the molar weight remains unchanged, and the rest is the same as in Example 1-1. 219.3 mg of the product 3-(4-fluoro-phenyl)thiazol-2(3H)-one was obtained as a white powder, with a yield of 75%.
[0064] Its structural formula is:
[0065] White powder 1 H NMR (500MHz, DMSO-d 6 )δ7.57(dd, J=8.5,5Hz,2H),7.35(t,J=9.0Hz,2H),7.30(d,J=5.5Hz,1H),6.65(d,J=5.5Hz,1H ). 13 C NMR (125MHz, DMSO-d 6 ) 170.26, 161.85, 159.9 132.98 (d, 3 J C,F =10Hz), 126.80(d, 2 J C,F =35Hz), 116.13(d, 1 J C,F =95Hz), 102.03HRMS(ESI):m / z calcd for C 9 h 6 FNOS[M+H]+:196.0232,found:196.0229.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com