Synthesis method of N-substituted hydantoin compound
A technology of hydantoin and synthesis method, which is applied in the field of synthesis of N-substituted hydantoin compounds, can solve the problems of unfriendly environment, and achieve the effect of lowering blood sugar and inhibiting uremic toxins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Add 0.6 mmol benzoyl azide compound, 0.5 mmol phenylglycine ethyl ester, and 0.75 mmol potassium carbonate to a 25 ml screw-top test tube successively, stir and react at 100°C for 8 hours, and cool to room temperature after the reaction , the crude product can be obtained, and the crude product is purified by column chromatography to obtain the product 3aa with a yield of 92% and a purity of 99%.
[0065] The synthetic route is:
[0066]
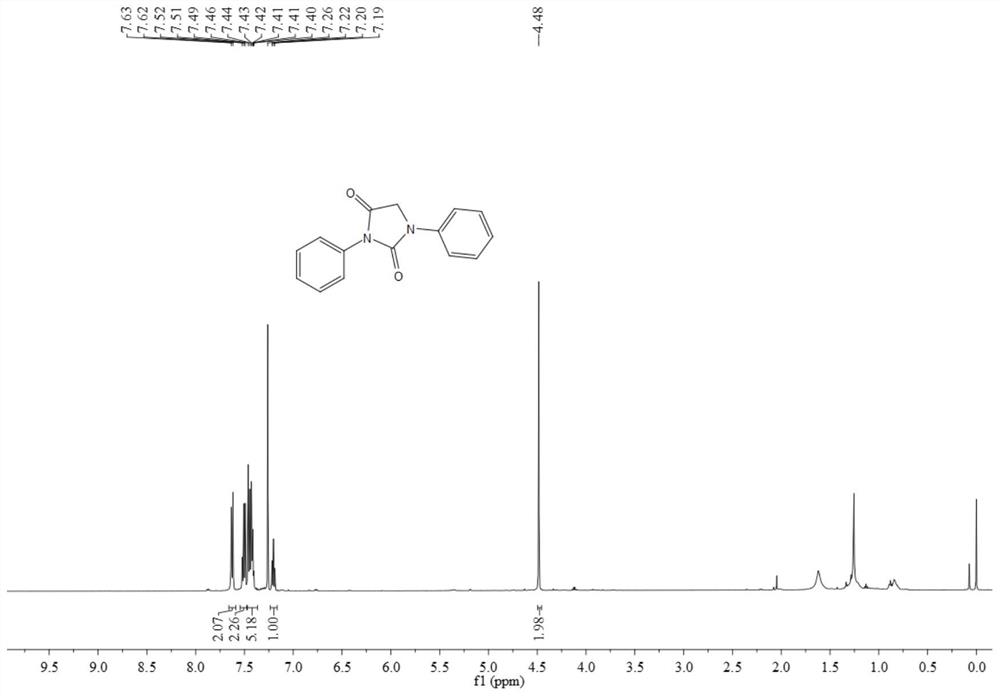
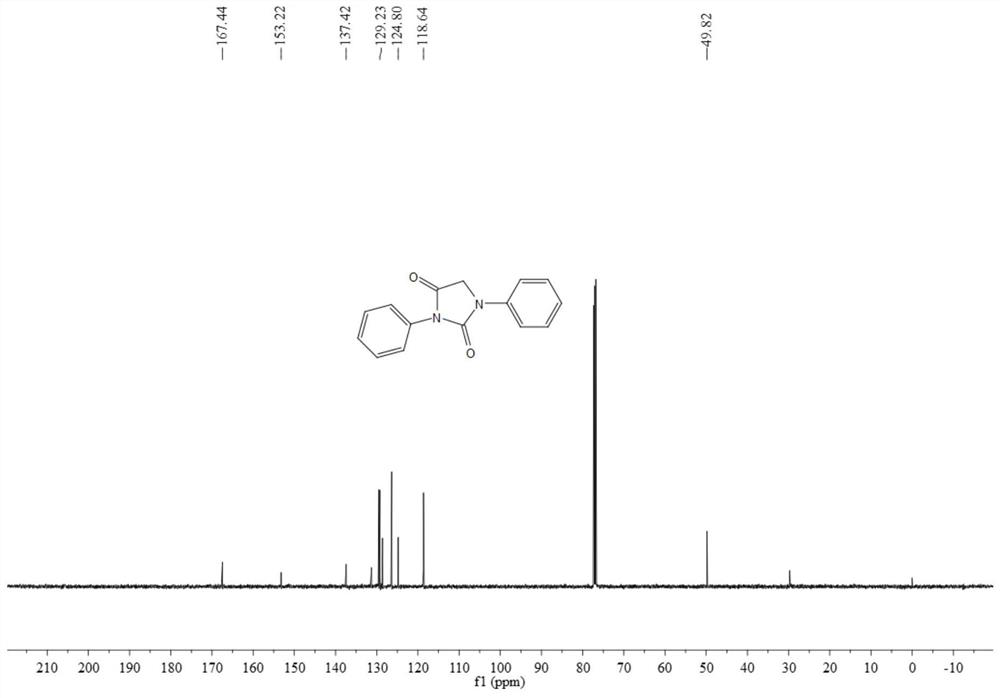
[0067] The resulting product 3aa is a yellow solid, and its hydrogen spectrum and carbon spectrum are as follows figure 1 and figure 2 As shown, the structural characterization data are as follows:
[0068] 1 H NMR (500MHz, CDCl 3 )δ7.63(d, J=7.9Hz, 2H), 7.52-7.49(m, 2H), 7.46-7.40(m, 5H), 7.20(t, J=7.4Hz, 1H), 7.20(t, J= 7.4Hz, 1H), 4.48(s, 2H); 13 C NMR (126MHz, CDCl 3 ) δ 187.0, 161.9, 158.7, 156.3, 146.5 132.2, 131.8, 130.7, 129.4, 125.2, 120.8, 49.8.
Embodiment 2
[0070] Add 0.6 mmol of 4-tert-butylbenzoyl azide, 0.5 mmol of ethyl phenylglycine, and 0.75 mmol of potassium carbonate to a 25 ml screw-top test tube in turn, stir and react at 100°C for 8 hours, and the reaction ends After cooling to room temperature, the crude product was obtained, which was purified by column chromatography to obtain the product 3ab with a yield of 75% and a purity of 99%.
[0071] The synthetic route is:
[0072]
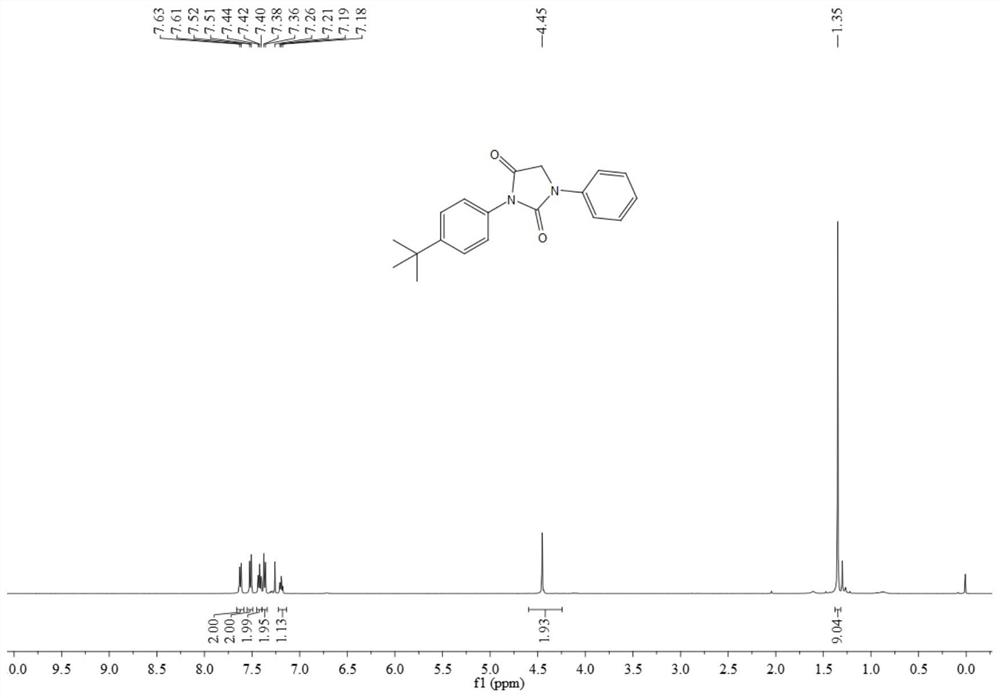
[0073] The resulting product 3ab is a light yellow solid, and its hydrogen spectrum and carbon spectrum are as follows image 3 and Figure 4 As shown, the structural characterization data are as follows:
[0074] 1 H NMR (500MHz, CDCl 3 )δ7.62(d, J=7.9Hz, 2H), 7.52(d, J=8.6Hz, 2H), 7.42(t, J=8.0Hz, 2H), 7.37(d, J=8.6Hz, 2H) ,7.19(t,J=7.4Hz,1H),4.45(s,2H),1.35(s,9H); 13 CNMR (126MHz, CDCl 3 ) δ 167.6, 153.4, 151.7, 137.5, 129.4, 128.5, 126.3, 125.9, 124.7, 118.6, 49.8, 34.8, 31.3.
Embodiment 3
[0076] Add 0.6 mmol of 4-methoxybenzoyl azide, 0.5 mmol of ethyl phenylglycine, and 0.75 mmol of potassium carbonate to a 25 ml screw-top test tube in turn, stir and react at 100°C for 8 hours, and the reaction ends After cooling to room temperature, the crude product can be obtained, and the crude product is purified by column chromatography to obtain the product 3ac with a yield of 80% and a purity of 99%.
[0077] The synthetic route is:
[0078]
[0079] The resulting product 3ac is a light yellow solid, and its hydrogen spectrum and carbon spectrum are as follows Figure 5 and Figure 6 As shown, the structural characterization data are as follows:
[0080] 1 H NMR (500MHz, CDCl 3 )δ7.66-7.58(m, 2H), 7.41(t, J=8.0Hz, 2H), 7.34(d, J=9.6Hz, 2H), 7.19(t, J=7.4Hz, 1H), 7.00( d, J=2.7Hz, 2H), 4.43(s, 2H), 3.98(s, 3H); 13 CNMR (126MHz, CDCl 3 ) δ 167.7, 159.5, 153.5, 137.5, 129.4, 127.7, 124.8, 123.9, 118.6, 114.6, 55.6, 49.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com