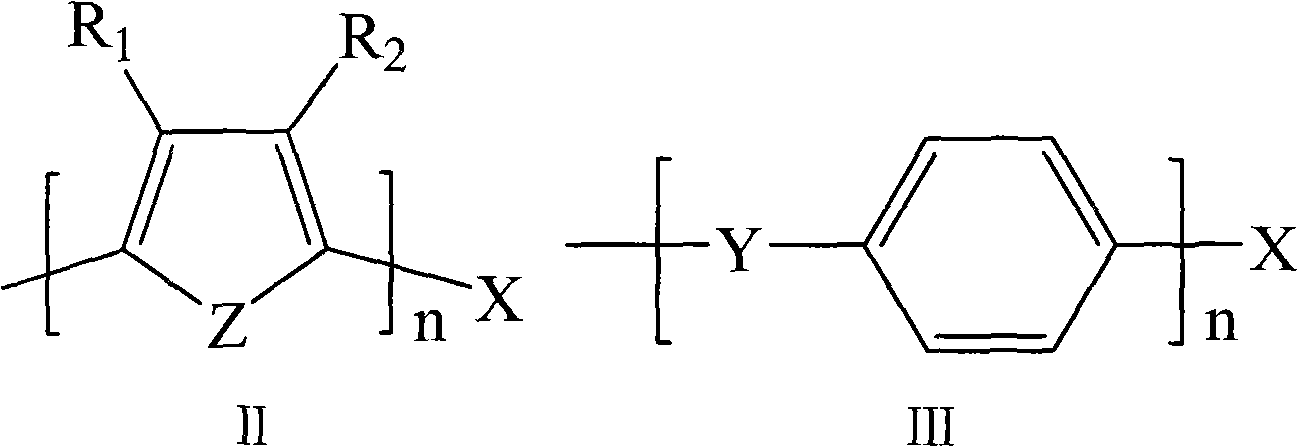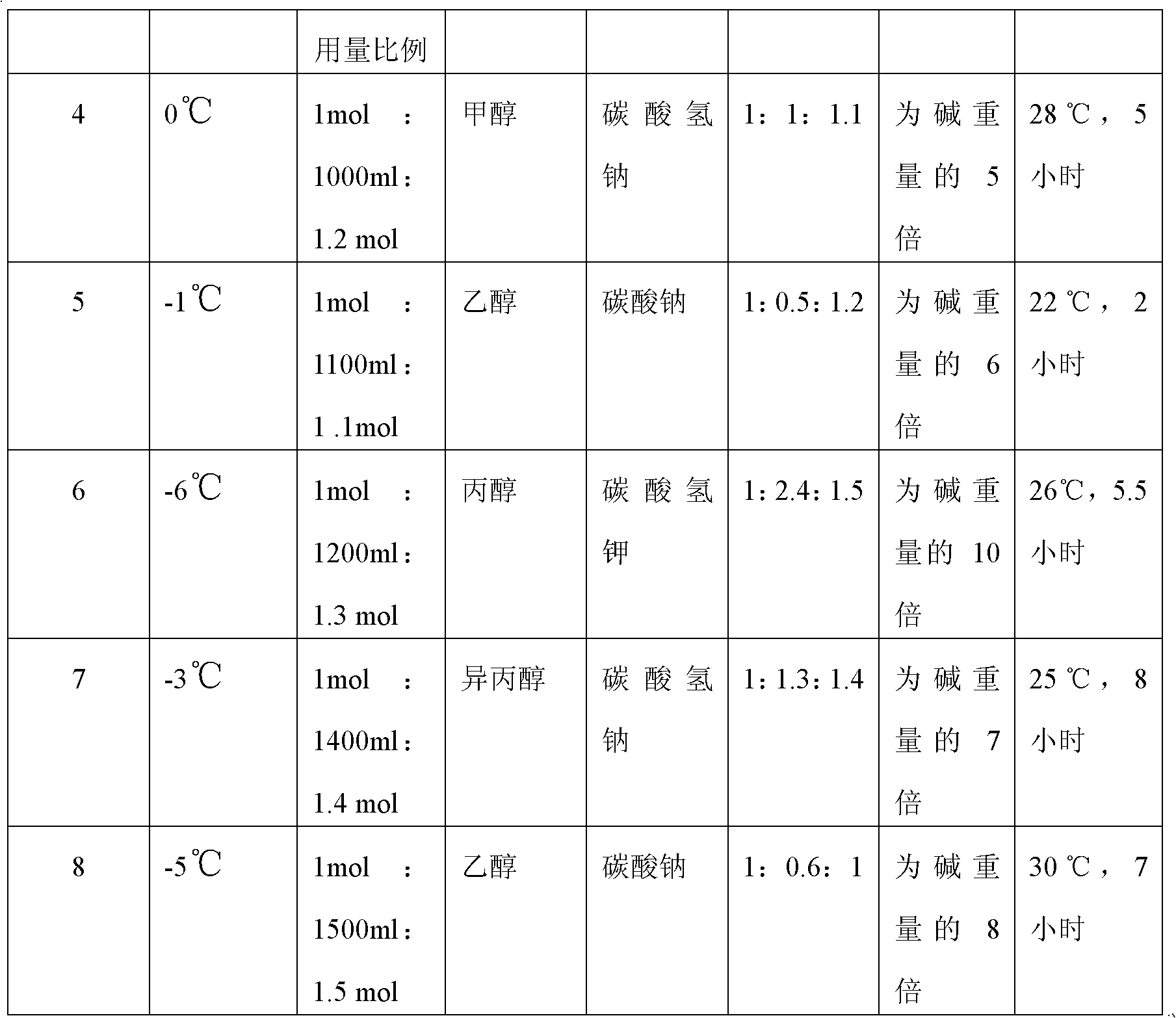Patents
Literature
107 results about "Glycine ethyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
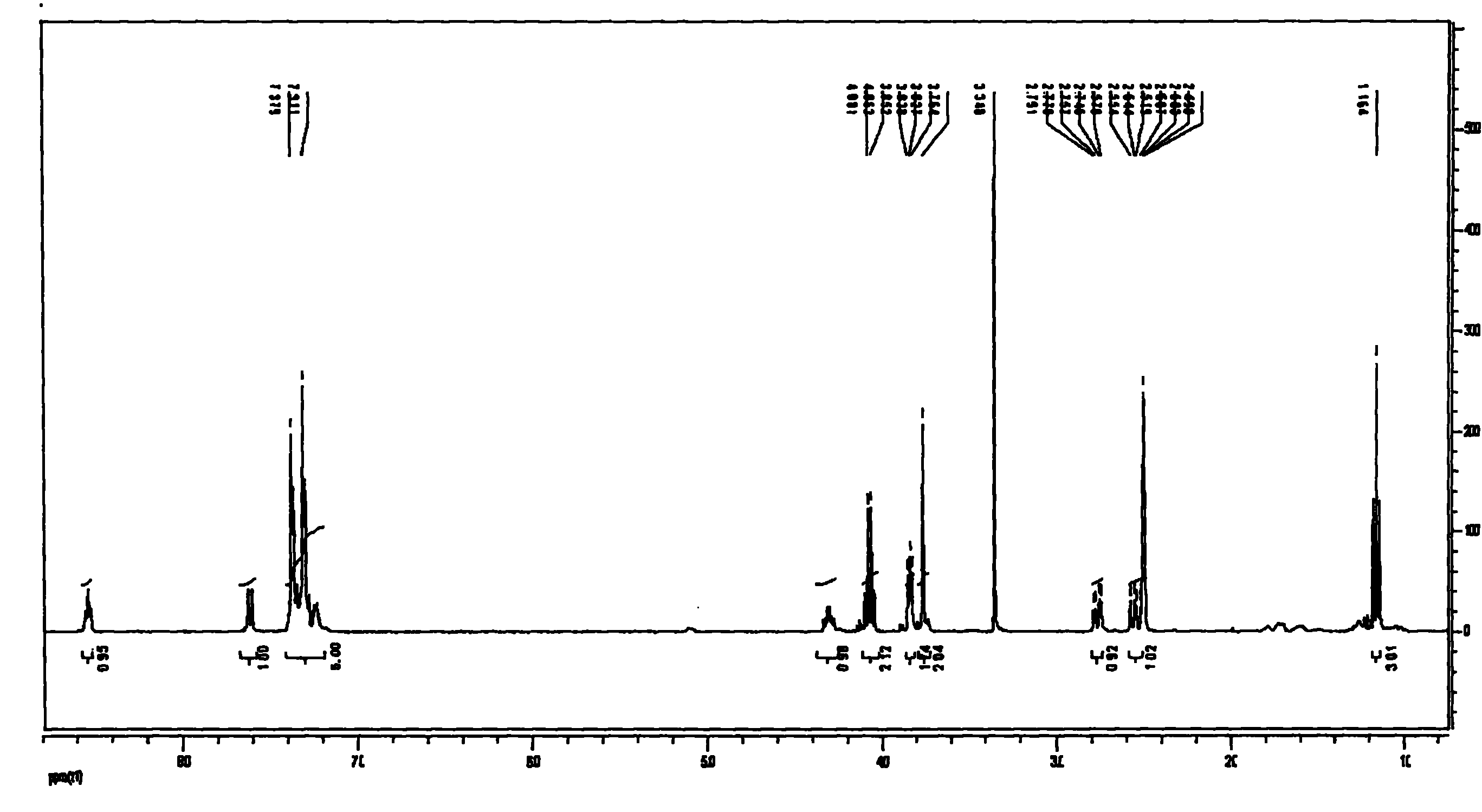
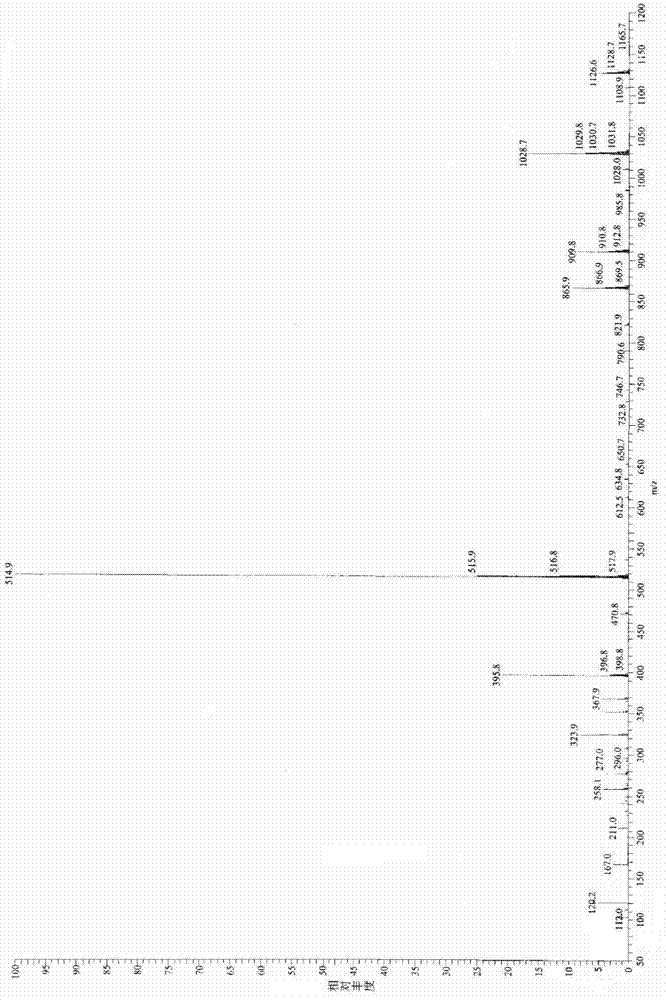
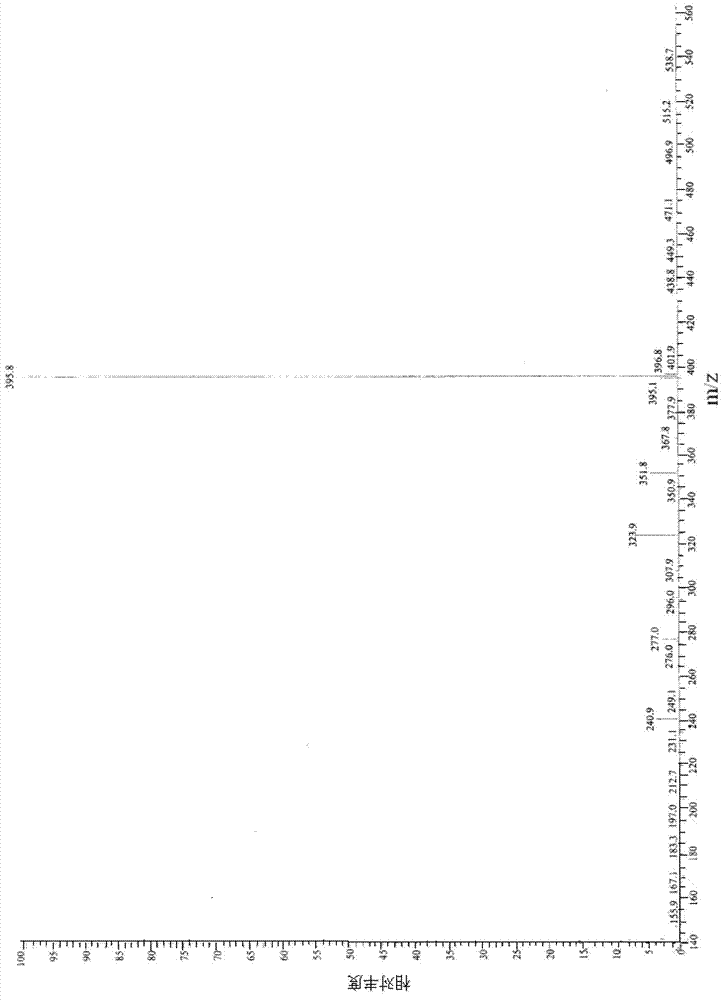
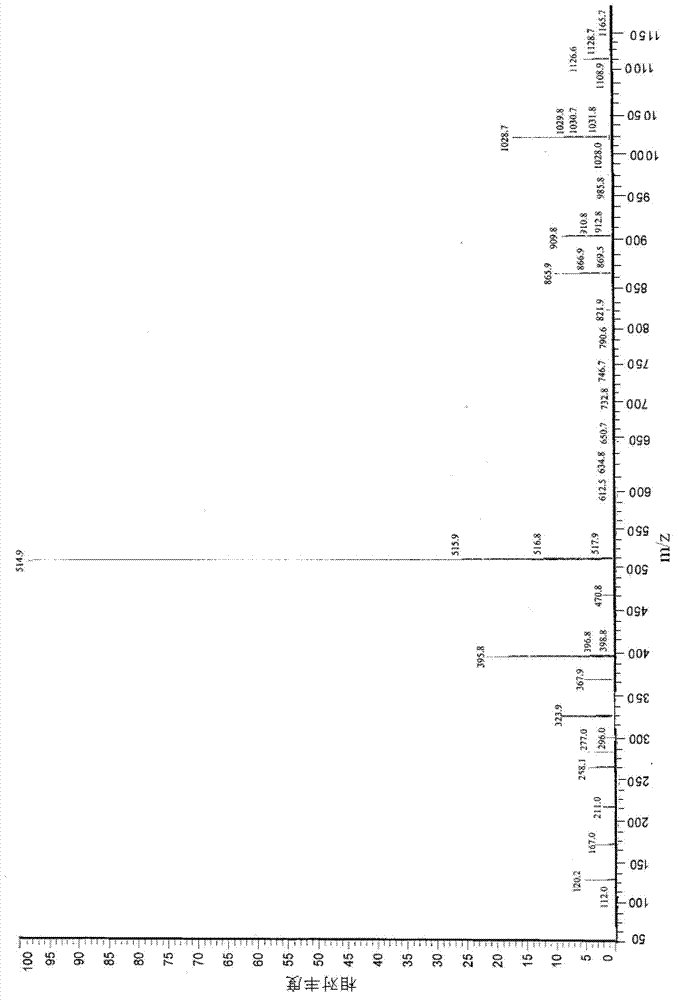
Application Glycine ethyl ester (GEE) is used in conjunction with N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) for carboxyl-footprinting studies of proteins.The GEE/EDC protocol effects specific derivatization of glutamate and aspartate carboxyl side chains on intact proteins.
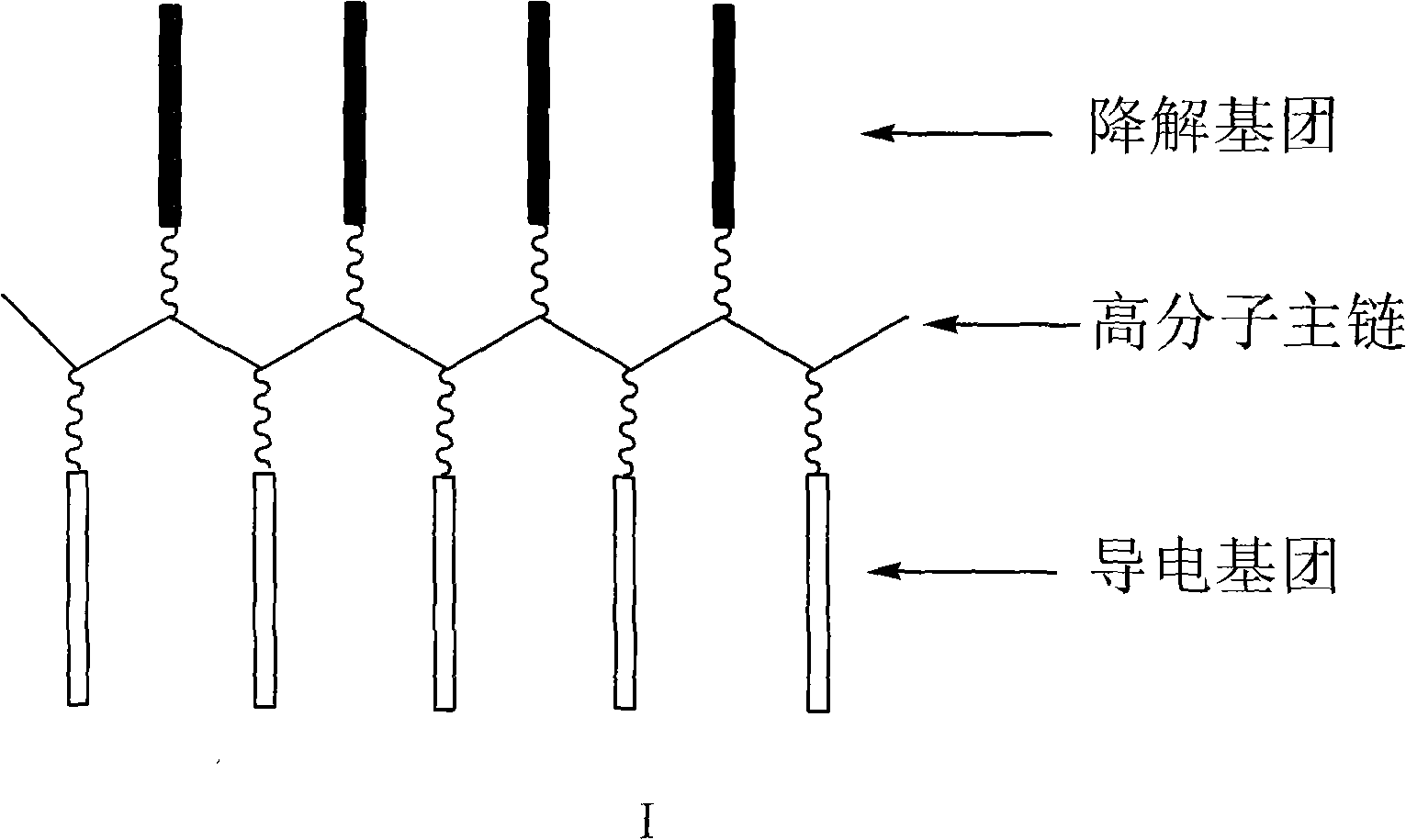
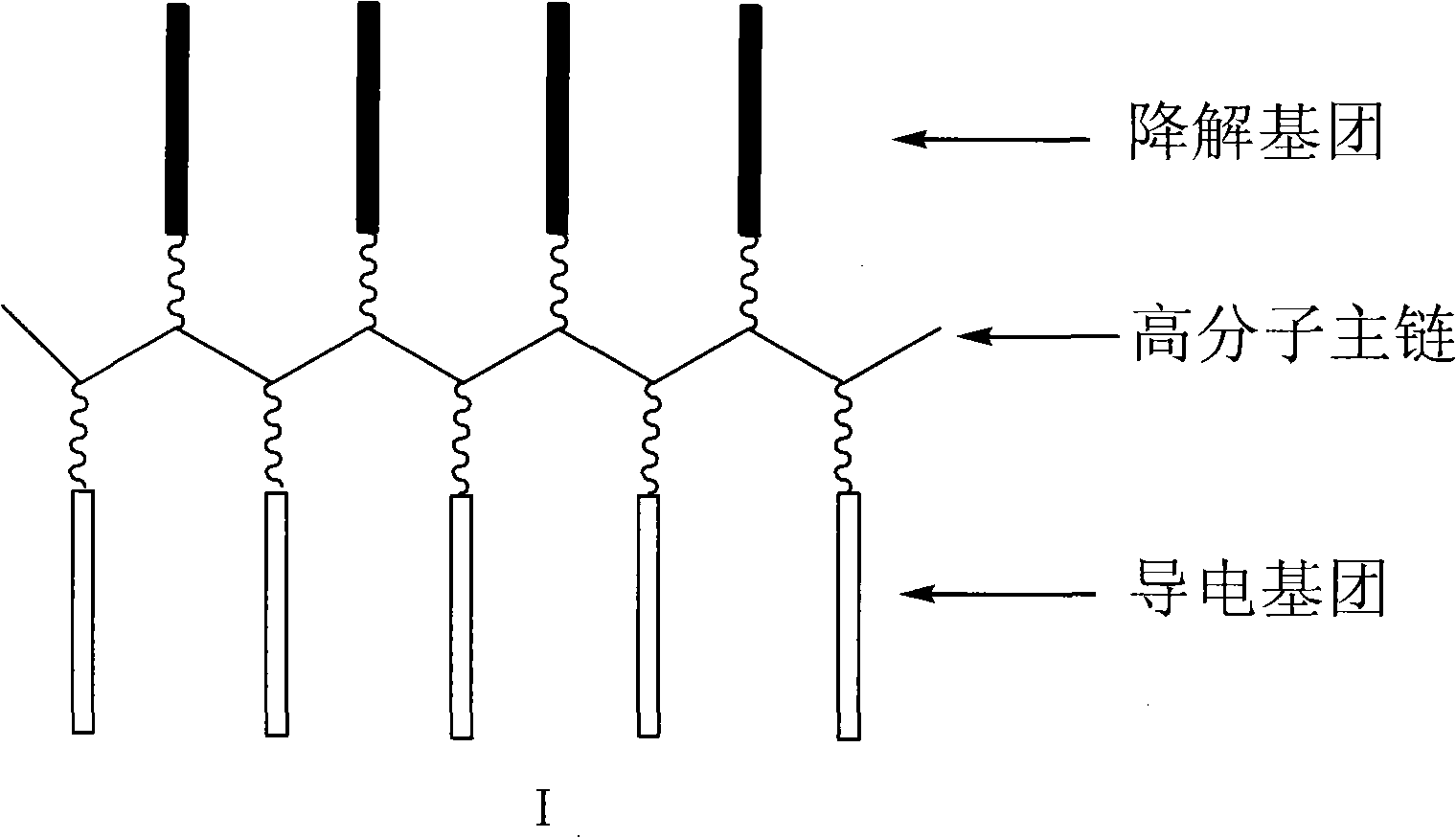
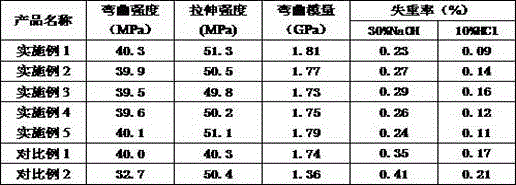
Degradable conductive biological medical polymer material
The invention discloses a degradable electricity-conducting biomedical polymer material, which has a molecular constitution as shown in formula I: the polymer material takes the polymer in the formula, which comprises polyphosphazene, chitosan, polyvinyl alcohol, poly-beta-malic acid or modified polyester, as the main chain, a conductive group and a degradable group are grafted, and the modified polyester is lactic acid, polyglycolide, polycarbonate, polyanhydride, polycaprolactone or copolymers between each two thereof; the conductive group comprises oligo-pyrrole, oligo-thiophene, oligo-aniline or copolymers between each two thereof; the degradable group mainly comprises: (1) amino: glycine ethyl ester, phenylalanine ethyl ester, imidazole and the like; and (2) alkoxy: glycerol, glucose, lactic acid, p-methyl phenol, glycolic acid and polyester. The polymer material has degradability and conductivity and can be prepared into guide pipes, suture threads, membranes, sheet bodies, block bodies and materials for frames of tissue engineering.
Owner:WUHAN UNIV OF TECH
Improved method for preparing glycine ethyl ester hydrochloride
ActiveCN108484421AReduce water contentEasy to handleOrganic compound preparationAmino-carboxyl compound preparationGlycineAlcohol
The invention discloses an improved method for preparing glycine ethyl ester hydrochloride. The method includes the steps of putting glycine, ethyl alcohol and hydrogen chloride according to a certainratio for salinization and esterification, adding dehydrant for evaporating water, conducting cooling, crystallizing, centrifugal separating and drying to obtain a product, adding a certain volume ofwater to a three-element system composed of the evaporated dehydrant, ethyl alcohol and water for standing and phase separating, directly recycling a phase layer of the dehydrant, conducting phase separating to obtain a water phase for recovering ethyl alcohol for use, and recycling a centrifugal mother solution for material putting. After salinization and esterification of glycine, the content of water in esterification liquid is reduced, glycine hydrochloride can continue to be esterified into glycine ethyl hydrochloride in the rectification process, the generated water is taken away at thesame time, and the product conversion rate is increased; the mother solution obtained after crystallization and centrifugation is recycled without distillation, neutralization and rectification treatment of each batch, the treatment amount of leftovers and waste salt is reduced, and energy consumption is also reduced. Meanwhile, the content of glycine hydrochloride is reduced, so the product quality is improved.
Owner:江苏优普生物化学科技股份有限公司
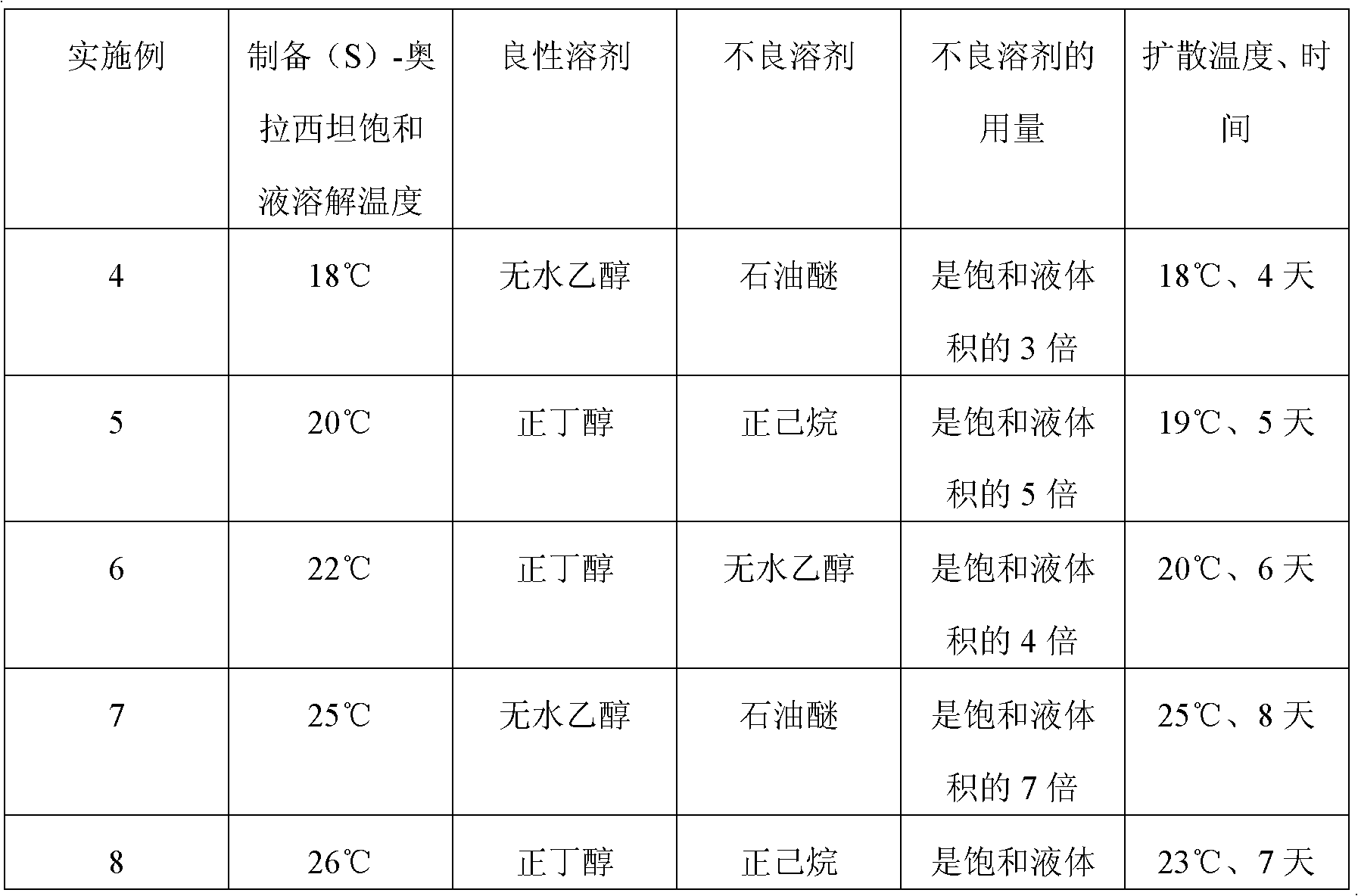
Preparation method of (S)-oxiracetam
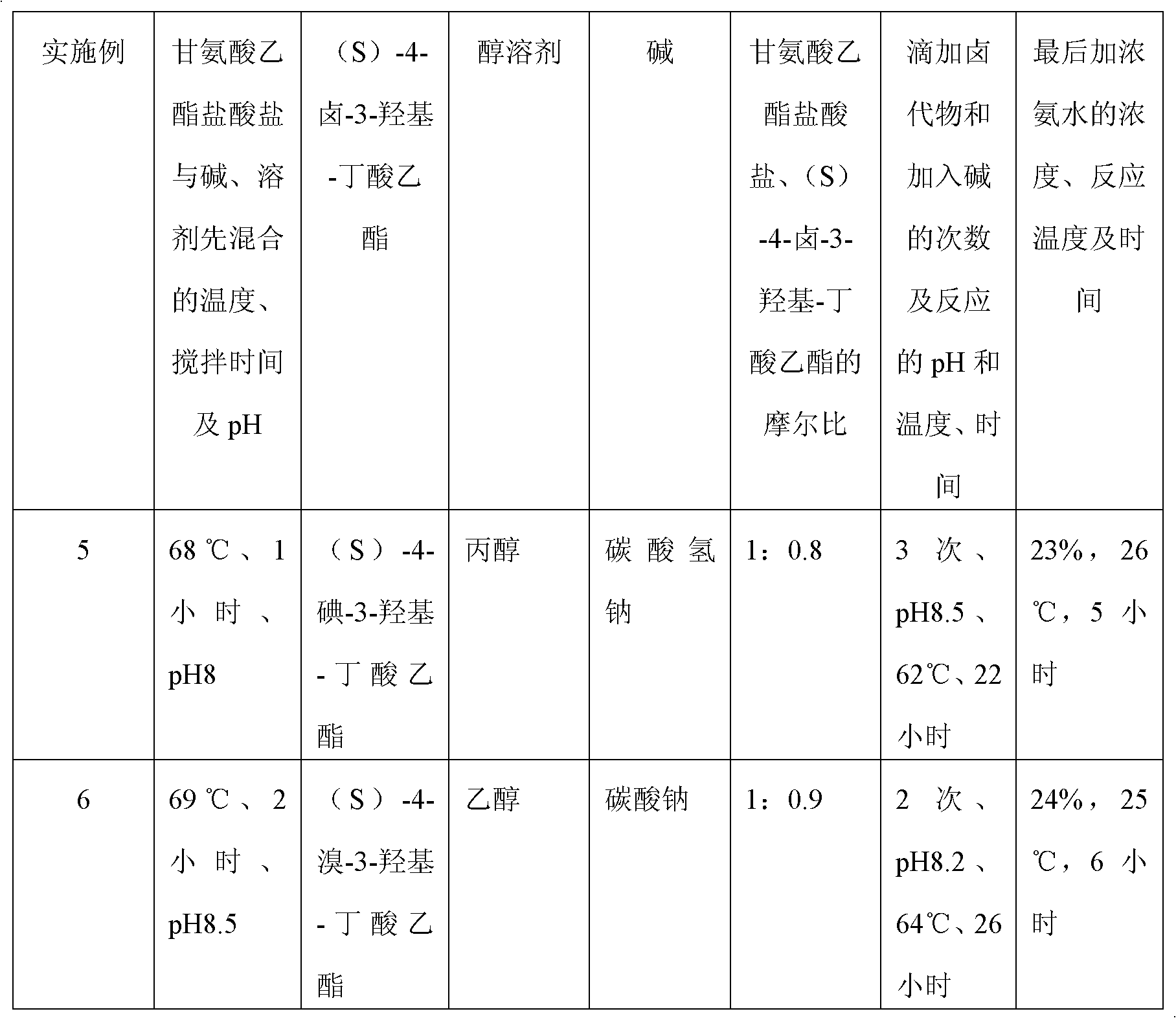
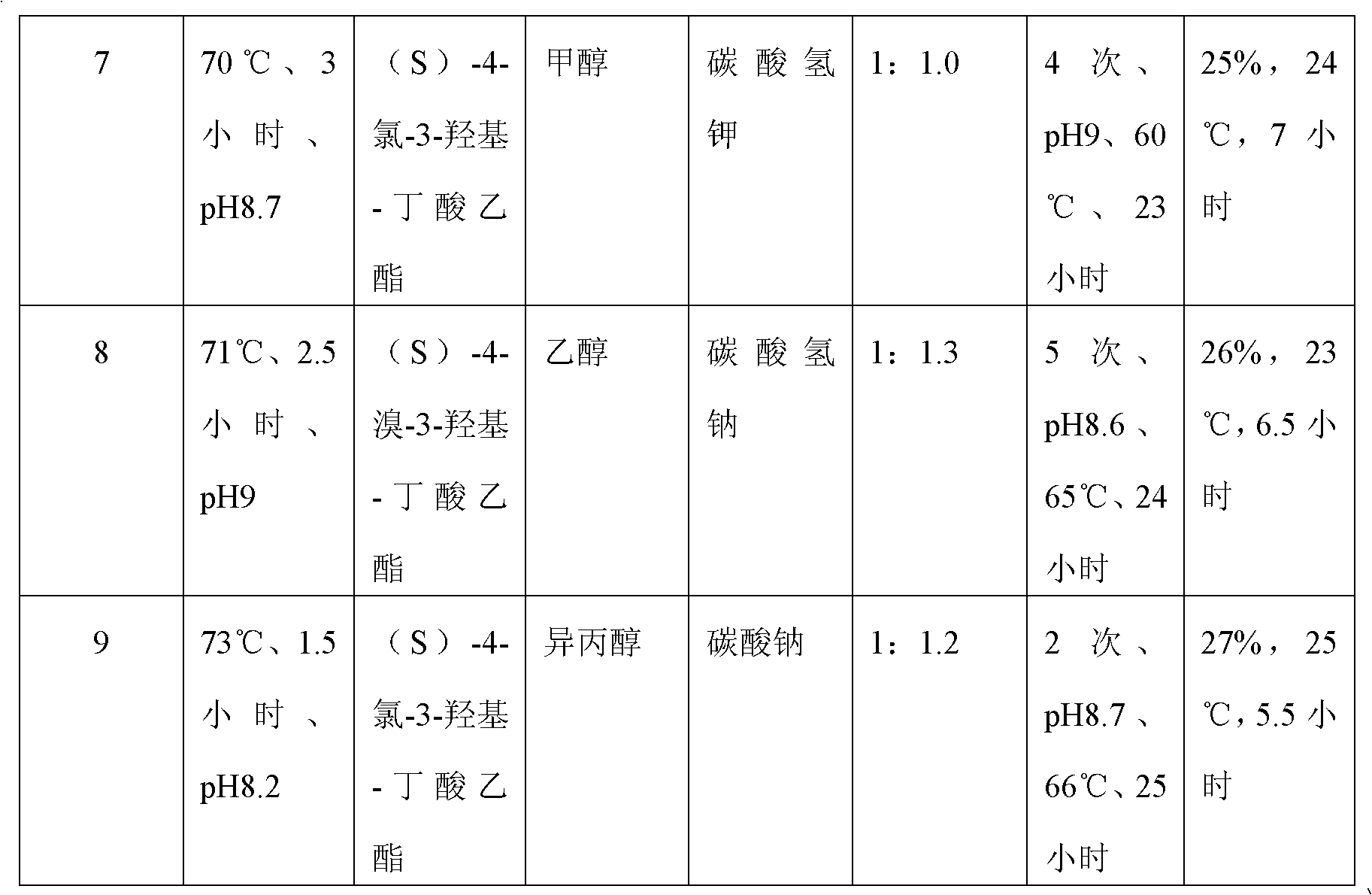
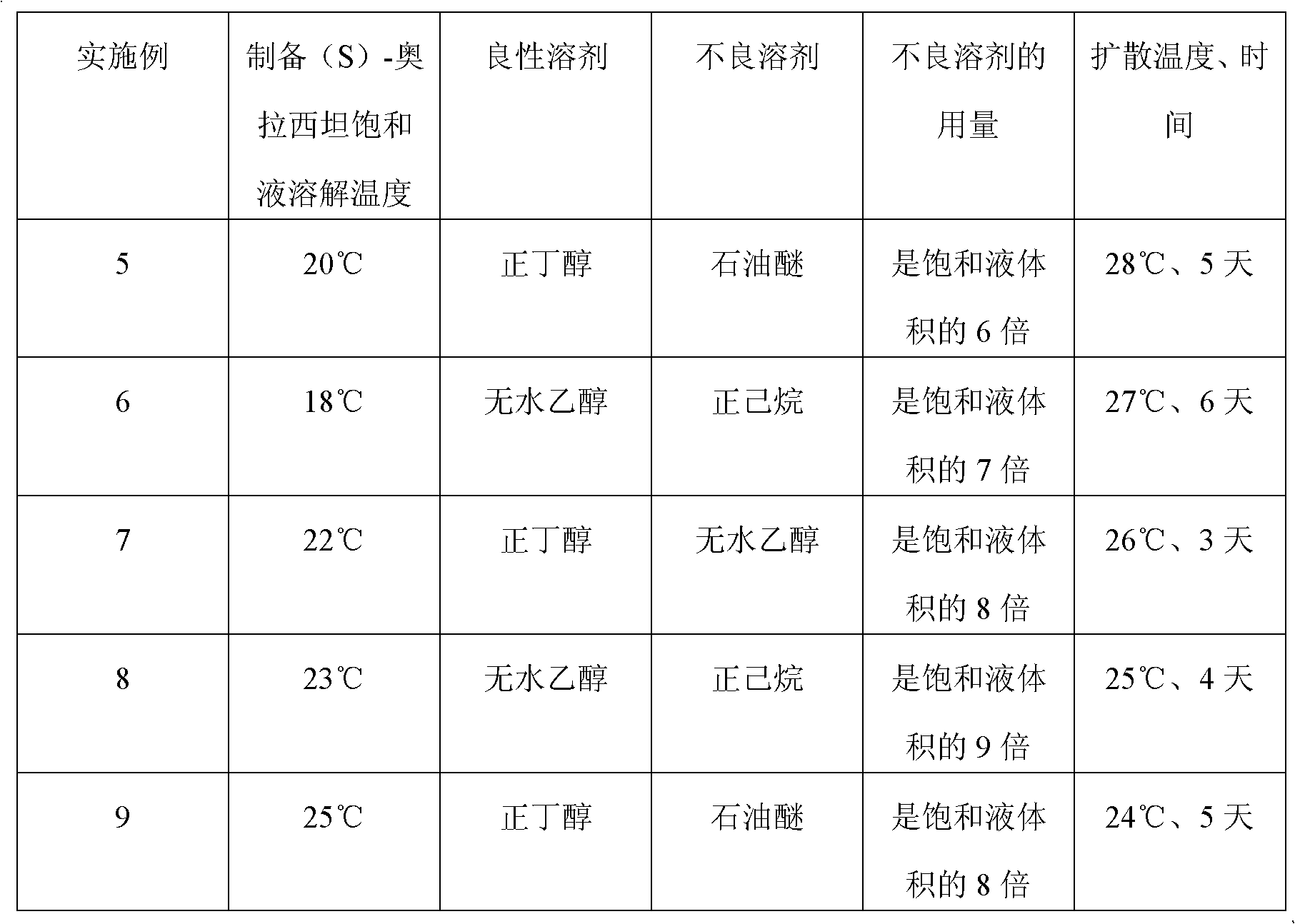
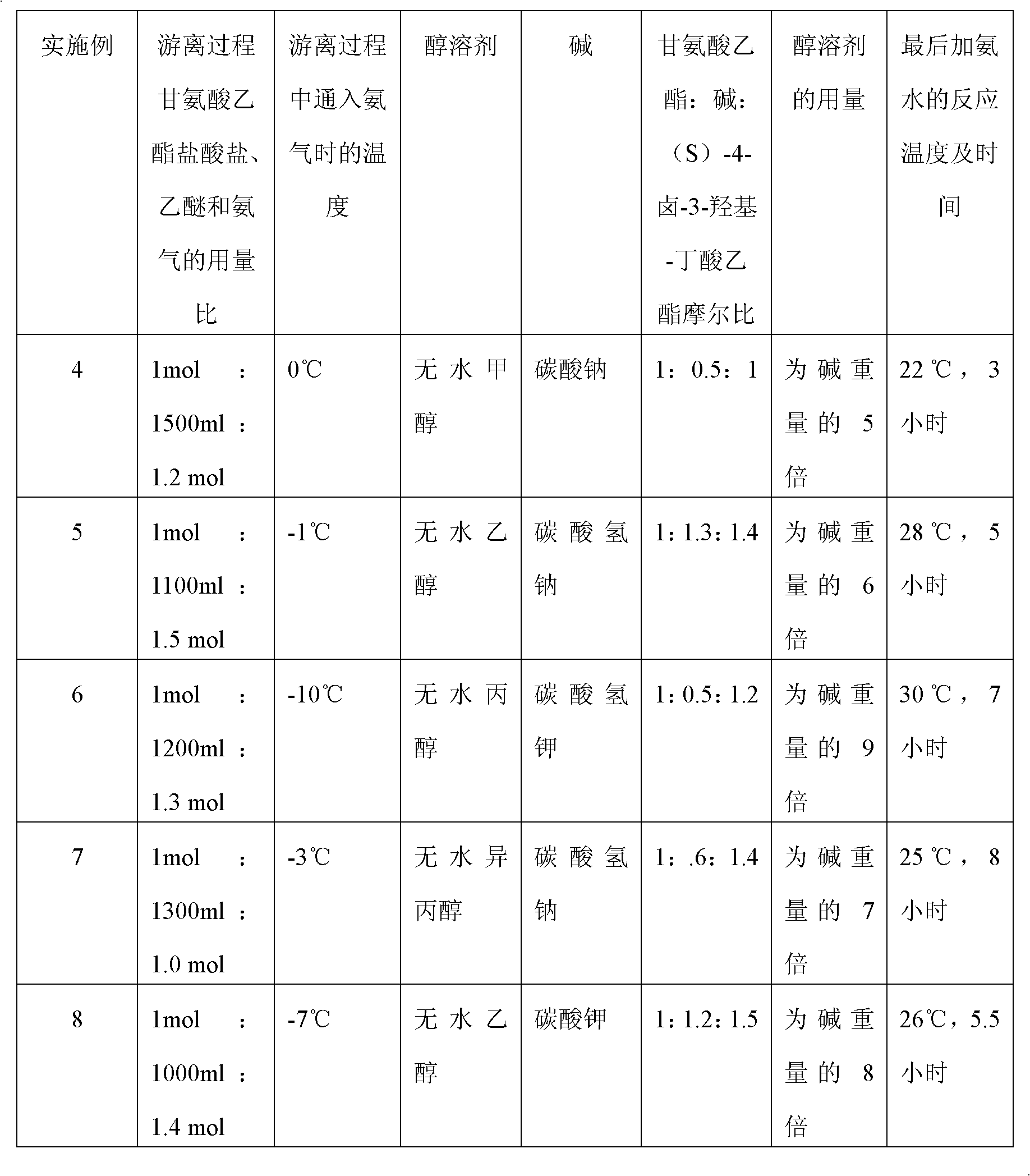
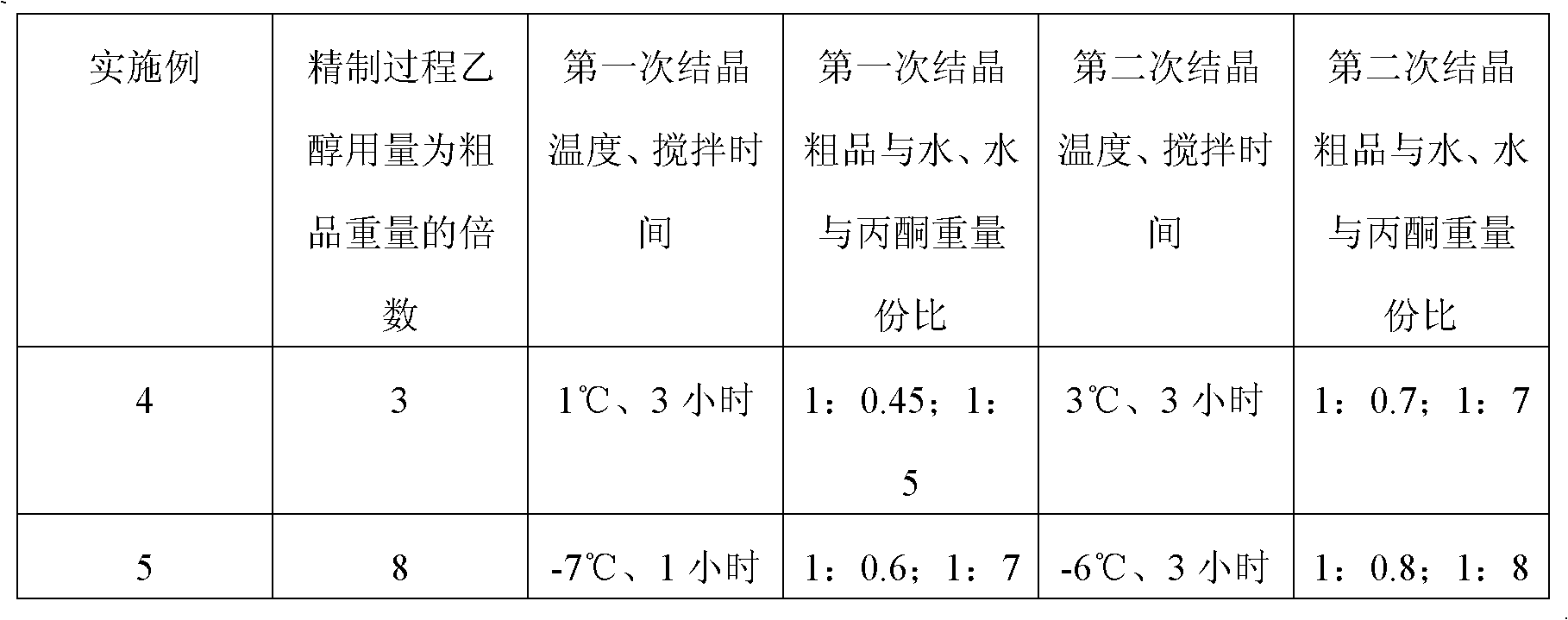
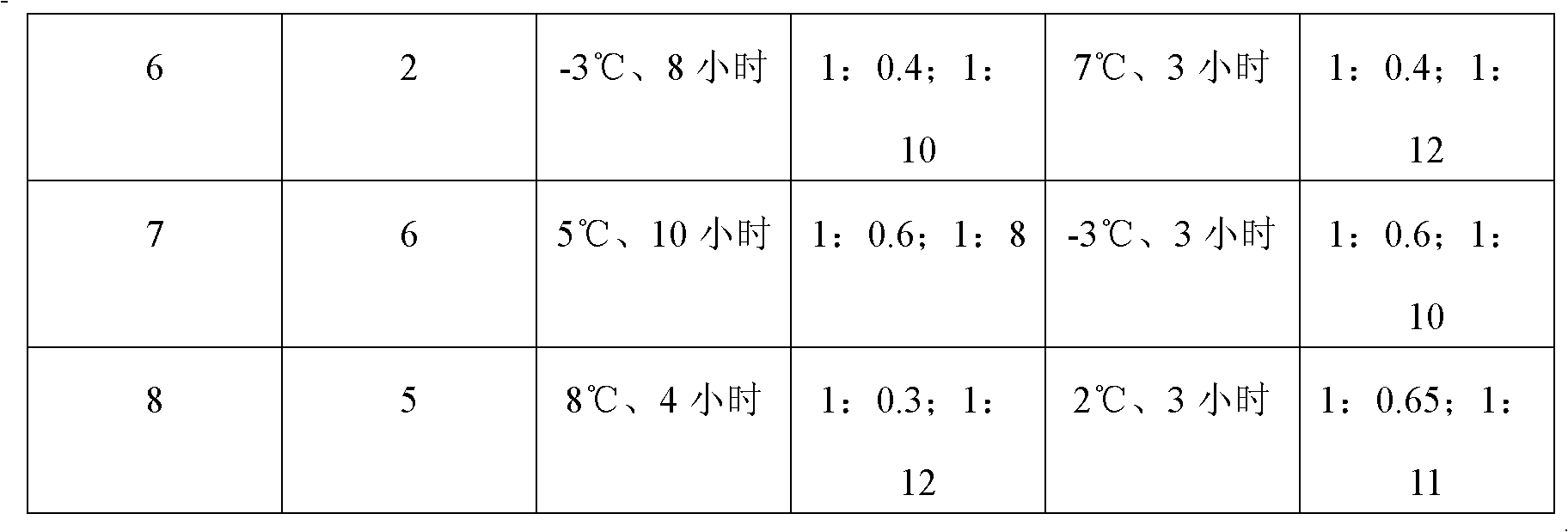
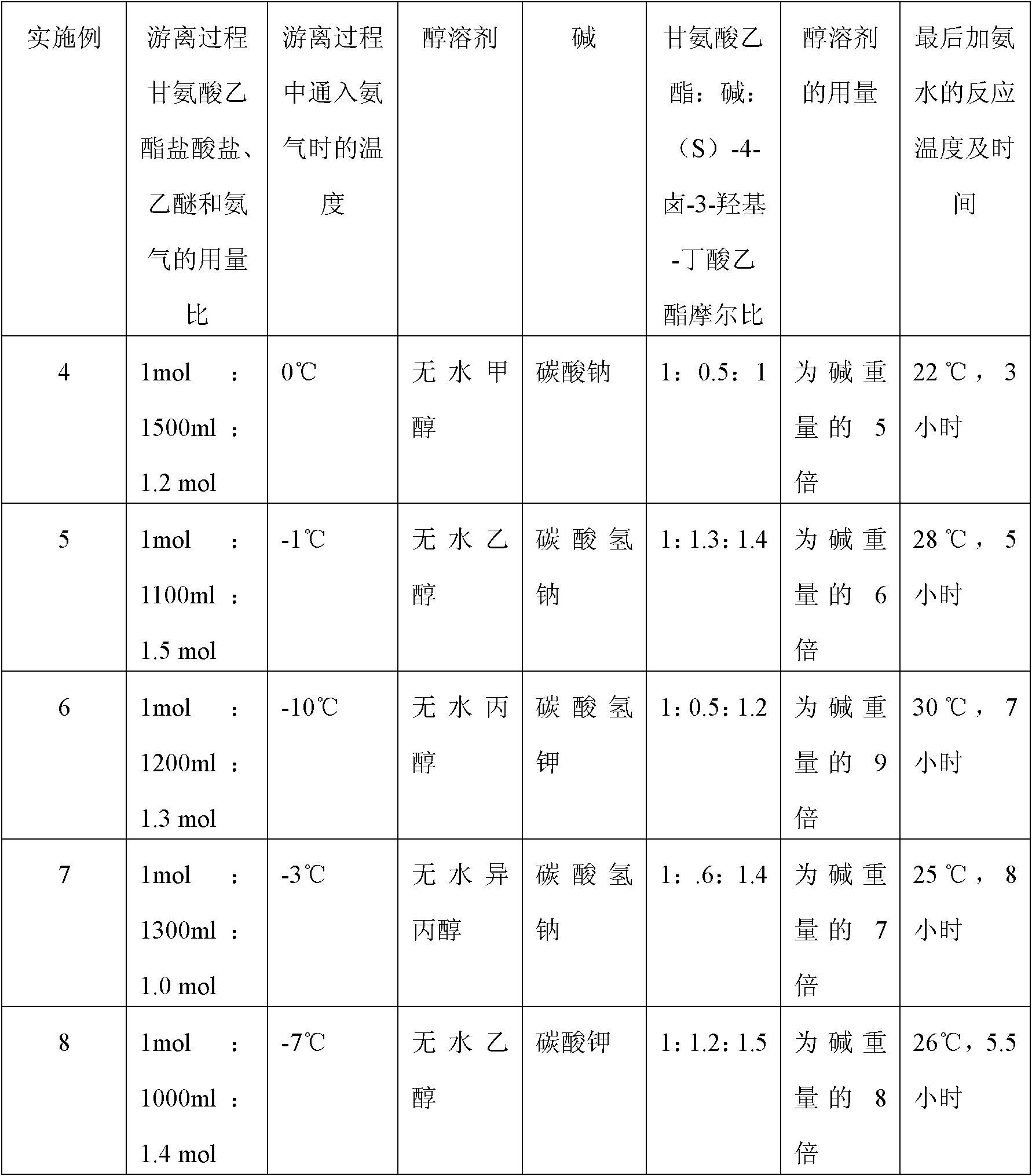
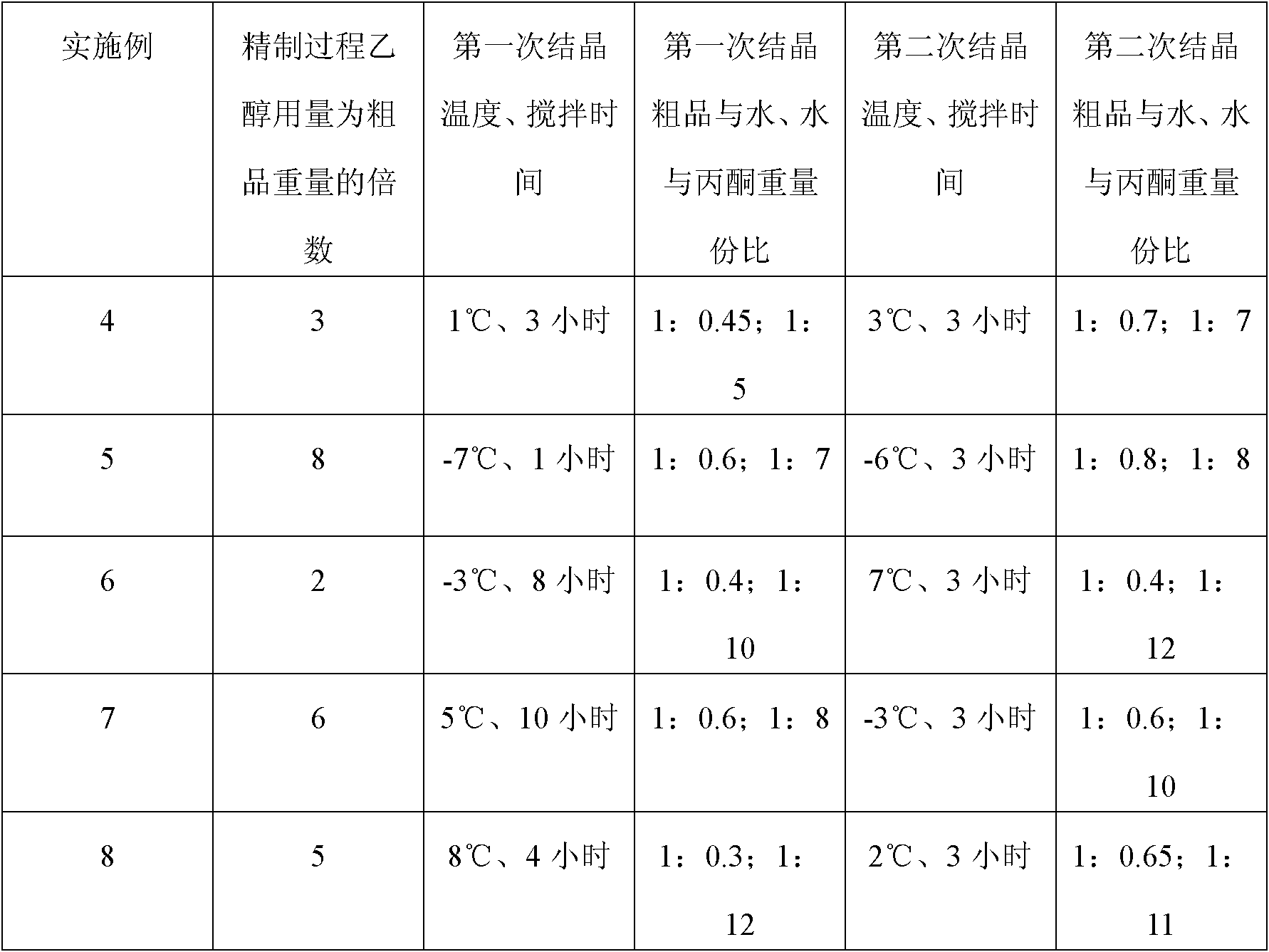
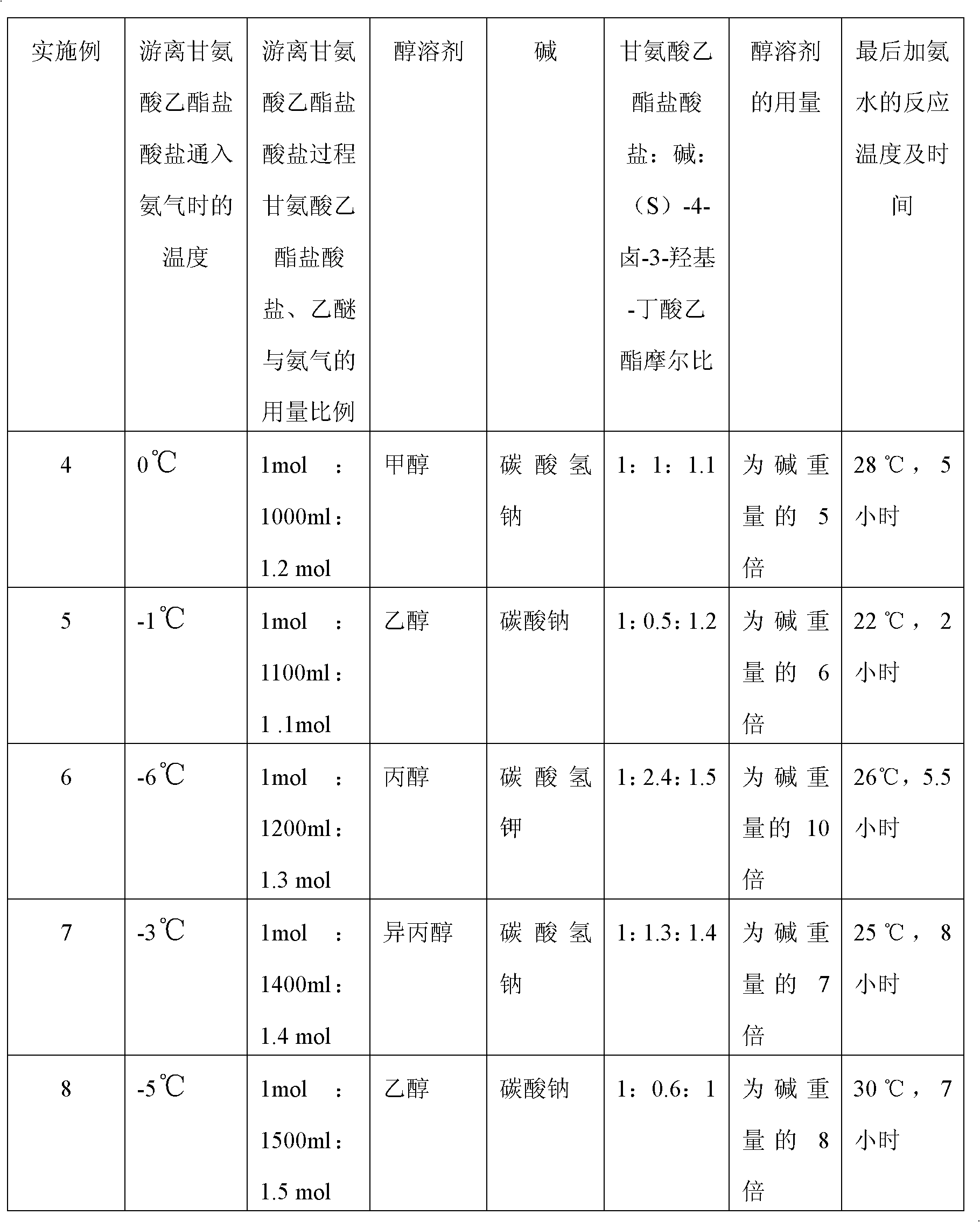
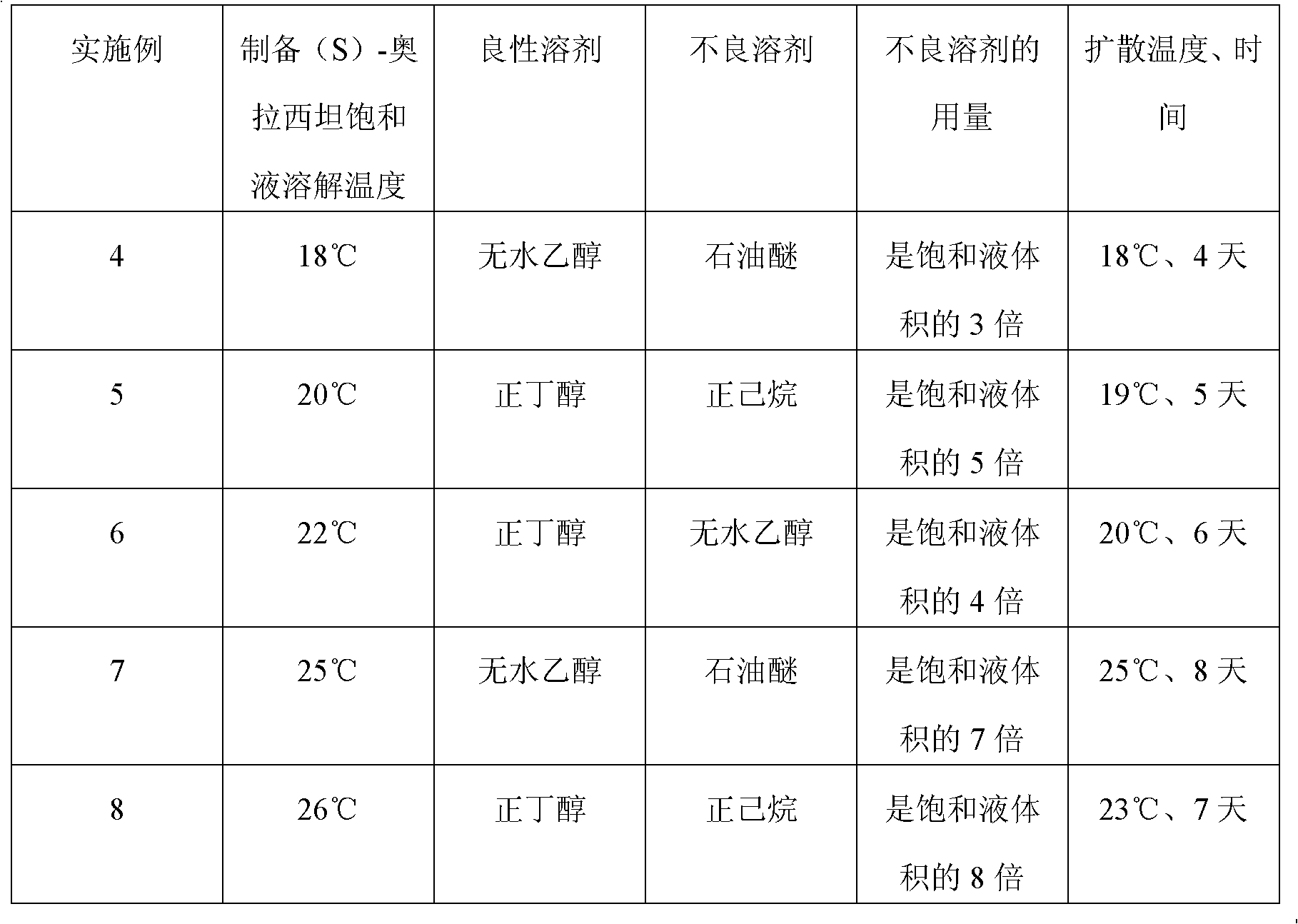
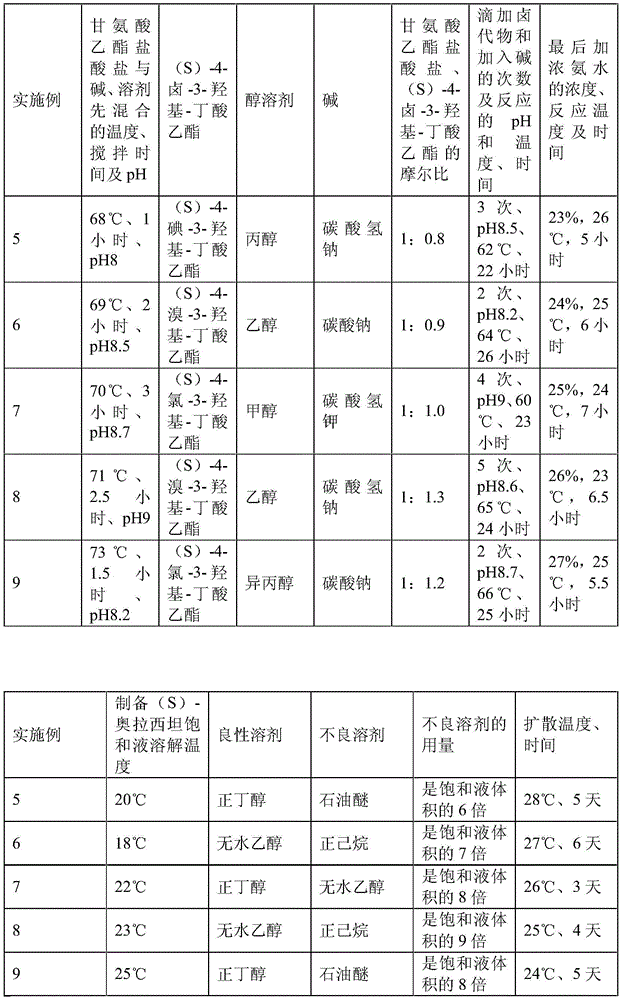
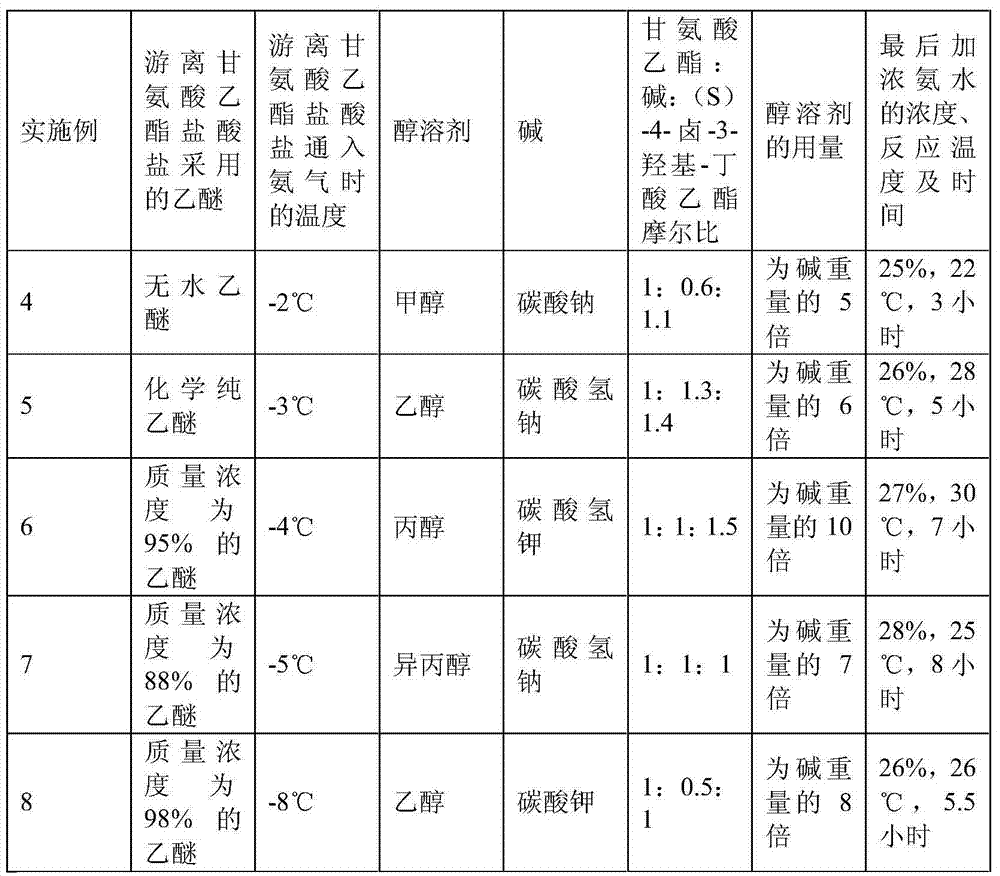
A preparation method of (S)-oxiracetam comprises the steps of conducting a reaction of a glycine ethyl ester hydrochloride with (S)-4-halgen-3-hydroxy-ethyl butyrate in an alcohol solvent under an alkaline condition, wherein the glycine ethyl ester hydrochloride and the (S)-4-halgen-3-hydroxy-ethyl butyrate are adopted as raw materials, filtering, washing with an inorganic alcohol, concentrating, then extracting, conducting water-phase concentration, introducing stronger ammonia water for a reaction so as to prepare a crude product of (S)-oxiracetam, and purifying the crude product; and mixing the glycine ethyl ester hydrochloride with alkali and the alcohol solvent firstly, then dropping the (S)-4-halgen-3-hydroxy-ethyl butyrate raw material in the mixture, and adding the alkali in times so as to control the pH value in the reaction to be 8-9. Purification treatment comprises the steps that the crude product of (S)-oxiracetam is dissolved in a benign solvent, a saturated solution is prepared at the room temperature, and then the saturated solution is dispersed in a closed environment by a poor solvent. The HPLC (high-performance liquid chromatography) purity of the prepared (S)-oxiracetam reaches up to more than 99.0%, the yield is high and reaches up to 33%, the reaction condition is moderate, the operation is simple, and the industrialized scale production is facilitated.
Owner:CHONGQING RUNZE PHARM CO LTD
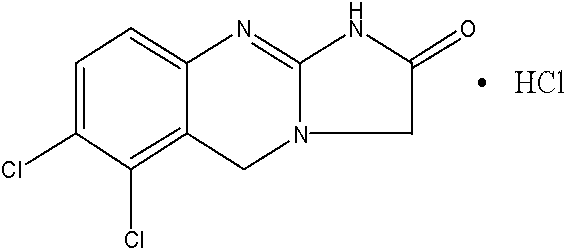
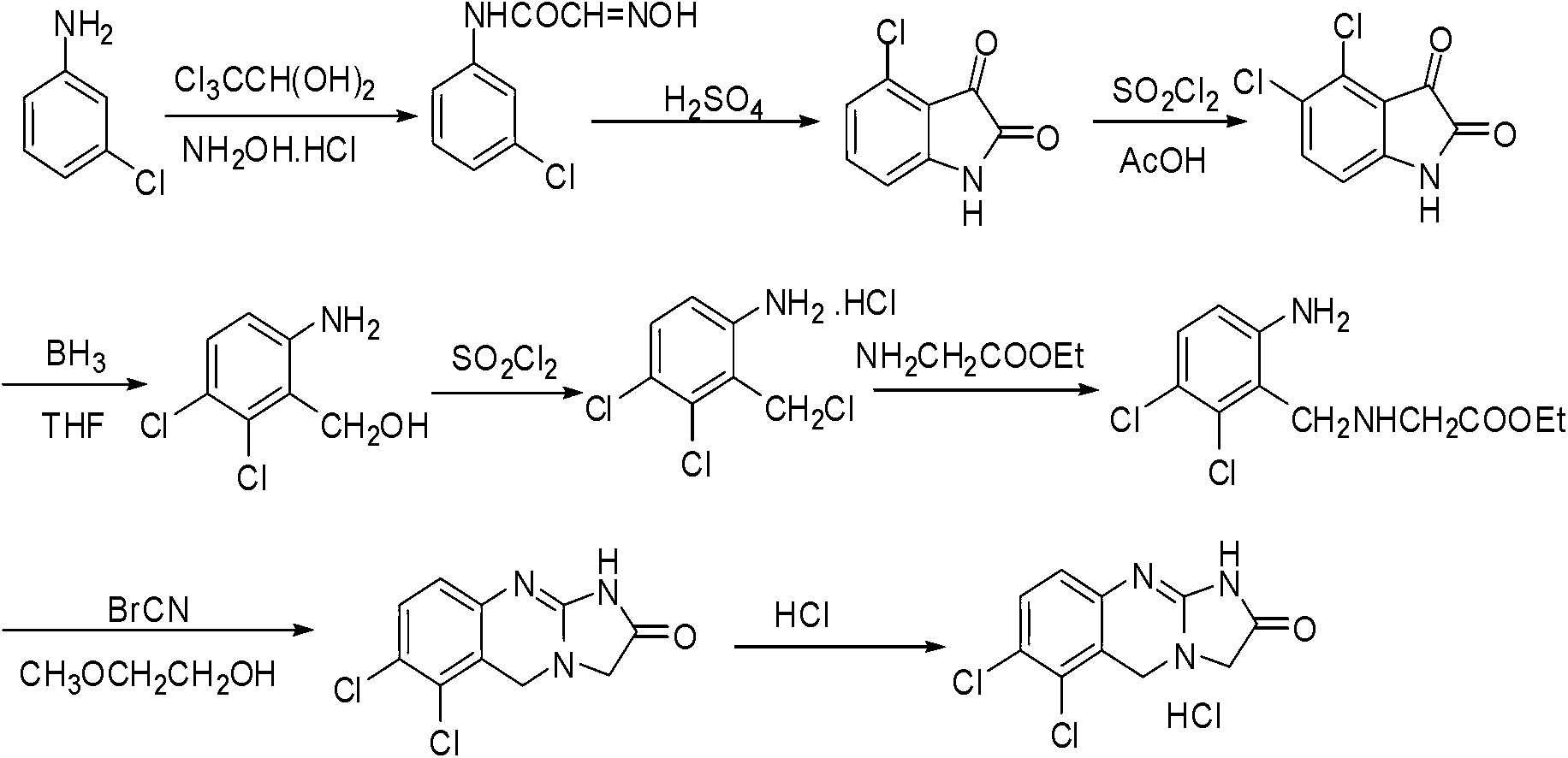
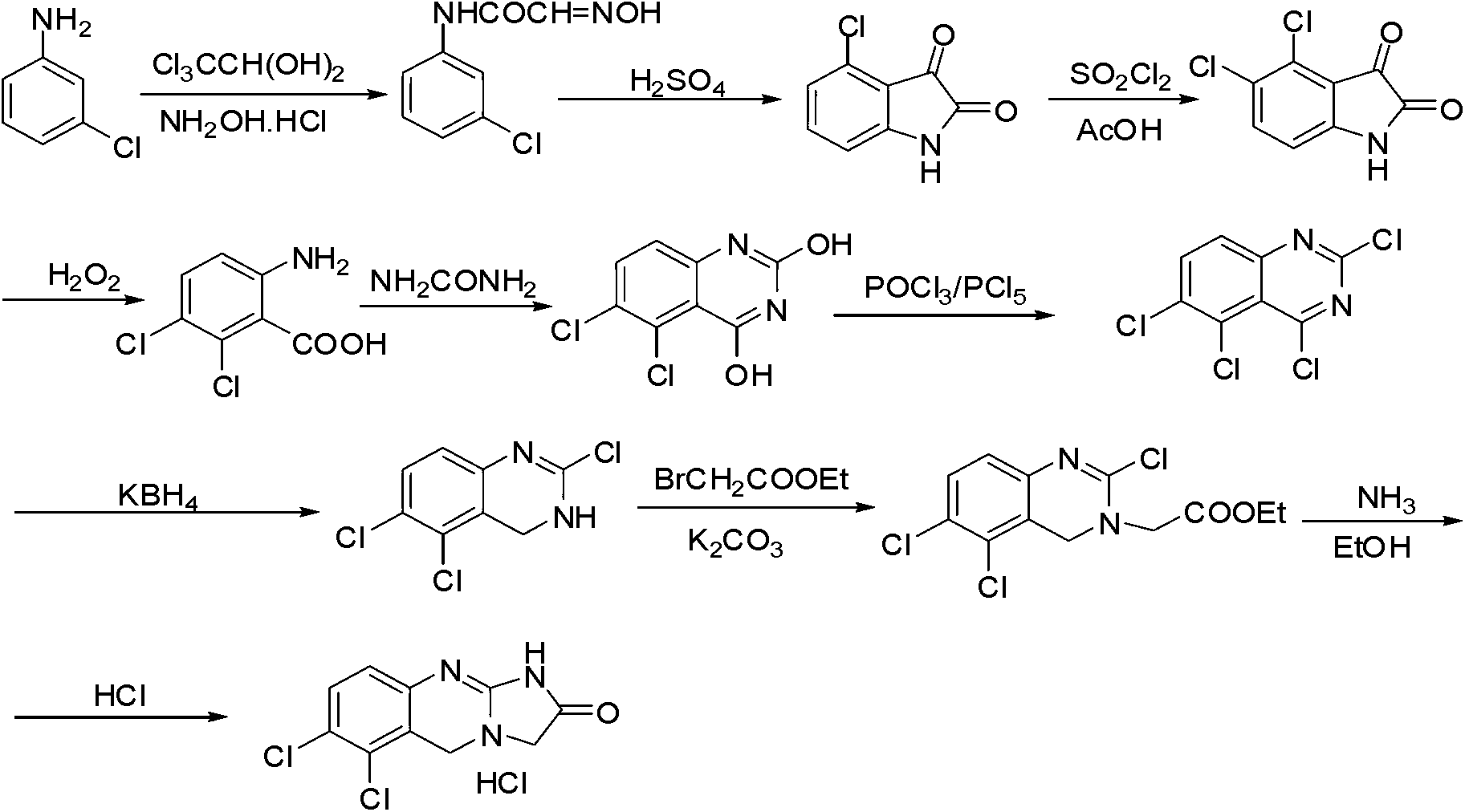
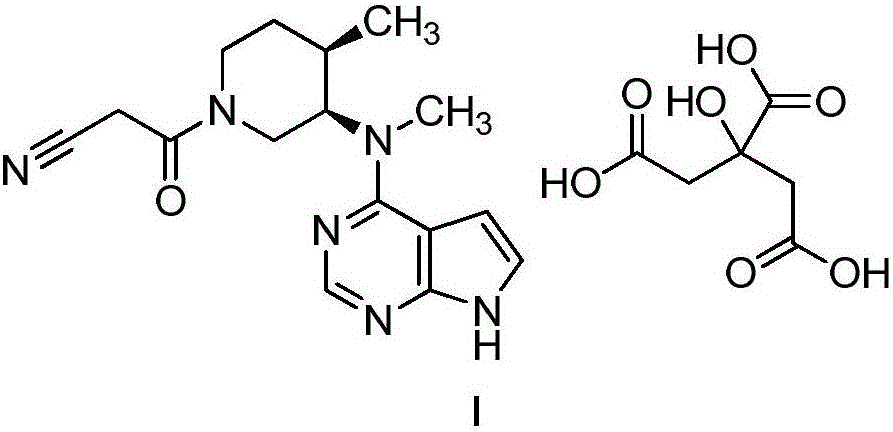
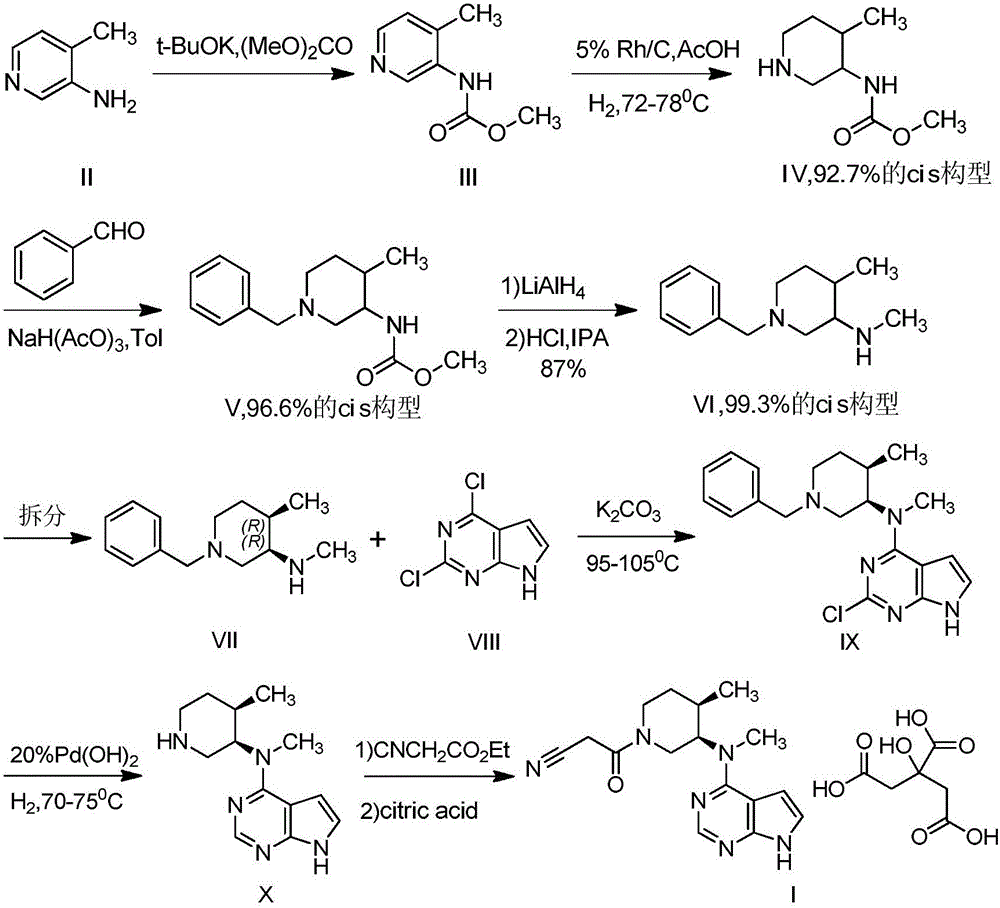
Preparation method of anagrelide hydrochloride
InactiveCN103254197AThe synthesis process is simpleShort synthetic routeOrganic chemistryBenzyl chlorideImpurity
The invention relates to a preparation method of anagrelide hydrochloride, and relates to preparation methods of chemical raw materials. The preparation method provided by the invention mainly solves the technical problems that an existing synthetic line of anagrelide hydrochloride is long, a toxic reagent, namely cuprous cyanide is used in the synthetic line, many impurities exist in the product, the product is hard to purify and the cost and unit consumption are high. The preparation method comprises the following steps: 1, preparing 2, 3-dichlorbenzyl alcohol; 2, preparing 2, 3-dichloro-6-nitrobenzyl alcohol; 3, preparing 2, 3-dichloro-6-nitryl-benzyl chloride; 4, preparing N-(2, 3-dichloro-6-nitrobenzyl) glycine ethyl ester hydrochloride; 5, preparing N-(6-amino-2, 3-dichlorobenzyl) glycine ethyl ester; 6, preparing an anagrelide hydrochloride crude product; and 7, preparing anagrelide hydrochloride. The preparation method provided by the invention is simple in synthetic process, shorter in synthetic line, low in cost and unit consumption, high in yield and less in three wastes generated in the reaction process, and is suitable for industrialized production. The preparation method provided by the invention is applied to the field of preparation of chemical raw materials.
Owner:HEILONGJIANG UNIV
Preparation method of (S)-oxiracetam
ActiveCN102603595AReduce dosageRaw materials are cheap, easy to obtain and environmentally friendlyOrganic chemistryEthyl butyrateSolvent
A preparation method of (S)-oxiracetam is characterized in that the preparation method comprises the steps of conducting a reaction of a glycine ethyl ester hydrochloride with a (S)-4-halogen-3-hydroxy-ethyl butyrate in an alcohol solvent under an alkaline condition, wherein the glycine ethyl ester hydrochloride and the glycine ethyl ester hydrochloride are taken as raw materials, filtering, washing with inorganic alcohol, concentrating, then extracting, separating, and introducing ammonia water to prepare a crude product of the (S) oxiracetam, and purifying the crude product. The glycine ethyl ester hydrochloride is dissociated into glycine ethyl ester by diethyl ether and ammonia gas firstly, and the purification of the crude product comprises recrystallization treatment by use of water and acetone as solvents, wherein a weight ratio of the water to acetone is 1:(5-20). According to the (S)-oxiracetam prepared by the preparation method, the yield is high and reaches up to 36%, the HPLC (high performance liquid chromatography) purity of the prepared (S)-oxiracetam reaches up to more than 99.4%, in addition, the reaction condition is moderate, the period is short, the operation is simple, and the industrialized scale production is facilitated.
Owner:CHONGQING RUNZE PHARM CO LTD
Synthesizing method of S-(+)-2, 2-dimethylcyclopropane carboxamide
ActiveCN101735099AShort reaction timeMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationGlycine ethyl esterCyclopropanation
The invention relates to a synthesizing method of S-(+)-2, 2-dimethylcyclopropane carboxamide which is synthezied by using glycine ethyl ester hydrochloride as main starting material and sequentially comprising the following steps of: diazotization reaction, cyclopropanation reaction, hydrolysis reaction, acylation reaction, resolution reaction and ammonolysis reaction. The synthesizing method has short reaction time, moderate reaction condition, simple process, overall yield more than 17 percent and ee more than 98 percent.
Owner:JIANGSU YUXIANG CHEM
Preparation method of (S)-oxiracetam
ActiveCN102603594AReduce dosageRaw materials are cheap, easy to obtain and environmentally friendlyOrganic chemistryEthyl butyrateDiethyl ether
A preparation method of (S)-oxiracetam is characterized in that the preparation method comprises the steps of conducting a reaction of a glycine ethyl ester hydrochloride with a (S)-4-halogen-3-hydroxy-ethyl butyrate in an alcohol solvent under an alkaline condition, wherein the glycine ethyl ester hydrochloride and the (S)-4-halogen-3-hydroxy-ethyl butyrate are taken as raw materials, filtering, washing with inorganic alcohol, concentrating, then extracting, separating, and introducing ammonia water to prepare a crude product of the (S) oxiracetam, and purifying the crude product. The glycine ethyl ester hydrochloride is dissociated into glycine ethyl ester by diethyl ether and ammonia gas firstly, and the purification of the crude product comprises recrystallization treatment by use of water and acetone as solvents, wherein a weight ratio of the water to acetone is 1:(5-20). According to the (S)-oxiracetam prepared by the preparation method, the yield is high and reaches up to 36%, the HPLC (high performance liquid chromatography) purity of the (S)-oxiracetam reaches up to more than 99.4%, in addition, the reaction condition is moderate, the period is short, the operation is simple, and the industrialized scale production is facilitated.
Owner:CHONGQING RUNZE PHARM CO LTD
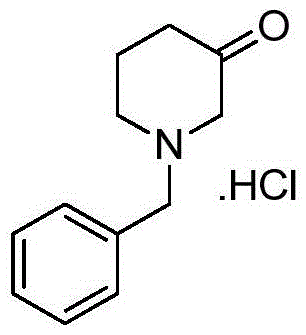
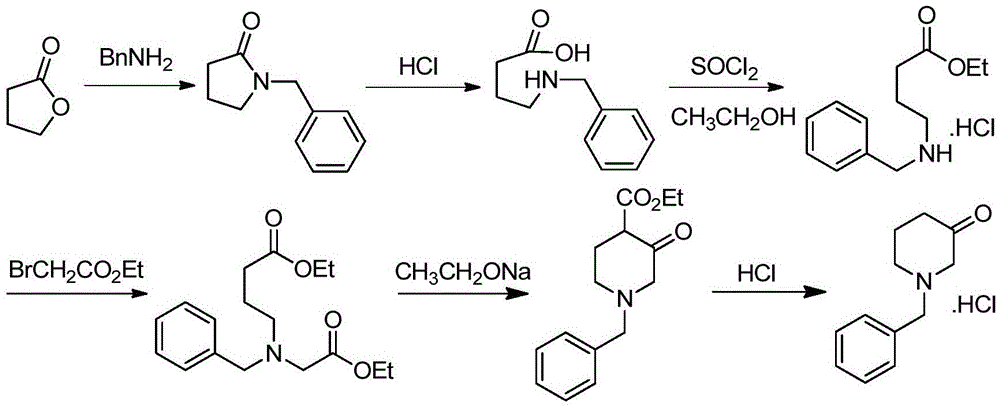
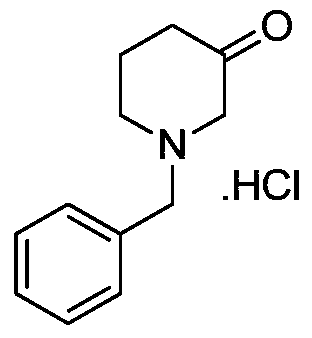
Preparation method for 1-benzyl-3-piperidone hydrochloride
ActiveCN105622444ALow costHigh purityOrganic compound preparationAmino-carboxyl compound preparationAcetic acidOrganic solvent
The invention discloses a preparation method for N-benzyl glycine ethyl ester. The method comprises the following steps: dissolving benzylamine in an organic solvent I, then adding 2-halogenated ethyl acetate, alkali and quaternary ammonium salt, and performing reaction to obtain the N-benzyl glycine ethyl ester. The invention also discloses a preparation method for 1-benzyl-3-piperidone hydrochloride. The method comprises the following specific steps: (1) preparing an intermediate IV (N-benzyl glycine ethyl ester); (2) dissolving the intermediate IV in an organic solvent II, then adding 4-halogenated ethyl acetate and alkali, and performing reaction to obtain an intermediate III; (3) performing reaction between the intermediate III and the alkali, reversely regulating a pH value to 6-8, performing concentration under reduced pressure, extracting by using ethyl acetate, performing washing and drying, and then performing concentration under reduced pressure to obtain an intermediate II; (4) performing reaction on the intermediate II and acid, performing rotary evaporation concentration, and adding a crystal solvent for crystallization to obtain a product. The route synthesis steps are short, the process is novel, the intermediates are high in purity, the product yield is high, and the cost is low.
Owner:CHONGQING WEIPENG PHARMA
Method for preparing (S)-oxiracetam
A method for preparing (S)-oxiracetam comprises the steps of conducting a reaction of a glycine ethyl ester hydrochloride with (S)-4-halogen-3-hydroxy-ethyl butyrate in an alcohol solvent in an alkaline condition, wherein the glycine ethyl ester hydrochloride and the (S)-4-halogen-3-hydroxy-ethyl butyrate are adopted as raw materials, filtering, washing filtrate with inorganic alcohol, concentrating, then extracting, separating and introducing ammonia water to prepare a crude product, and purifying the crude product; and dissociating the glycine ethyl ester hydrochloride into glycine ethyl ester by diethyl ether and ammonia gas firstly. Purification treatment comprises the steps that the crude product of (S)-oxiracetam is dissolved in a benign solvent to prepare a saturated solution, and then the saturated solution is dispersed in a closed environment by a poor solvent. According to the method, the main raw materials are low in cost, easy to obtain and environment-friendly; the glycine ethyl ester hydrochloride is adopted for dissociation, so that the using amount of the materials in the reaction is reduced effectively, the cost is lowered, and in addition, the glycine ethyl ester hydrochloride plays an active role in the reaction yield. The HPLC (high-performance liquid chromatography) purity of the prepared (S)-oxiracetam reaches up to more than 99.0%, the yield is high and reaches up to 36%, the reaction condition is moderate, the operation is simple, and the industrialized scale production is facilitated.
Owner:CHONGQING RUNZE PHARM CO LTD
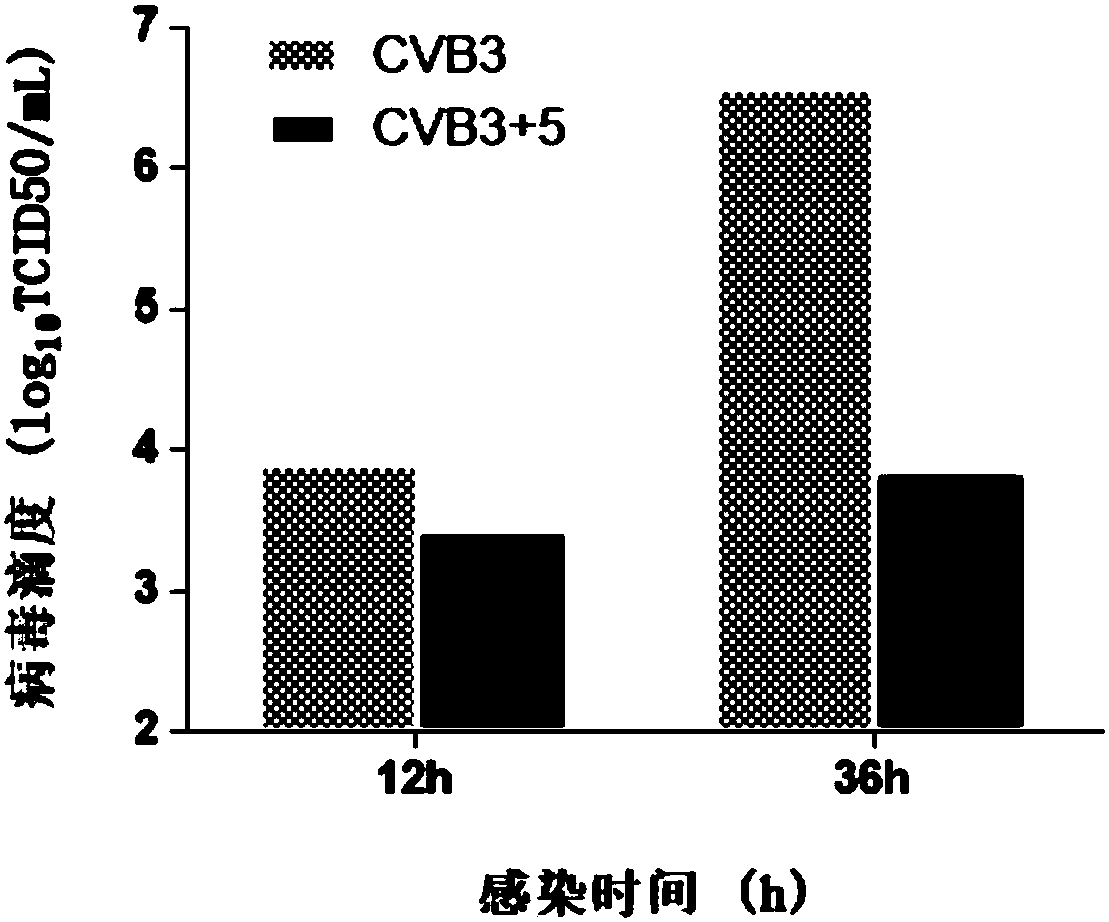
Application of amino acid ester compounds in preparation of anti-CVB3 (anti-coxsackie B virus 3) drugs
The invention discloses application of amino acid ester compounds in the preparation of anti-CVB3 (anti-coxsackie B virus 3) drugs and belongs to the technical field of antiviral drugs. Verification by massive biological experiments shows that amino acid ester compounds (glycine ethyl ester, L-leucine methyl ester, L-alanine methyl ester, tert-butyl L-alaninate, beta-alanine ethyl ester, D-leucinemethyl ester, tert-butyl glycinate, beta-alanine tert-butyl ester, and L-leucine tert-butyl ester) can inhibit replication and proliferation of CVB3 in cells, can strongly inhibit cytopathic effect due to CVB3 and increase the survival rate of infected cells, and is applicable to the preparation of anti-CVB3 drugs or drugs to treat diseases due to CVB3 infections. Novel pharmaceutical applicationof amino acid ester compounds is discovered herein, and a new direction is provided for the development of anti-CVB3 drugs.
Owner:HUBEI UNIV OF TECH
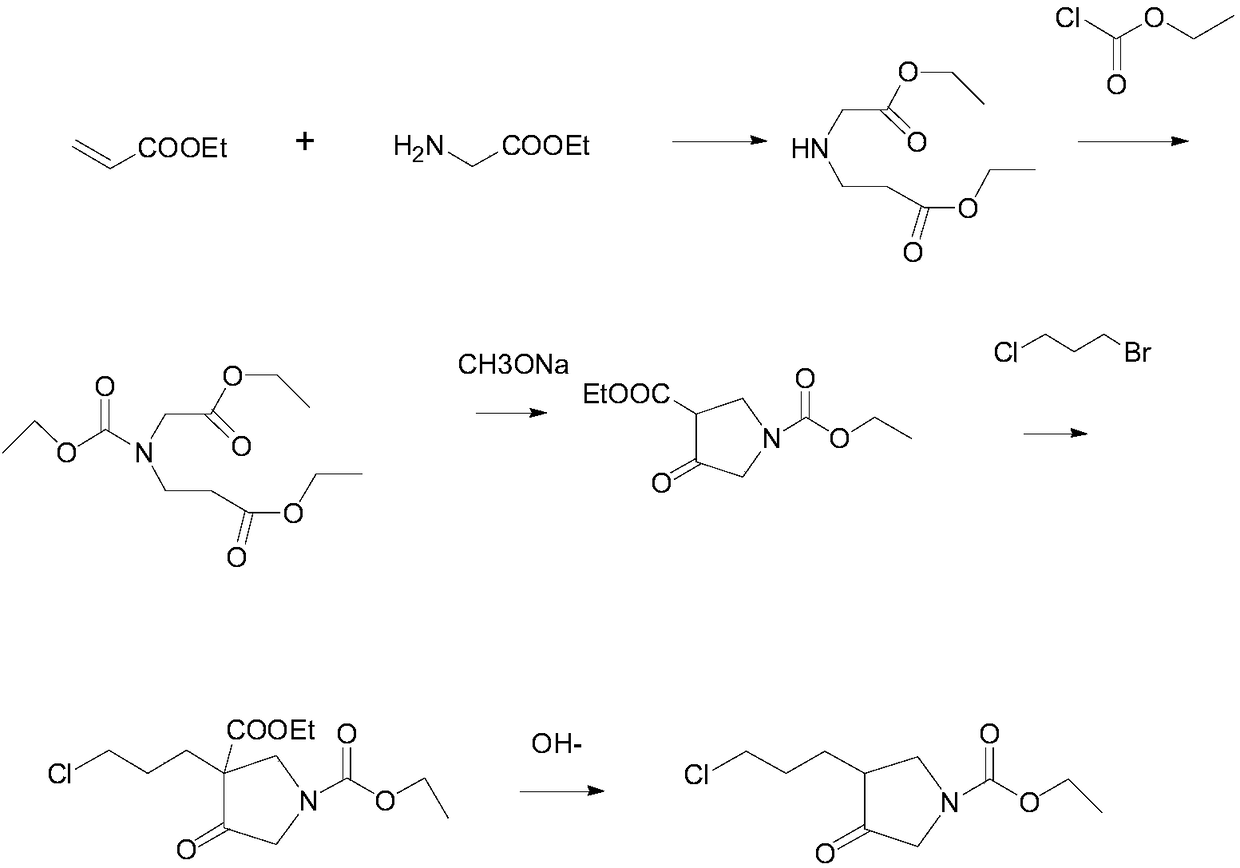
Method for preparing 3-(3-chloropropyl)-4-oxopyrrolidine-1-ethyl carboxylate
InactiveCN108863886AEasy to operateShorten the production cycleOrganic chemistryEcological environmentSynthesis methods
The invention provides a novel method for preparing 3-(3-chloropropyl)-4-oxopyrrolidine-1-ethyl carboxylate, aiming at solving the technical problems of a traditional 3-(3-chloropropyl)-4-oxopyrrolidine-1-ethyl carboxylate synthesis method in the prior art that a flow is complicated, the environmental hazards are great, the safety is low and the yield is low. The technology takes glycine ethyl ester and ethyl acrylate as starting raw materials, and steps of taking benzylamine as a raw material and carrying out debenzylation in a subsequent process are reduced; a process for preparing the 3-(3-chloropropyl)-4-oxopyrrolidine-1-ethyl carboxylate is greatly simplified; an existing technology needs 6-step reaction, and the technology only needs 5-step reaction, so that the operation is simplified and a production period is shortened; the method is applicable to large-scale industrial production. The technology does not take the benzylamine as the starting raw material, and the generation ofa cancer-causing high-hazard substance, i.e., benzyl chloride, is directly stopped from the source; harms to an ecological environment and body health of people are effectively avoided; a reaction process is safe and accords with environmental protection requirements of China better; the total yield is greatly improved and the yield is greater than or equal to 50 percent.
Owner:江苏八巨药业有限公司
A preparation method of (s)-oxiracetam
The invention relates to a preparation method of (S)-oxiracetam, which comprises the following steps: reacting raw materials glycine ethyl ester hydrochloride and ethyl (S)-4-halo-hydroxy-butyrate in an alcohol solvent under alkaline conditions, filtering, washing with inorganic alcohol, concentrating, extracting, concentrating the water phase, introducing stronger ammonia water to react to obtain a (S)-oxiracetam crude product, and carrying out purification treatment on the crude product. The glycine ethyl ester hydrochloride is firstly mixed with the alkali and alcohol solvent, and the raw material ethyl (S)-4-halo-hydroxy-butyrate is dropwisely added and the alkali is added in different batches to control the pH value of the reaction at 8-9. The purification treatment comprises the following steps: dissolving the (S)-oxiracetam crude product in a benign solvent to prepare a saturated solution at room temperature, and dispersing with a poor solvent in a closed environment. The HPLC (high performance liquid chromatography) purity of the prepared (S)-oxiracetam is up to 99.0% %, and the yield can be up to 33%; and the method is mild in reaction conditions, simple to operate and beneficial to industrial large-scale production.
Owner:CHONGQING RUNZE PHARM CO LTD
Novel wide-temperature-range lubricating oil and preparation method thereof
InactiveCN108893180AWide temperature rangeImprove the lubrication effectLubricant compositionChlorobenzeneGlycine ethyl ester
The invention discloses novel wide-temperature-range lubricating oil and a preparation method thereof. The lubricating oil is prepared from, by mass, 75-98 parts of base oil, 0.8-3.5 parts of a lubricant, 0.05-0.8 part of N-(2,3-dichloro-6-amino-benzyl)glycine ethyl ester, 0.05-2.5 parts of triethanolamine, 0.5-2.0 parts of sodium benzoate and 0.05-0.2 part of chlorobenzene. The lubricating oil has advantages of wide utilization temperature range, high flashing point, high kinematic viscosity, small trail diameter, excellent lubricating performances and high abrasion resistance under a high-load condition.
Owner:张毅
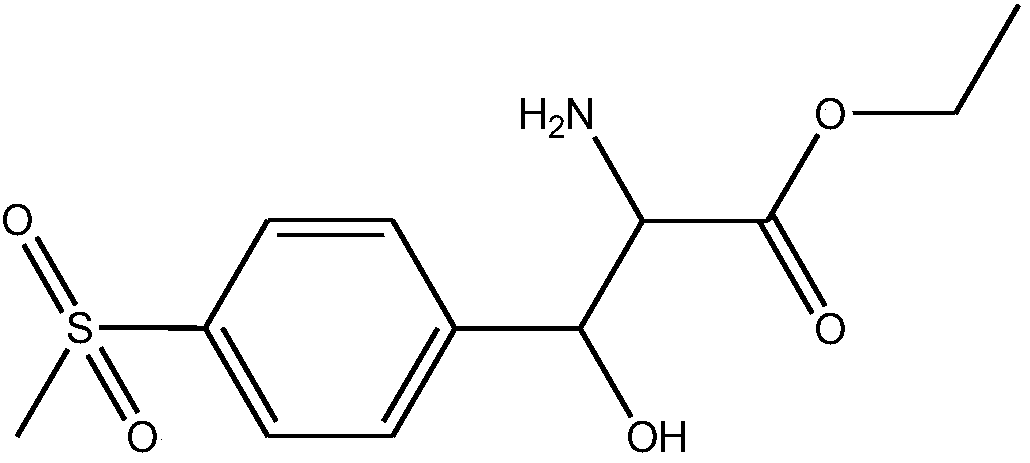
DL-p-methylsulfonylphenyl serine ethyl ester preparation method
InactiveCN110776444AAvoid dangerAvoid it happening againPhysical/chemical process catalystsOrganic chemistryIce waterBenzaldehyde
The invention discloses a DL-p-methylsulfonylphenyl serine ethyl ester preparation method, which comprises: 1) adding glycine ethyl ester hydrochloride into ethanol, and carrying out heating reflux; 2) dissolving basic cupric carbonate in ammonia water, and adding into the solution obtained in the step 1); 3) adding KOH into the solution obtained in the step 2) to adjust the pH value of the solution, and adding p-methylsulphonyl benzaldehyde, carrying out a reaction; and 4) after the reaction is finished, recovering ethanol in the reacted substance, adding ammonia water into the residue, regulating the pH value, cooling the solution, filtering, washing with ice water, and drying to obtain the p-methylsulfonylphenyl serine ethyl ester. According to the invention, the target product is generated by carrying out a one-step reaction on glycine ethyl ester hydrochloride and p-methylsulphonyl benzaldehyde under the catalysis of basic cupric carbonate and an alkali, wherein the reaction process does not require the use of concentrated sulfuric acid so as to completely avoid the generation of strong acid wastewater, protect and the environment, avoid the possible danger in the sulfuric acid transportation and production process, achieve the safety and easily improve the operation efficiency.
Owner:JIANGSU YUXIANG CHEM
Antibacterial anticorrosive pipeline material and preparation method thereof
InactiveCN105754234AImprove mechanical propertiesImprove anti-corrosion performancePolybutyleneFatty alcohol
The invention provides an antibacterial anticorrosive pipeline material and a preparation method thereof. The antibacterial anticorrosive pipeline material is prepared by the following steps: (1) mixing titanium dioxide, nano sliver, nano aluminum oxide, dipentaerythritol diphosphite and water to obtain a mixture, and slowly stirring the mixture for 1-2 hours at a temperature of 50-60 DEG C; (2) drying the mixture in a vacuum drying oven after filtering; (3) mixing a dried object with polybutylene, polypropylene, basalt fibers, a polyvinyl acetate emulsion, liquid acrylonitrile butadiene rubber, calcium lignosulphonate, fatty alcohol-polyoxyethylene ether, N-glycine ethyl ester, N-bromo-succinimide, chlorinated paraffin, silicone oil, 1-methyl pentanol and guar gum, and dispersing in a low-speed dispersing machine; and (4) adding the mixture into a twin-screw extruder to carry out extrusion molding. The antibacterial anticorrosive pipeline material has good mechanical property, and has good corrosion resistance and anti-bacterial property.
Owner:SUZHOU FUZHONG PLASTIC CO LTD
Fertilizer for promoting growth of tea leaf and preparation method of fertilizer
InactiveCN107244963ANutritional supplementsPromote growthExcrement fertilisersBioloigcal waste fertilisersAdditive ingredientRapeseed
The invention discloses a fertilizer for promoting growth of a tea leaf and a preparation method of the fertilizer. The fertilizer comprises the following ingredients in parts by weight: 10-15 parts of vinasse, 5-10 parts of rapeseed meal, 5-10 parts of wheat bran, 10-15 parts of animal waste, 10-15 parts of plant straw, 2-7 parts of biological bacteria, 10-20 parts of water, 0.8-1.5 parts of an insecticide, 1-3 parts of microelements, 2-10 parts of kaolin powder, 0.2-0.5 part of vinyl acetate, and 0.01-0.03 part of glycine ethyl ester hydrochloride. The fertilizer has the beneficial effects that the main ingredients of the fertilizer are mainly from the domestic wastes, are easy to degrade in the natural environment, do not pollute the environment, and improve the quality of the tea leaf; and synthesizing the protease by a plant is promoted, the agrochemical residuals are naturally removed, the nutritional element is adsorbed, the nutritional ingredient needed by the tea leaf is complemented, an injurious insect is killed, the soil is improved, the soil hardening is prevented, and the preparation method is simple.
Owner:新昌县南翔食品科技有限公司
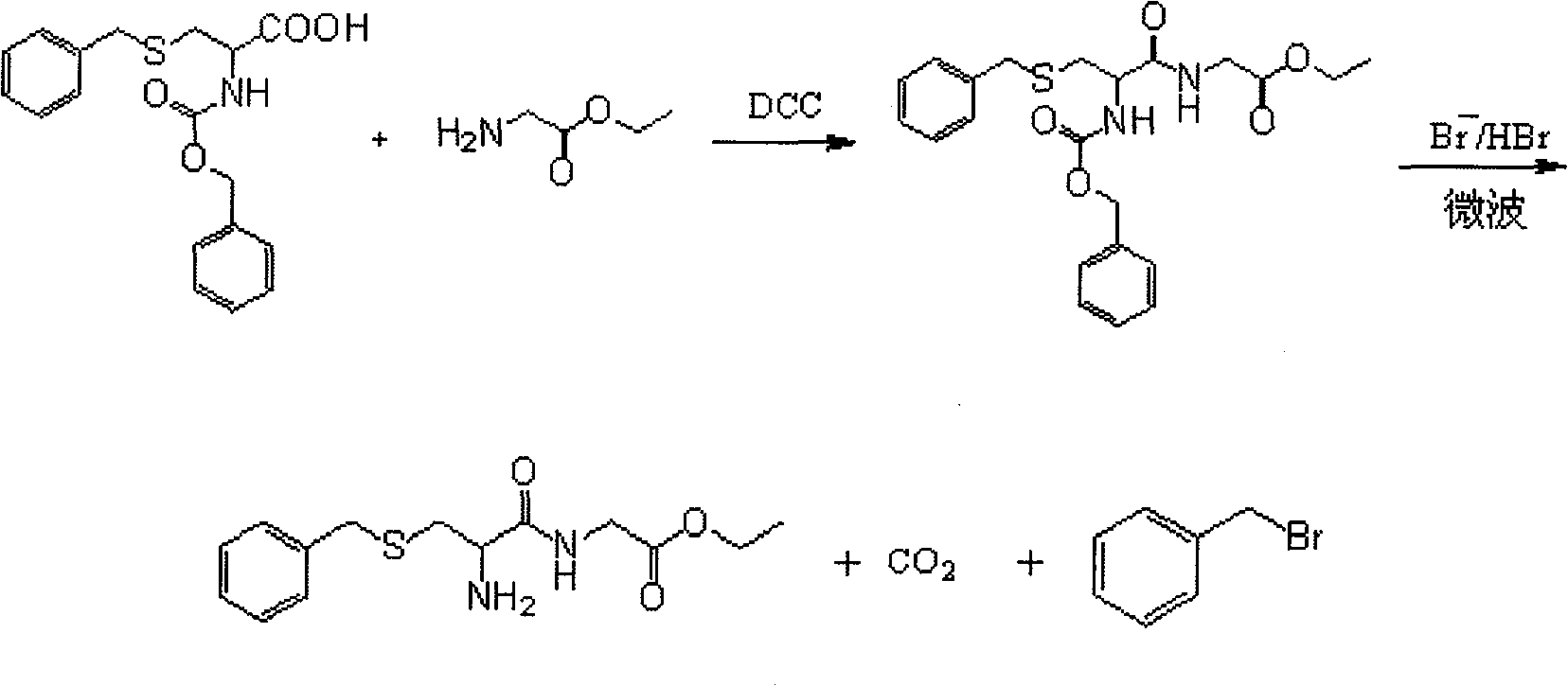
Method for preparing S-benzyl cysteinyl glycine ethyl ester under microwave condition
InactiveCN101898986AEasy to prepareShort reaction timeSulfide preparationTetrahydrofuranPhotochemistry
The invention relates to a method for preparing S-benzyl cysteinyl glycine ethyl ester under the microwave condition, which comprises the steps of: (1) dissolving glycine ethyl ester hydrochloride into methylene dichloride, adding tetrahydrofuran and triethylamine, then adding S-benzyl-N-benzoxy carbonyl cysteine, controlling the temperature to be -2 to 0 DEG C, additionally taking N, N'-dicyclohexyl carbodiimide DCC and dissolving in the methylene dichloride, and slowly dripping into the mixed solution under the stirring condition; and (2) adding S-benzyl-N-benzoxy carbonyl cysteinyl glycineethyl ester into a three-neck flask provided with a backflow condenser pipe and putting into a microwave instrument, then, adding saturated hydrogen bromide acetum, leading reactant to be rapidly dissolved, discharging gas, carrying reaction for 15-20min, reducing the pressure, extracting hydrogen bromide and glacial acetic acid, washing and drying. The method is simple, short in reaction time, high in yield and suitable for industrialized production.
Owner:DONGHUA UNIV
Preparation method for industrial amplified production of anagrelide hydrochloride active pharmaceutical ingredient
The invention relates to a preparation method of an active pharmaceutical ingredient, particularly to a preparation method for industrial amplified production of an anagrelide hydrochloride active ingredient, and aims to solve the problems that the synthetic route of the existing anagrelide hydrochloride is long, more impurities are produced, products are difficult to purify, and the cost is high. The method includes preparing 2, 3-dichloro-6-nitrobenzonitrile, preparing N-(2, 3- dichloro-6-nitrobenzene) glycine ethyl ester, preparing N-(6-amino-2, 3-dichlorobenzene) glycine ethyl ester, and preparing the anagrelide hydrochloride. According to the anagrelide hydrochloride, unit consumption of the cost is low, the yield is 71.20%, products contain fewer impurities after purified, the content of single impurity is low, impurity profiles are simple, and the whole process is simple in operation and prone to industrialization. The preparation method is applied to the preparation field of the anagrelide hydrochloride.
Owner:黑龙江天宏药业股份有限公司
Production method of N-benzyl glycine ethyl ester
InactiveCN1477095ASimple processFew reaction stepsOrganic compound preparationAmino-carboxyl compound preparationOrganic solventGlycine ethyl ester
The present invention discloses a production method of N-benzyl glycine ethyl ester. In the presence of catalyst and organic solvent it makes the benzyl chloride and glycine ethyl ester produce reaction to obtain N-benzyl glycine ethyl ester.
Owner:江苏优普生物化学科技股份有限公司
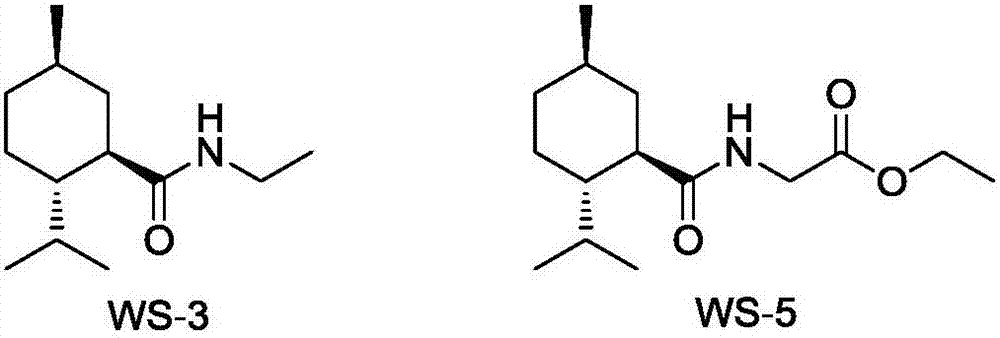
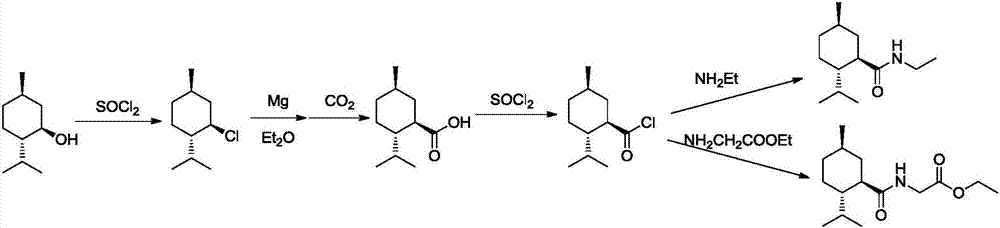
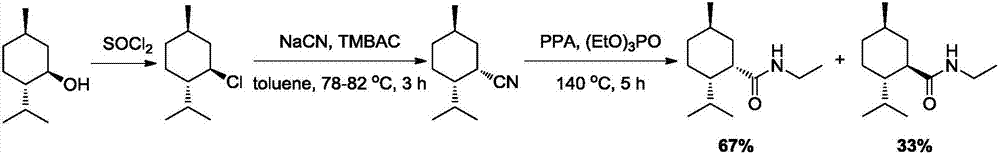
Preparation method of L-N-ethyl-p-menthane-3-carboxamide compound
ActiveCN106946729ALow priceObvious cost advantageOrganic compound preparationOrganic chemistry methodsDistillationGlycine ethyl ester
The invention relates to a preparation method of an L-N-ethyl-p-menthane-3-carboxamide compound. The preparation method comprises the following steps: (1) Friedel-Crafts reaction: preparing 2-isopropyl-5-methyl benzoyl chloride from cymene and phosgene; (2) condensation reaction: under the effect of alkalis, reacting RNH2 with 2-isopropyl-5-methyl benzoyl chloride to prepare 2-isopropyl-5-methyl benzoyl amide, wherein R represents -Et or -CH2COOEt; and (3) reduction reaction: hydrogenating 2-isopropyl-5-methyl benzoyl amide to obtain reaction products containing the L-N-ethyl-p-menthane-3-carboxamide compound. The reactions products are subjected to distillation, rectification, melting, and crystallization to obtain N-ethyl-2-isopropyl-5-methylcyclohexanecarboxamide or L-N-[[5-methyl-2-(1-methylethyl)cyclohexyl]carbonyl]glycine ethyl ester. The highest total yield is 18%, the raw material cost is reduced by 60%, compared with a conventional technology, and the cost advantage is obvious.
Owner:WANHUA CHEM GRP CO LTD
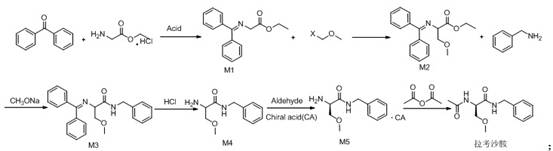
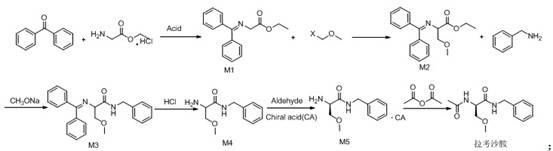
Synthetic route of lacosamide
ActiveCN112574058AAtom economy is highReduce usageOrganic compound preparationCarboxylic acid amides optical isomer preparationAcetic anhydrideChloroformic acid
The invention discloses a new synthesis route of lacosamide. The new synthesis route comprises the following steps: taking glycine ethyl ester hydrochloride as an initial raw material to react with methylbenzene, benzophenone and p-toluenesulfonic acid to obtain a compound of formula M1; reacting the compound of formula M1 with Xmethyl methyl ether to generate a compound of formula M2; reacting the compound of formula M2 with benzylamine under the catalytic action of sodium ethoxide to generate a compound of formula M3; reacting the compound of formula M3 under the action of acid to generate acompound of formula M4; reacting the compound of formula M4 with Ltartaric acid to generate a compound of formula M5; and enabling the compound of formula M5 to react with acetic anhydride to generate the lacosamide compound. The synthesis route has the advantages that the atom economy is high, the use of isopropyl chloroformate highly toxic products for preparing amide is avoided, the use of methylation reagents methyl iodide or dimethyl sulfate is avoided, the yield is high, and the like.
Owner:珠海润都制药股份有限公司
Preparation methods of N-phenylacetyl-L-proline, and N-(1-(phenylacetyl)-L-prolyl)glycine ethyl ester
The invention provides a preparation method of N-phenylacetyl-L-proline. The preparation method comprises following steps: 1) L-proline is mixed with a strong base solution a, and phenylacetyl chloride and a strong base solution b are added via double dropwise adding for reaction; 2) after reaction of the step 1), ethyl acetate is used for extraction, and an ethyl acetate layer is removed so as to obtain a product aqueous layer; 3) pH value of the product aqueous layer obtained via step 2) is adjusted to be lower than 7, dichloromethane is used for extraction, and a water layer is removed; and 4) a neutral drying agent is added, an obtained mixture is filtered, and an obtained filtrate is subjected to distillation and cooling so as to obtain N-phenylacetyl-L-proline. The invention also discloses a preparation method of N-(1-(phenylacetyl)-L-prolyl)glycine ethyl ester. Operation of the preparation methods is simple; and preparation efficiency is high.
Owner:SHANGHAI XUXIN CHEM
Fenofibric acid glycine ethyl ester crystal form and preparation method thereof
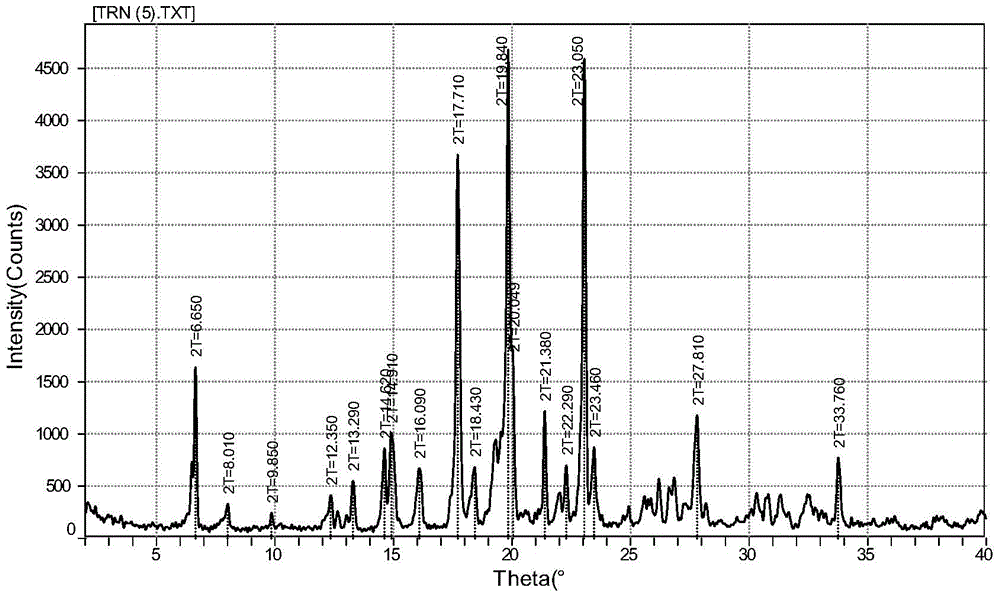
InactiveCN104803842AImprove stabilityImprove bioavailabilityMetabolism disorderOrganic compound preparationSecondary hyperlipidemiaFENOFIBRIC ACID
The invention discloses a fenofibric acid glycine ethyl ester crystal form and a preparation method thereof. The crystal form is the fenofibric acid glycine ethyl ester crystal form shown in a structural formula (I) and is represented through a 2theta angle in an X-ray powder diffraction map, and X-ray powder diffraction peaks exist at the angles of 6.650 degrees, 8.010 degrees, 9.850 degrees, 12.350 degrees, 13.290 degrees, 14.620 degrees, 14.910 degrees, 16.090 degrees, 17.710 degrees, 18.430 degrees, 19.840 degrees, 20.049 degrees, 21.380 degrees, 22.290 degrees, 23.050 degrees, 23.460 degrees, 27.810 degrees and 33.760 degrees. The fenofibric acid glycine ethyl ester crystal form has high stability, the stability and the bioavailability of fenofibric acid glycine ethyl ester are greatly improved, and the fenofibric acid glycine ethyl ester has a broad application prospect in the hyperlipidemia treatment process.
Owner:TETRANOV PHARMA CO LTD
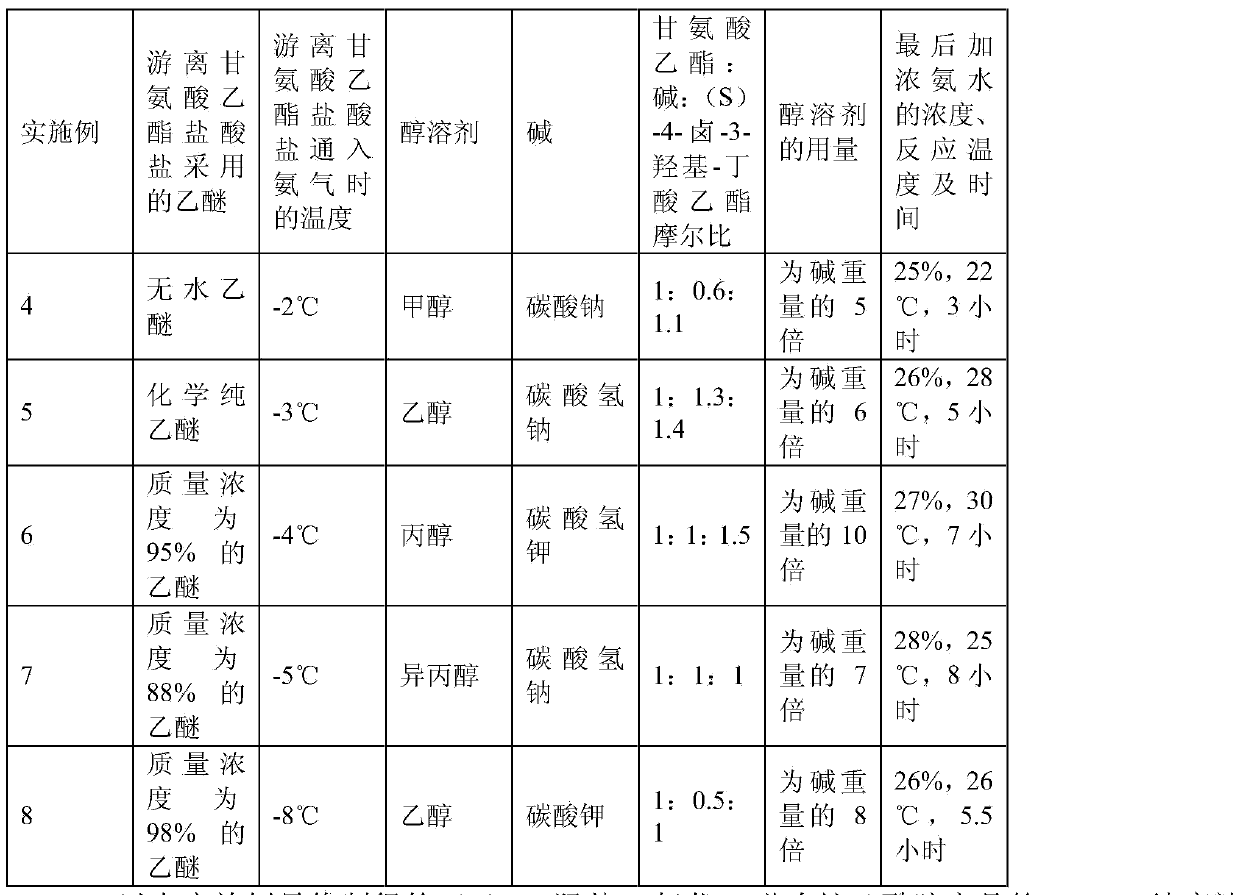
Preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
The invention discloses a preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide. The method comprises the following steps: reacting glycine ethyl ester hydrochloride and (S)-4-halo-3-hydroxy-ethyl butyrate serving as raw materials in the presence of an alcohol solvent under an alkaline condition; filtering, washing, concentrating, extracting, separating, introducing ammonia water to obtain a crude product, and purifying the crude product, wherein the glycine ethyl ester hydrochloride is dissociated into glycine ethyl ester by using diethyl ether and ammonia gas, the alcohol solvent is absolute methanol or absolute ethyl alcohol, and the alkali is sodium carbonate or sodium bicarbonate. The (S)-4-halo-3-hydroxy-ethyl butyrate and the glycine ethyl ester hydrochloride are taken as major raw materials, so that the raw materials are cheap, readily available and environmentally friendly; the glycine ethyl ester hydrochloride is dissociated, so that the using quantities of the materials in reaction are reduced, the cost is reduced, and meanwhile the reaction yield is increased. The preparation method is low in preparation cost, the yield can be up to 36 percent, the reaction conditions are mild, the industrial mass production is facilitated, and the HPLC (High Performance Liquid Chromatography) purity of an obtained (S)-oxiracetam product is over 98.5 percent.
Owner:CHONGQING RUNZE PHARM CO LTD
Method for preparing (S)-oxiracetam
A method for preparing (S)-oxiracetam comprises the steps of conducting a reaction of a glycine ethyl ester hydrochloride with (S)-4-halogen-3-hydroxy-ethyl butyrate under an alkaline condition, wherein the glycine ethyl ester hydrochloride and the (S)-4-halogen-3-hydroxy-ethyl butyrate are adopted as raw materials, filtering, washing filtrate with inorganic alcohol, concentrating, then extracting, separating and introducing ammonia water to prepare a crude product, and purifying the crude product; and dissociating the glycine ethyl ester hydrochloride into glycine ethyl ester by diethyl ether and ammonia gas firstly. Purification treatment comprises the steps that the crude product of (S)-oxiracetam is dissolved in a benign solvent to prepare a saturated solution, and then the saturated solution is dispersed in a closed environment by a poor solvent. According to the method, the main raw materials are low in cost, easy to obtain and environment-friendly; the glycine ethyl ester hydrochloride is adopted for dissociation, so that the using amount of the materials in the reaction is reduced effectively, the cost is lowered, and in addition, the glycine ethyl ester hydrochloride plays an active role in the reaction yield. The HPLC (high-performance liquid chromatography) purity of the prepared (S)-oxiracetam reaches up to more than 99.0%, the yield is high and reaches up to 36%, the reaction condition is moderate, the operation is simple, and the industrialized scale production is facilitated.
Owner:CHONGQING RUNZE PHARM CO LTD
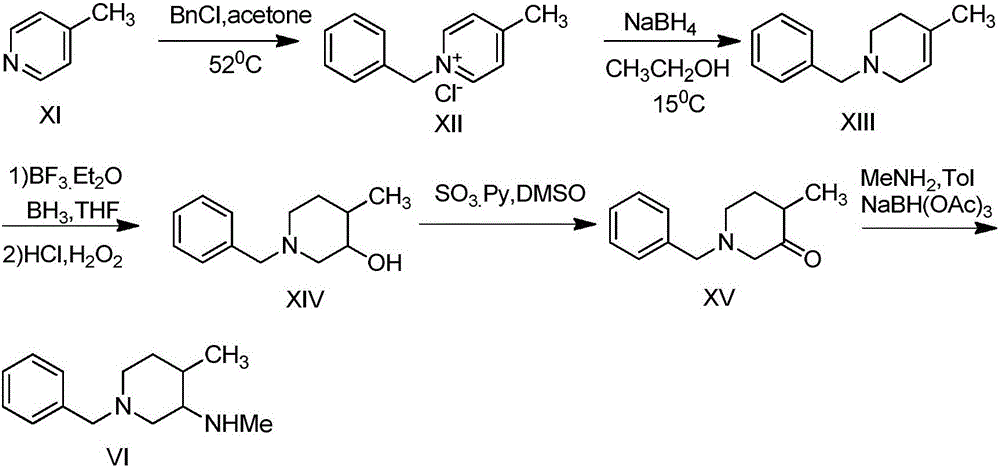
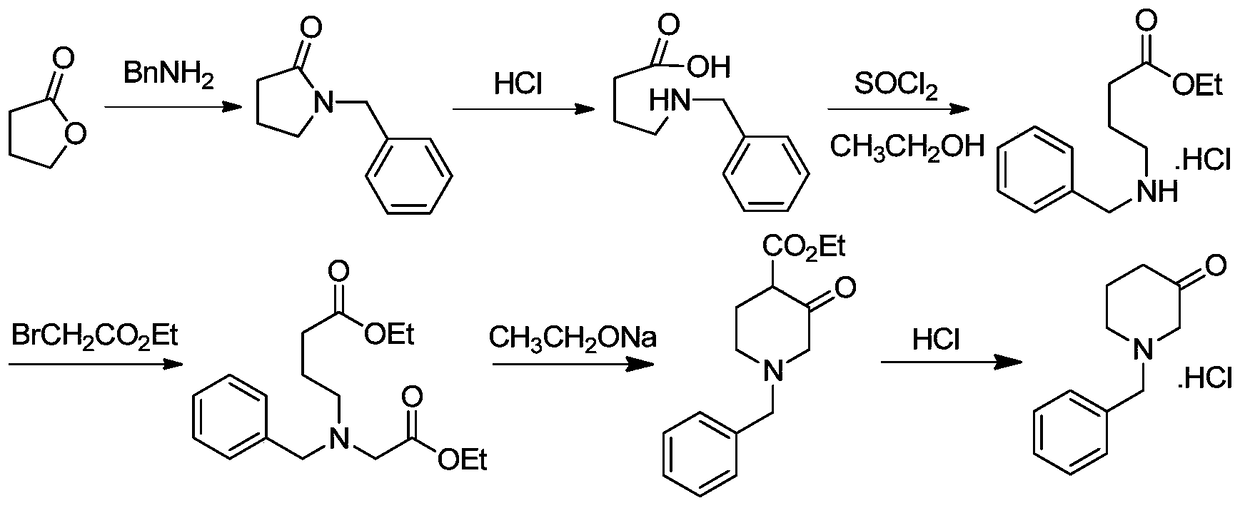
Synthesis method of N-benzyl-4-methylpiperidine-3-one hydrochloride
The invention discloses a synthesis method of N-benzyl-4-methylpiperidine-3-one hydrochloride. The synthesis method includes the steps of: a) with N-benzyl glycine ethyl ester as a raw material, performing a condensation reaction to the raw material with 2-methyl-4-halogenated ethyl acetate; and b) after the reaction is finished, performing an intramolecular cyclization reaction and finally performing hydrolytic decarboxylation to prepare the product. Compared with methods reported in references, the method establishes a novel synthesis route which is short in process, is novel in technology, employs low-cost raw materials and simple process operations, has good product quality and high total yield, and is suitable for industrial production.
Owner:CHONGQING WORLD HAORUI PHARM CHEM
The preparation method of 1-benzyl-3-piperidone hydrochloride
ActiveCN105622444BLow costHigh purityOrganic compound preparationAmino-carboxyl compound preparationAcetic acidOrganic solvent
The invention discloses a preparation method for N-benzyl glycine ethyl ester. The method comprises the following steps: dissolving benzylamine in an organic solvent I, then adding 2-halogenated ethyl acetate, alkali and quaternary ammonium salt, and performing reaction to obtain the N-benzyl glycine ethyl ester. The invention also discloses a preparation method for 1-benzyl-3-piperidone hydrochloride. The method comprises the following specific steps: (1) preparing an intermediate IV (N-benzyl glycine ethyl ester); (2) dissolving the intermediate IV in an organic solvent II, then adding 4-halogenated ethyl acetate and alkali, and performing reaction to obtain an intermediate III; (3) performing reaction between the intermediate III and the alkali, reversely regulating a pH value to 6-8, performing concentration under reduced pressure, extracting by using ethyl acetate, performing washing and drying, and then performing concentration under reduced pressure to obtain an intermediate II; (4) performing reaction on the intermediate II and acid, performing rotary evaporation concentration, and adding a crystal solvent for crystallization to obtain a product. The route synthesis steps are short, the process is novel, the intermediates are high in purity, the product yield is high, and the cost is low.
Owner:CHONGQING WEIPENG PHARMA
Absorbable medical hemostatic material and preparation process thereof
InactiveCN106139236AShort hemostatic timeHigh hemostasis rateSurgical adhesivesPharmaceutical delivery mechanismBiocompatibility TestingIn vivo
The invention discloses an absorbable medical hemostatic material and a preparation process thereof. The hemostatic material is prepared from the following components: fish scale collagen protein, maltodextrin, oligomannuronate, potassium alginate, D-threitol, polycaprolactone, 0.7 mol / L radix angelicae and alcohol extract, oxaloacetic acid, carboxyl glucan, gamma-polyglutamic acid, glycine ethyl ester, sodium D-glucuronate, microcrystalline cellulose 101, L-pentaguluronic acid pentasodium salt, citric acid and distilled water. The hemostatic material provided by the invention can stop bleeding quickly, is high in hemostasis rate, is higher in degrading speed in vivo, is non-toxic and high in biocompatibility, does not have immunoreactions, is high in capacity of healing and recovering a wound, can be used safely and reliably, and has a good market application prospect.
Owner:林春梅
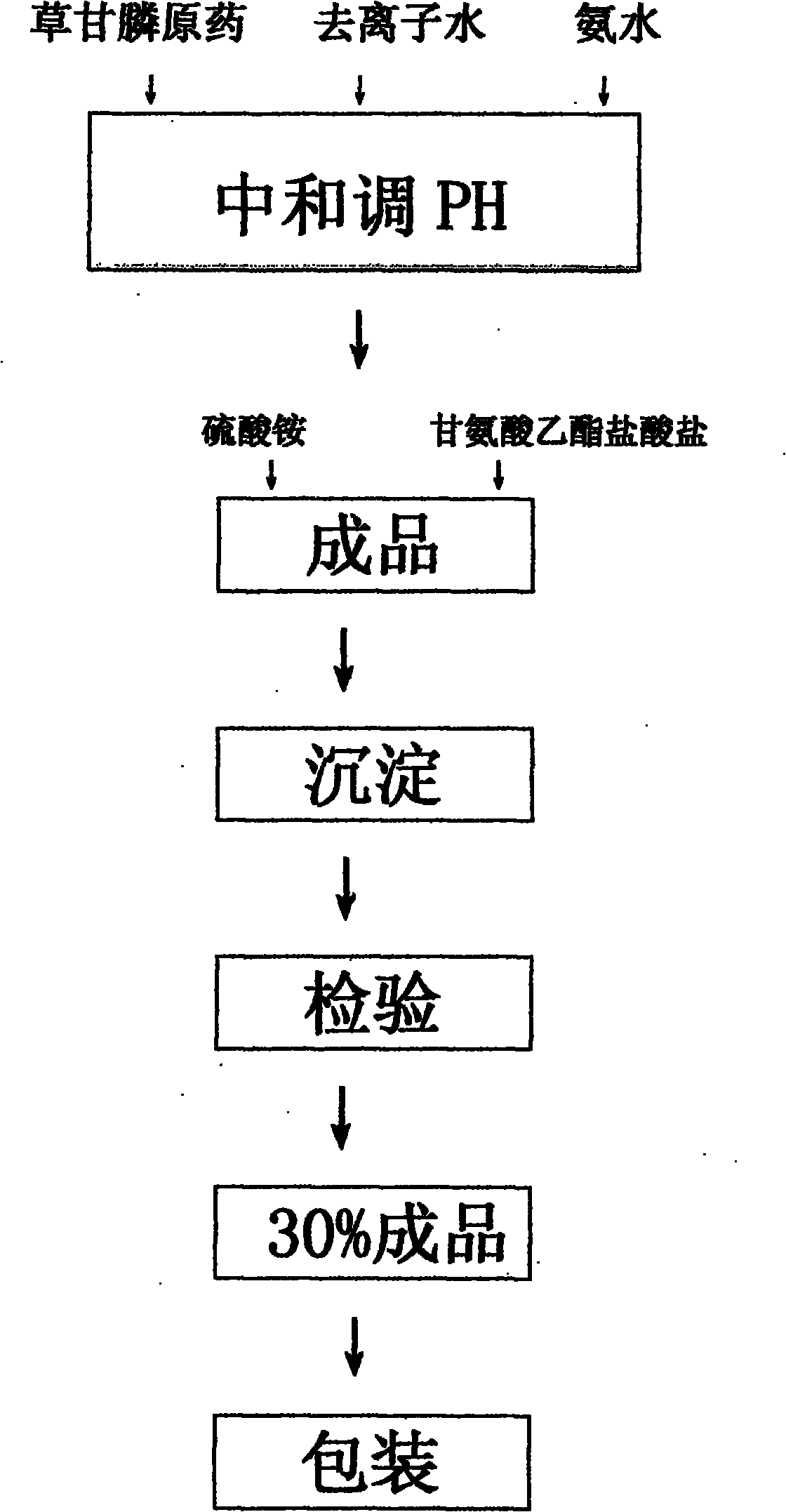
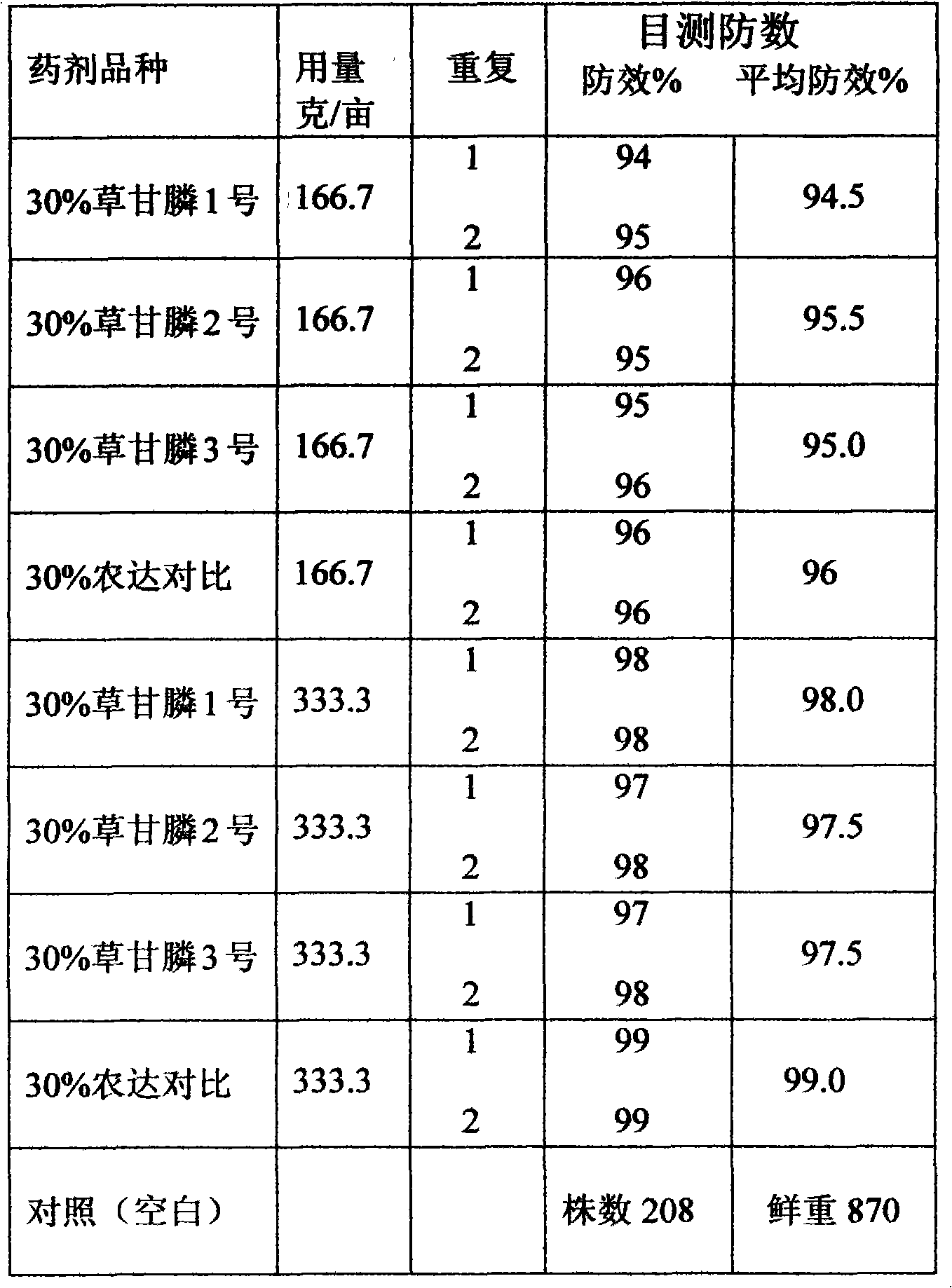
Method for preparing gyphosate solution from glyphosate raw material
InactiveCN102067879ANo pollution in the processChange dependenciesBiocideAnimal repellantsBULK ACTIVE INGREDIENTSolvent
The invention relates to a method for preparing gyphosate solution from glyphosate raw material, comprising the following steps of: preparing materials according to 30% of gyphosate solution per ton, and feeding raw material comprising glyphosate of 95% of 320 kg; then taking deionized water of 160 L as a solvent, dropping industrial ammonia water with a concentration of 15% to 25% in stirring condition, and controlling the temperature between 35 DEG C and 60 DEG C for about half an hour; and then preserving the temperature between 50 DEG C and 65 DEG C for one hour, regulating the Ph value of the product to a scope of 6.5 to 7.0 when the reaction is ended, feeding ammonium sulfate of 50 kg as a solubilizer and a synergist, feeding glycine ethyl ester hydrochloride of 40 kg as an assistant, and finally feeding an industrial general pigment and diluting by using deionized water so as to obtain a gyphosate solution product with an active ingredient content of 30%. Compared with conventional method, the method provided by the invention has the advantages that the production device is simple, the reaction conditions are easy to be controlled, the security is high, the energy consumption is low, the yield is high, the three wastes are less and are easy to be treated, the economic benefit is obvious, and the method is suitable for the production of enterprises.
Owner:陈运谋
Method for preparing (S)-4-hydroxy-2-oxo-1-pyrrolidine acetamide
The invention discloses a method for preparing (S)-4-hydroxy-2-oxo-1-pyrrolidine acetamide. The method comprises the following steps: reacting glycine ethyl ester hydrochloride and (S)-4-halo-3-hydroxy-ethyl butyrate as raw materials in an alcohol solvent under an alkaline condition, washing by inorganic alcohol, concentrating, subsequently separating, feeding ammonia water so as to obtain a crude product, and performing purification treatment on the crude product, wherein the glycine ethyl ester hydrochloride needs to be freed into glycine ethyl ester by diethyl ether and ammonia gas. The (S)-4-halo-3-hydroxy-ethyl butyrate and glycine ethyl ester hydrochloride are used as the main raw materials which are low in price, easily available, environmentally friendly and free of pollution; as the glycine ethyl ester hydrochloride is firstly subjected to free treatment, the use amount of the raw materials in the reaction is effectively reduced, the cost is lowered, and meanwhile, the yield of the reaction is beneficially improved. With the method, the cost for preparing (S)-oxiracetam is low, and the yield can be as high as 36%; the reaction condition is mild, and thus the method is beneficial to industrial scale production; the HPLC (High Performance Liquid Chromatography) purity of the prepared (S)-oxiracetam product is greater than 98.5%.
Owner:CHONGQING RUNZE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com