Preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
A technology for pyrrolidineacetamide and hydroxyl, which is applied in the field of preparation of -4-hydroxy-2-oxo-1-pyrrolidineacetamide, and can solve the problems of increasing reaction steps, low synthesis yield, and decreased total yield , to achieve the effect of short cycle, low pollution and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
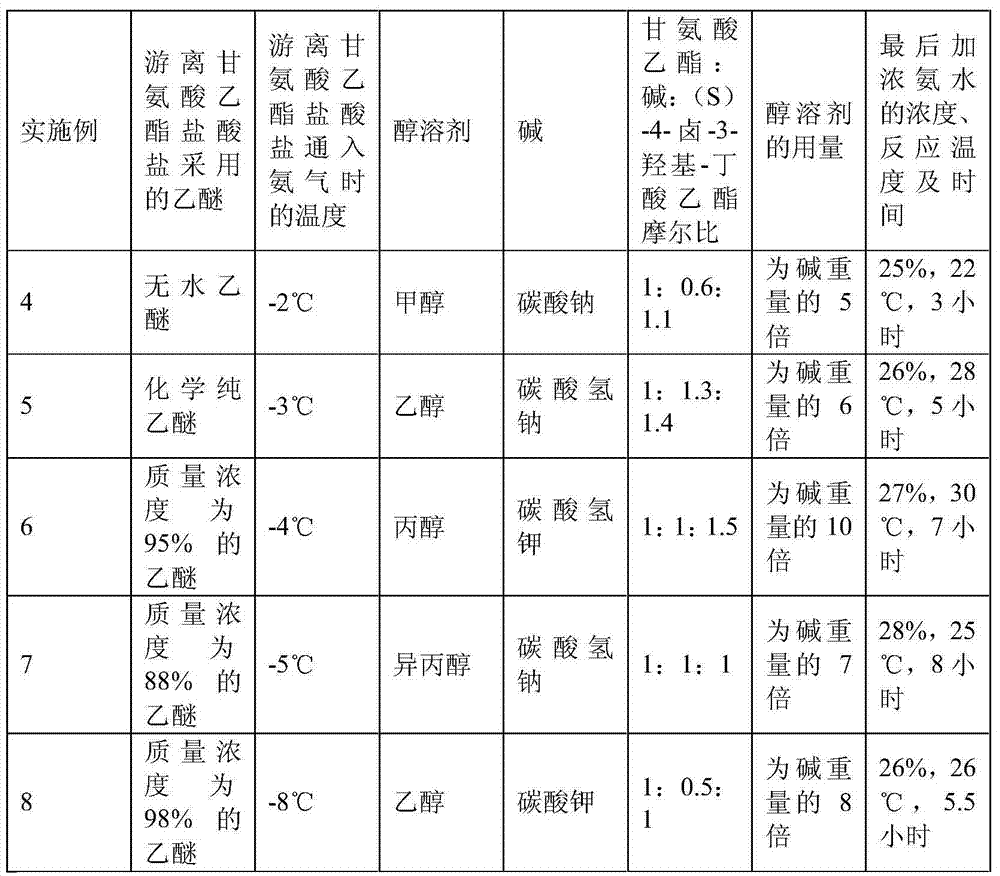
[0025] The preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide is carried out as follows:
[0026] 1. Preparation of crude product:
[0027] (a) Add 139.6 g of ethyl glycine hydrochloride to 1,300 ml of anhydrous ether, cool to -2°C and blow in 22.1 g of ammonia gas to dissociate ethyl glycine hydrochloride into ethyl glycine, wherein ethyl glycine hydrochloride Salt: anhydrous ether: 1mol of ammonia: 1300m1: 1.3mol;
[0028] (b) Add absolute ethanol 672ml, sodium bicarbonate 84.0g, dropwise (S)-4-chloro-3-hydroxyl-butyric acid ethyl ester 250.0g in the above-mentioned product, and described dropping time is 2.5 hours, in React at pH 8.2 and temperature 66°C for 28 hours;
[0029] (c) Filtrate, fully wash the filtrate with ethanol, concentrate, dissolve the concentrate in water, add ethyl acetate 7 times the weight of the filtrate to extract, concentrate the water phase, and separate by column chromatography; the final mass percentage concentration is 26% Aque...
Embodiment 2
[0036] The preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide is carried out as follows:
[0037] 1. Preparation of crude product:
[0038] (a) Add glycine ethyl ester hydrochloride to anhydrous ether, cool to 1°C and pass through ammonia gas to dissociate glycine ethyl ester hydrochloride into glycine ethyl ester, wherein glycine ethyl ester hydrochloride: anhydrous ether: Ammonia is 1mol: 1395ml: 1.5mol;
[0039] (b) Add anhydrous methanol, sodium carbonate, (S)-4-iodo-3-hydroxy-butyric acid ethyl ester to the above product, and react for 30 hours at a pH of 8 and a temperature of 70°C;
[0040] (c) Filtrate, fully wash the filtrate with ethanol, concentrate, dissolve the concentrate in water, then add 6 times the weight of the filtrate in dichloromethane for extraction, concentrate the water phase, and separate by column chromatography; finally add a mass percentage concentration of 25% Aqueous ammonia was reacted at 20° C. for 5 hours to obtain the crude p...
Embodiment 3
[0046] The preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide is carried out as follows:
[0047] 1. Preparation of crude product:
[0048] (a) ethyl glycine hydrochloride is suspended in chemically pure ether, then feed ammonia to make ethyl glycine hydrochloride free into ethyl glycine;
[0049] (b) Add absolute ethanol, sodium bicarbonate, dropwise (S)-4-bromo-3-hydroxy-butyric acid ethyl ester to the above product;
[0050] (c) Then filter, fully wash the filtrate with ethanol, concentrate, dissolve the concentrate in water, then add 4 times the weight of the filtrate in chloroform for extraction, concentrate and separate the water phase; finally add concentrated ammonia water and react at 25°C for 6 hours to prepare Obtain the crude product of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide;
[0051] 2. Purification of crude product:
[0052] (a) dissolve the above-mentioned crude product with water, pass through 732# strongly acidic cation exchange resin, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com