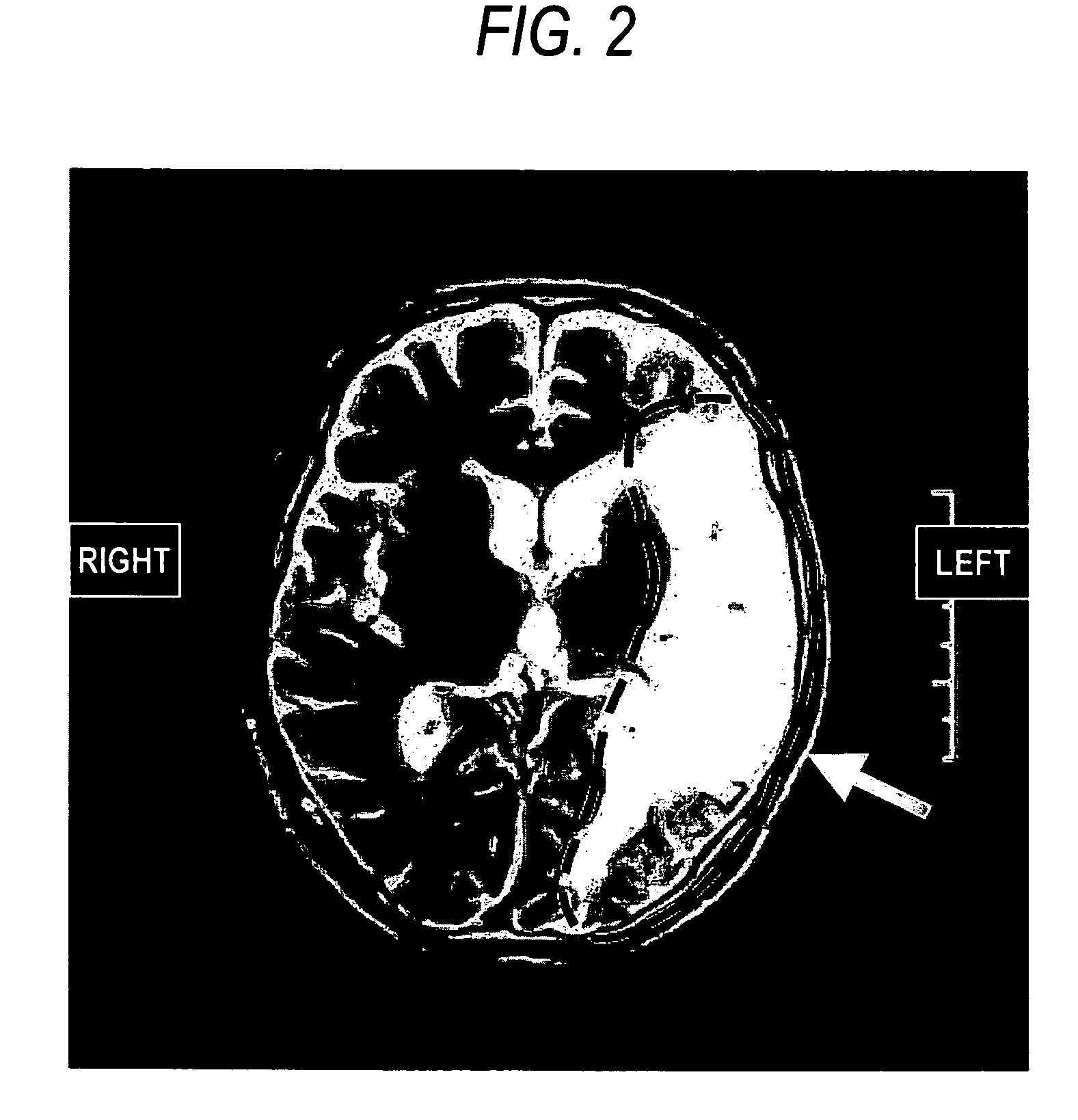
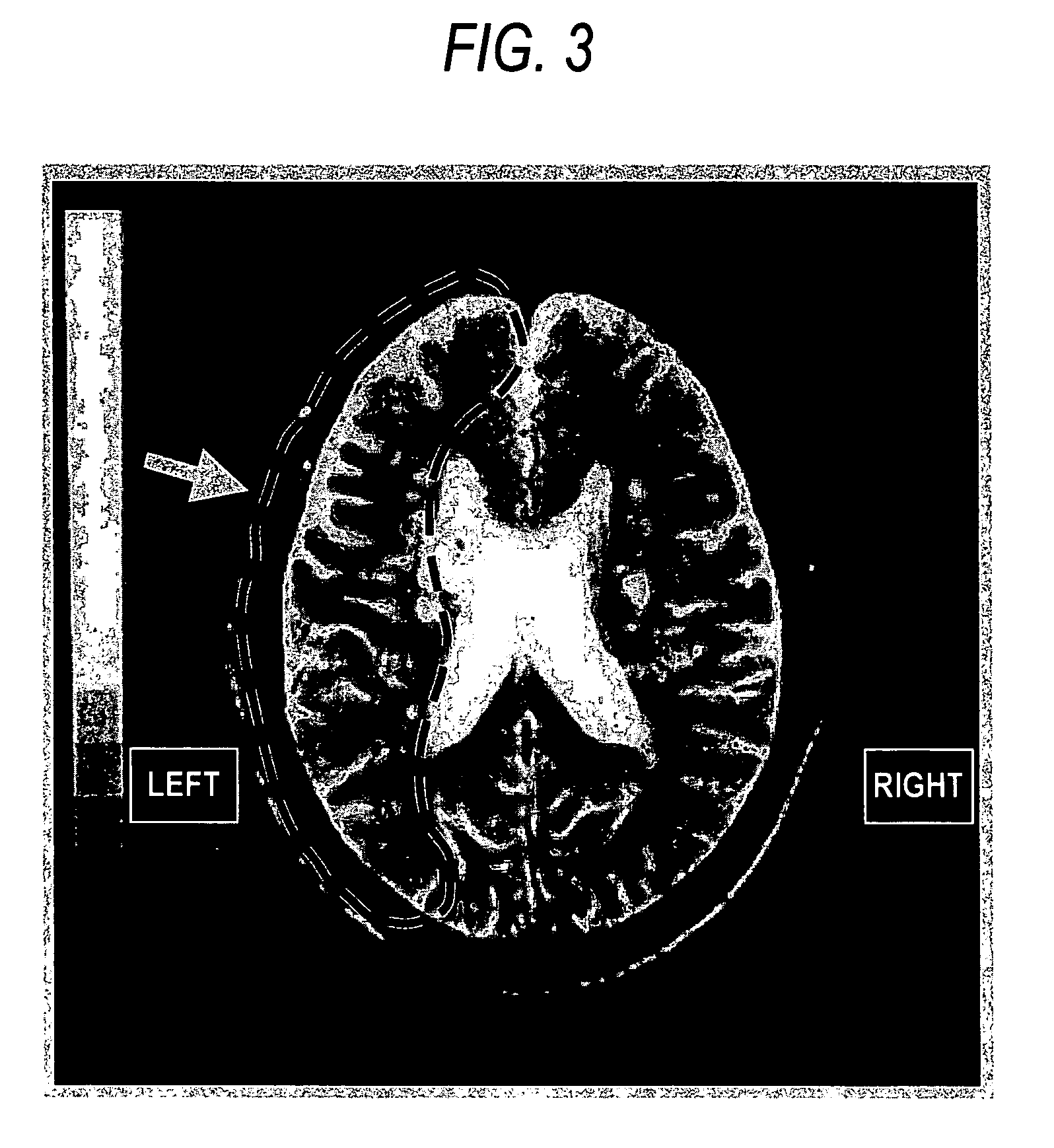
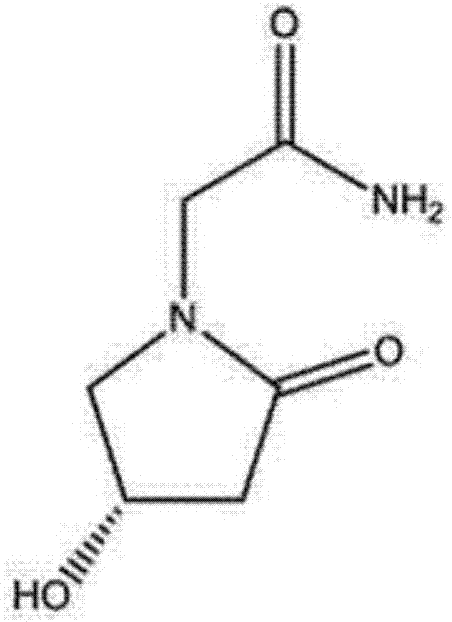
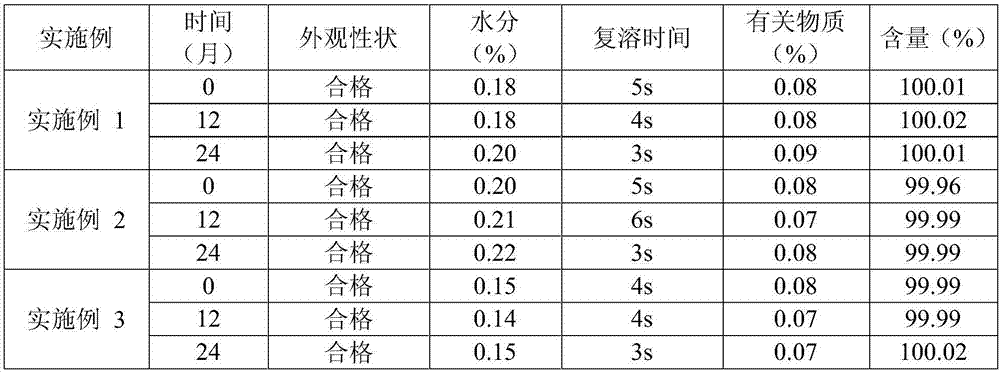
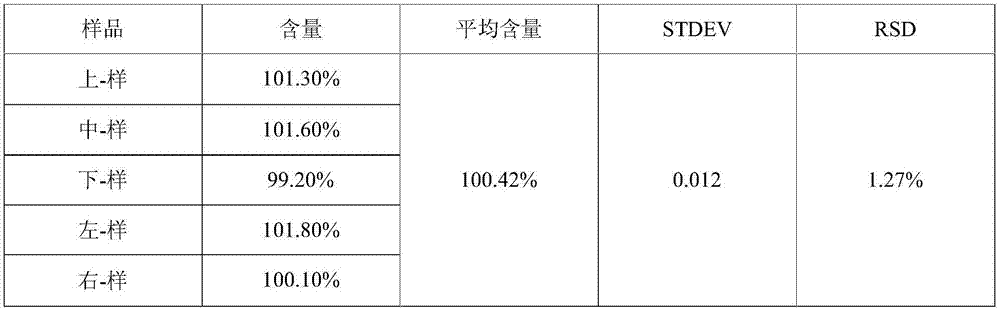
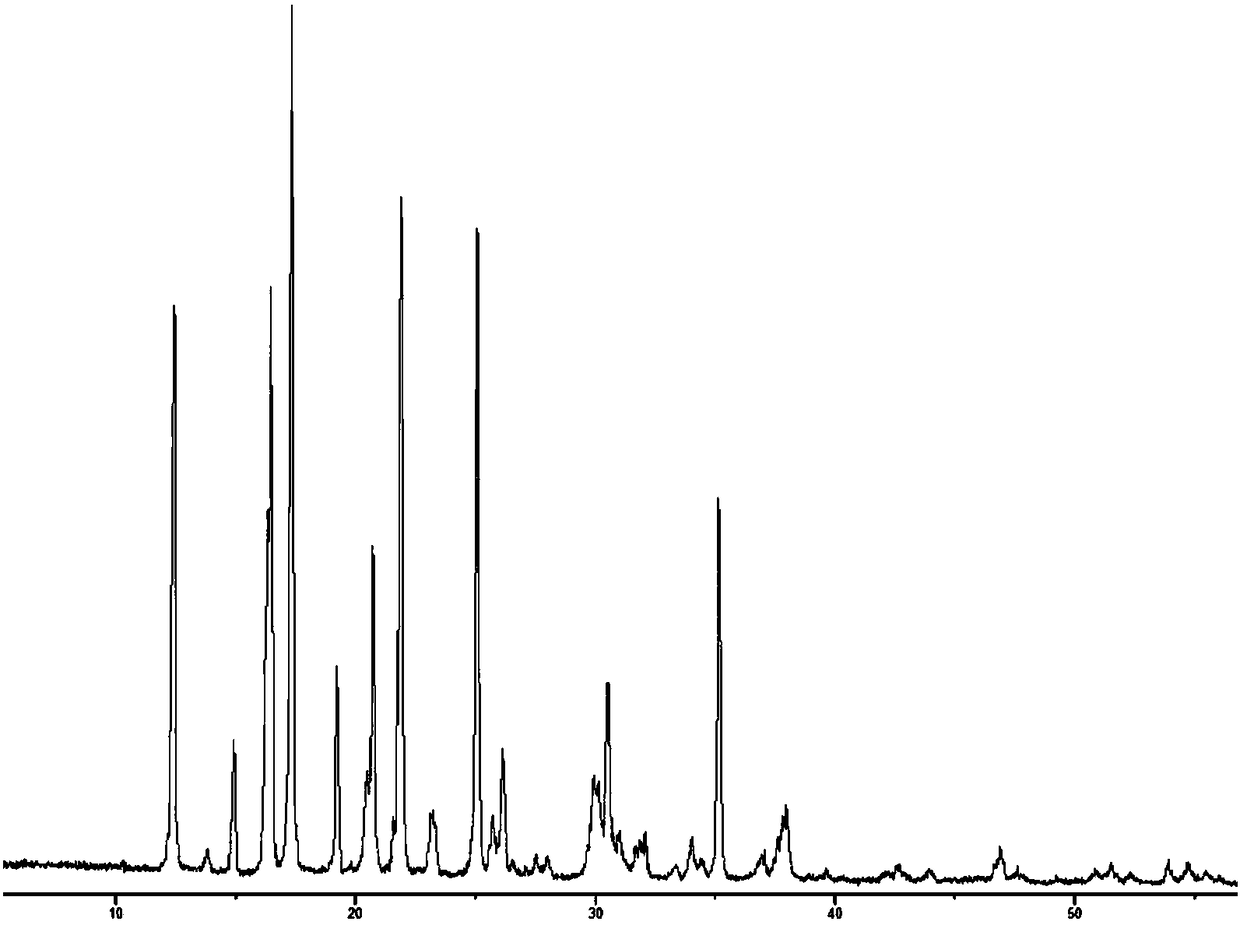
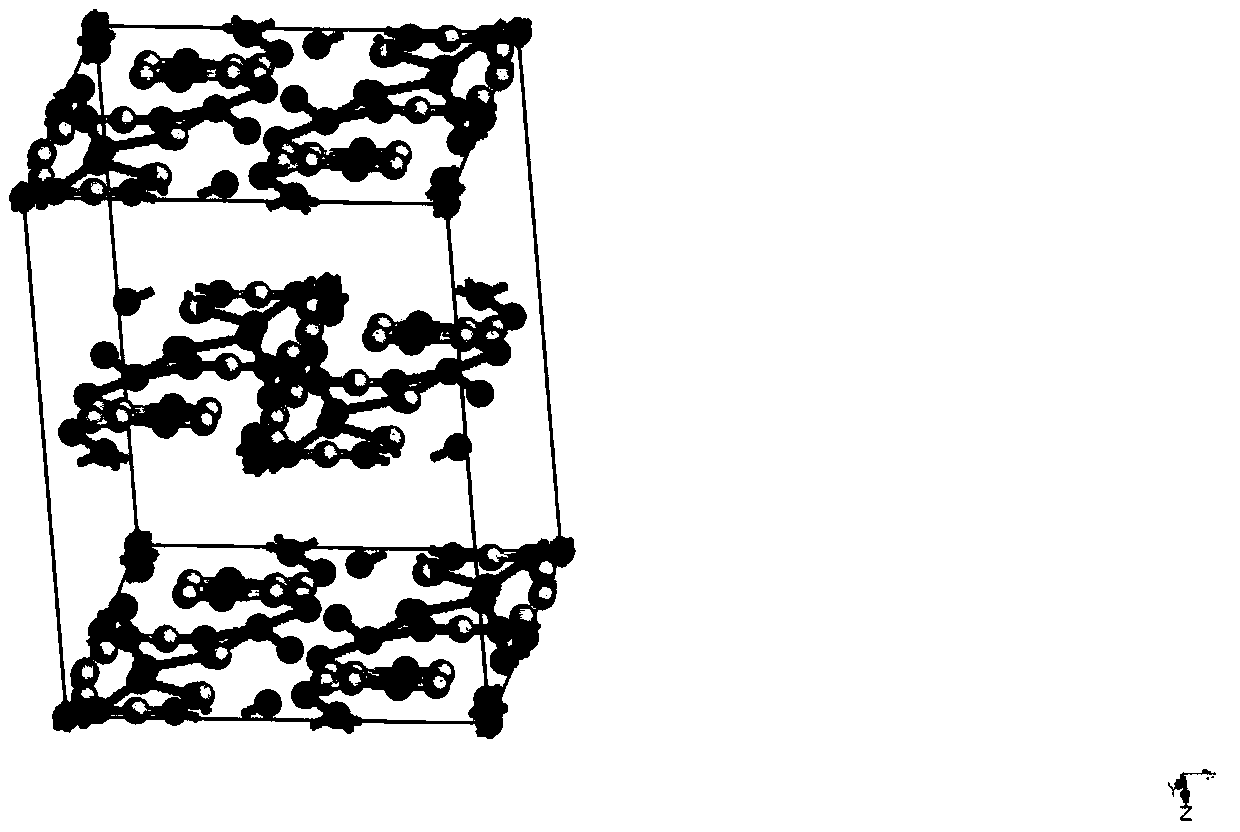
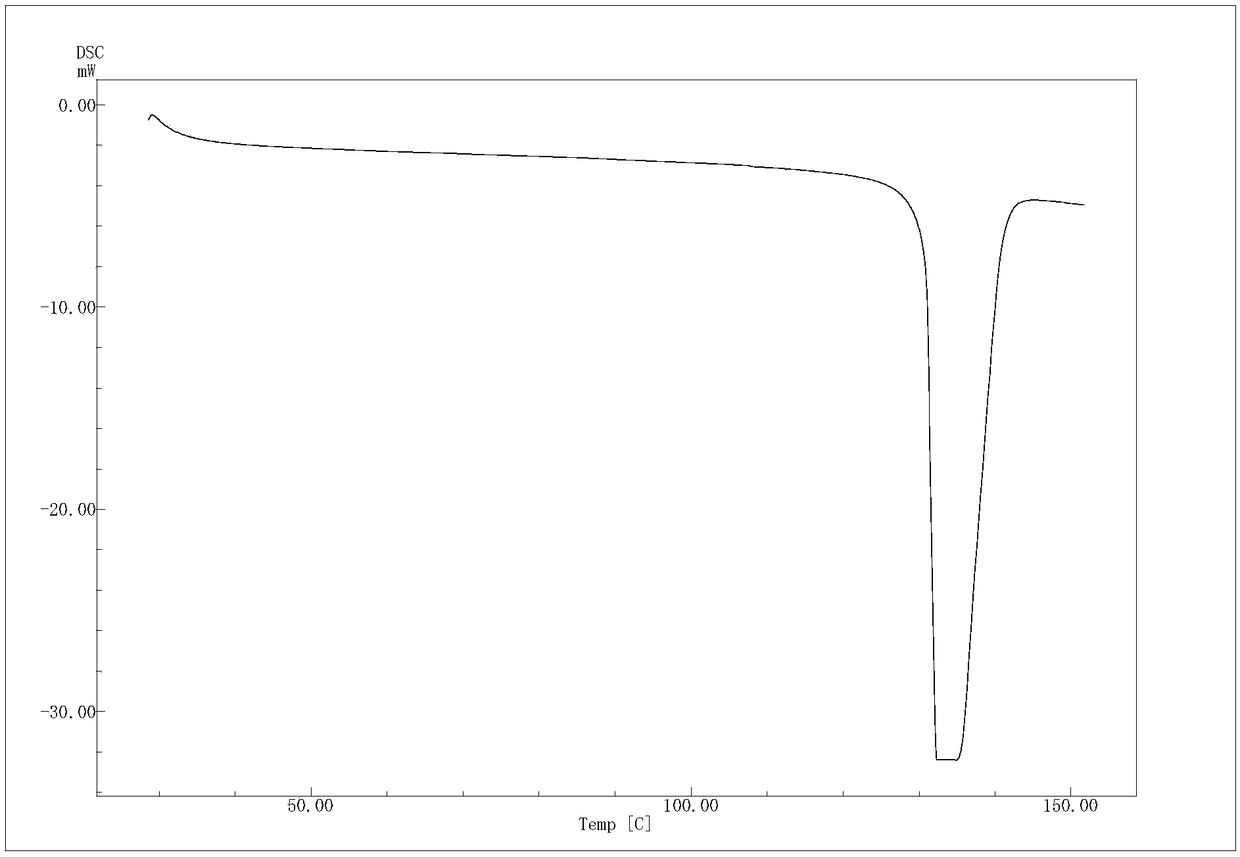
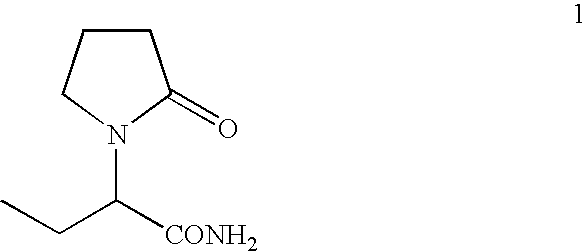Patents
Literature
36 results about "1-pyrrolidineacetamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for synthesizing high-purity levetiracetam
InactiveCN101885696AHigh optical purityHigh chemical purityOrganic chemistryChemical synthesisAcetic acid
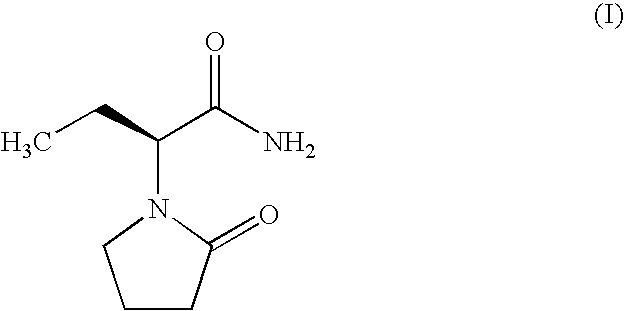
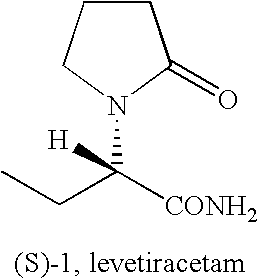
The invention relates to a method for synthesizing high-purity levetiracetam, belongs to the field of chemical synthesis and particularly relates to a method for preparing high-purity levetiracetam by substituting diisopropylethylamine for triethylamine in a reaction for preparing (S)-a-ethyl-2-oxo-1-pyrrolidineacetamide (levetiracetam) by ammoniation of (S)-a-ethyl-2-oxo-1-pyrrolidineacetic acid.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
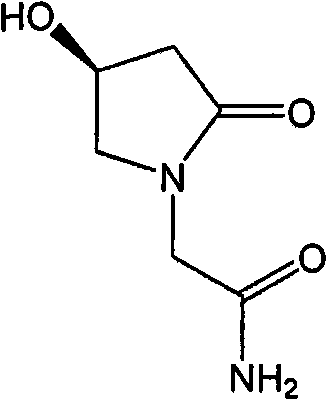
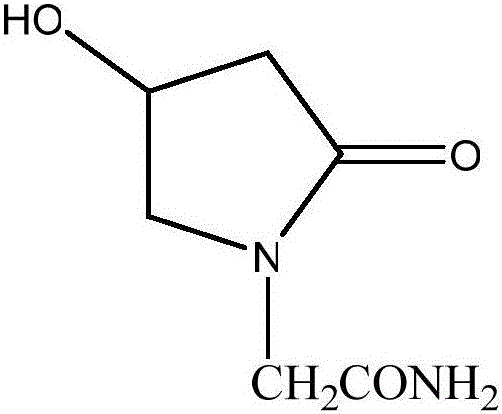
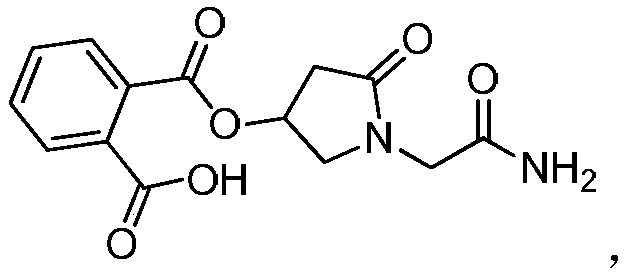
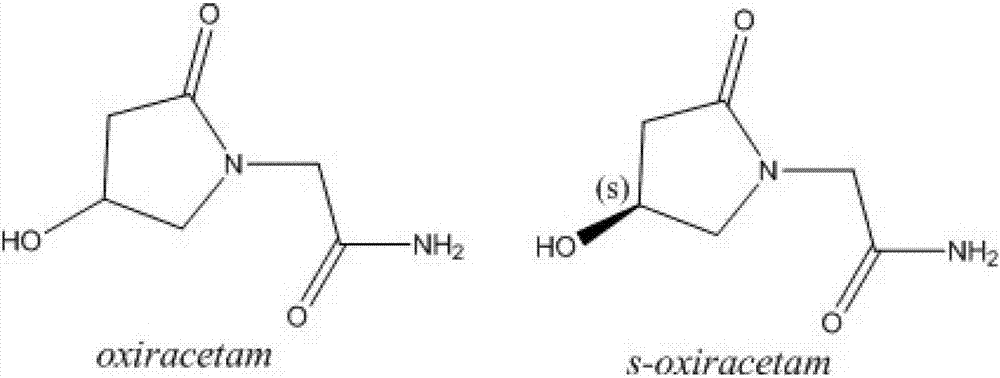
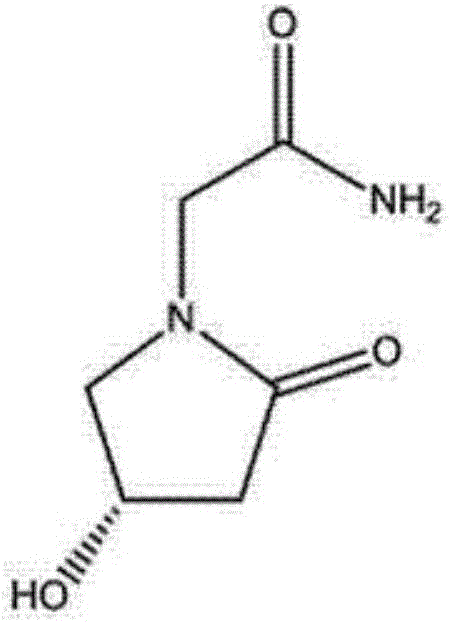
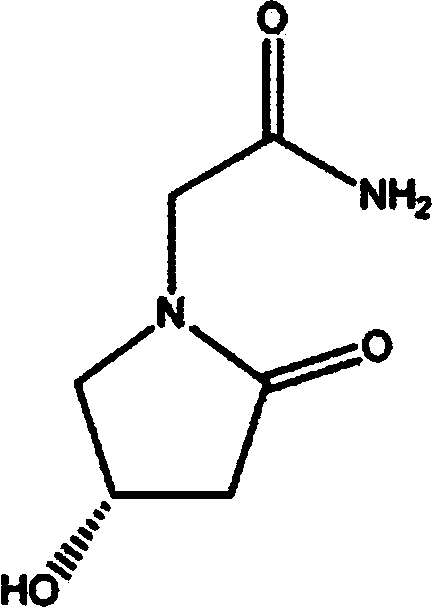
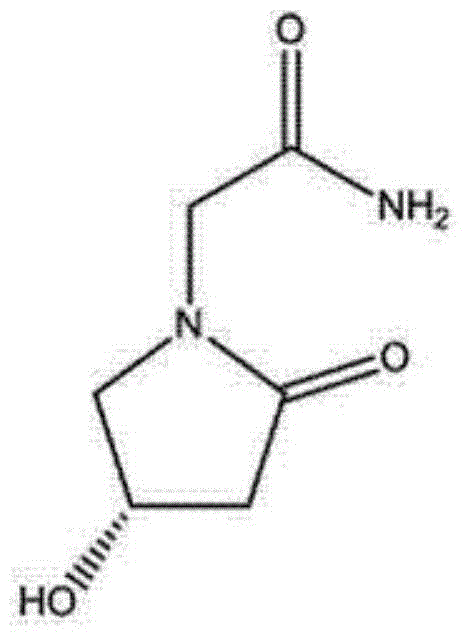
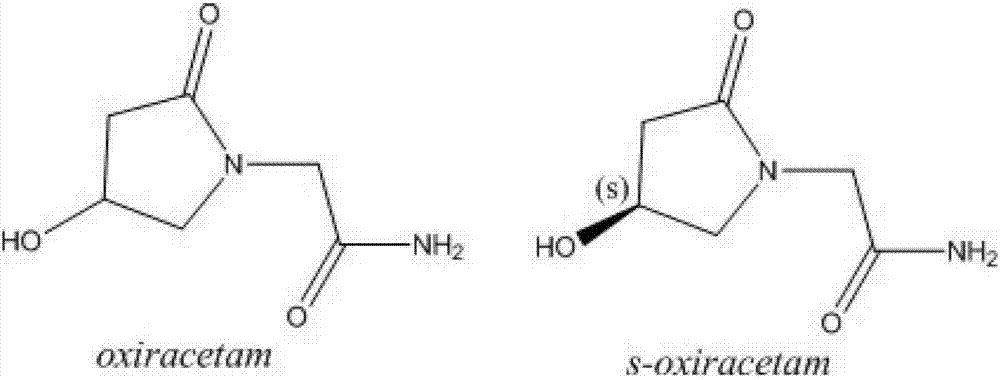
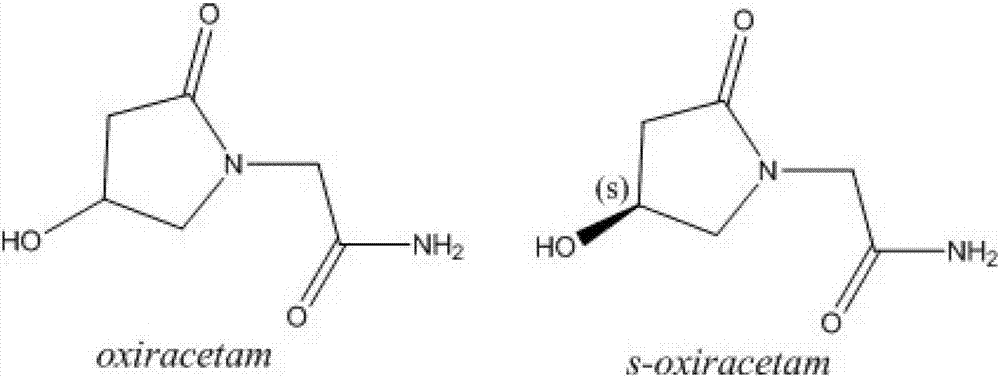
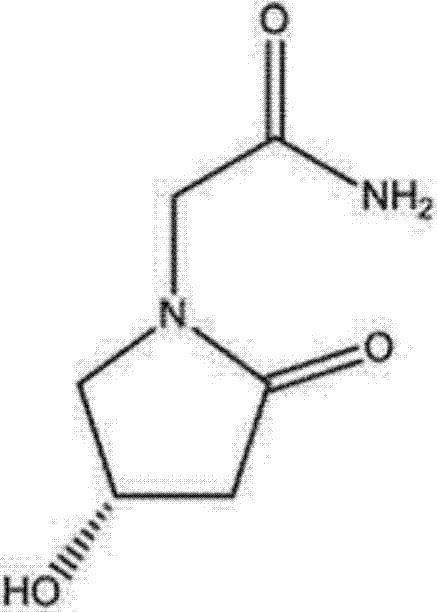
Preparation method of (s)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
A preparation method of (s)-4-hydroxy-2-oxo-1-pyrrolidineacetamide. The preparation method comprises the following steps of crude product preparation and crystallization, wherein the step of crystallization adopts acetone and water as solvents. Levo-oxiracetam prepared through the preparation method has high purity above 99.3 wt% and low impurity content of 0 to 0.5 wt%. The preparation method has the advantages that a material addition mode adopted by the preparation method realizes that frequency of addition of an inorganic base is reduced; operation is simple; and the preparation method is in favor of industrialized production.
Owner:CHONGQING RUNZE PHARM CO LTD
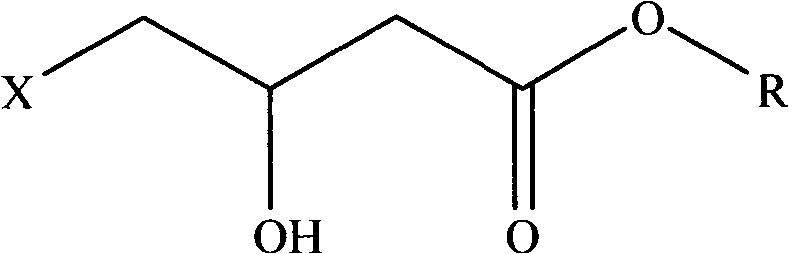
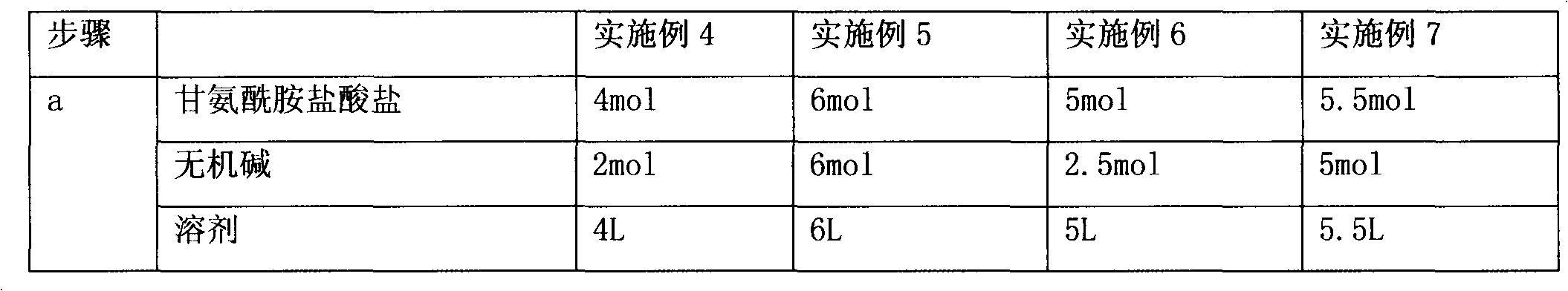
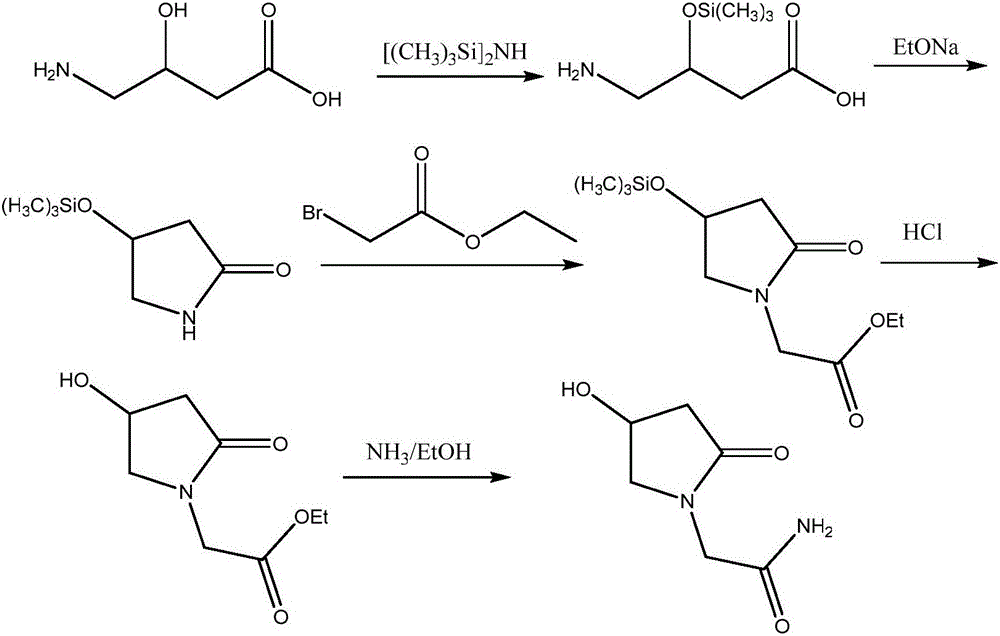
Preparation method of (R,S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
InactiveCN102690222ALower synthesis costReduce loadOrganic chemistry1-pyrrolidineacetamidePyrrolidine
The invention provides a preparation method of (R,S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide. The preparation method comprises that (R,S)-4-halogeno-3-hydroxybutyrate as a raw material undergoes a reaction in the presence of one or more polar solvents under alkaline conditions to produce a crude product of (R,S)-4-hydroxy-2-oxo-1-pyrrolidinylacetamide and the crude product of (R,S)-4-hydroxy-2-oxo-1-pyrrolidinylacetamide is purified. The preparation method is characterized in that through using an inorganic base of which molar weight is 1 to 1.2 times molar weight of glycinamide hydrochloride in the reaction under alkaline conditions in batches or at one time, the alkaline conditions for keeping the reaction can be controlled so that the reaction is fully and unnecessary waste caused by the large excess of the inorganic base is prevented; and when a target product of (R,S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide ((R,S)-oxiracetam) produced by a reflux reaction in one or more polar solvents under ordinary pressure has the maximum concentration, the reaction is stopped or is blocked and then continues at a temperature of 90 DEG C for 1 hour. Through pressurization, a reaction yield is improved by about 15% and a cost is greatly reduced.
Owner:TIANJIN JIUHAI MEDICAL TECH
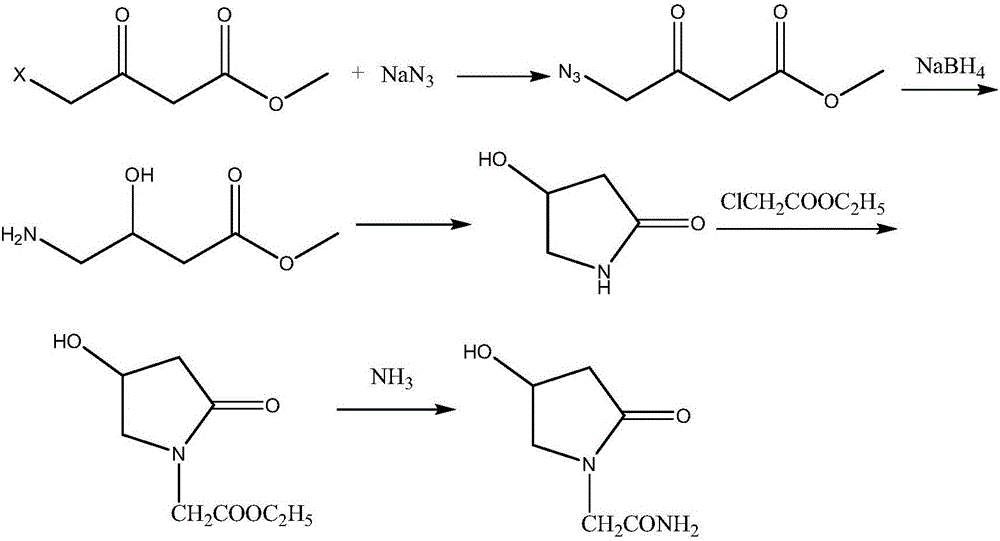
Oxiracetam synthesis technology
The invention belongs to the field of pharmaceutical chemicals and particularly relates to an oxiracetam synthesis technology, ethyl acetoacetate and glycine are taken as the raw materials, the ethyl acetoacetate is changed into 4-halogeneated ethyl acetoacetate by halogenation reaction, the 4-halogeneated ethyl acetoacetate and glycine ester formed by the glycine are cyclized to form 2,4-dioxo-1-pyrrolidine acetate, and 4-hydroxy-2-oxo-1-pyrrolidineacetamide is obtained after hydrolysis and aminolysis. According to the oxiracetam synthesis technology, the related raw materials are easy to obtain, and the synthesis technology is simple and has good industrial values.
Owner:WUHAN INSTITUTE OF TECHNOLOGY +1
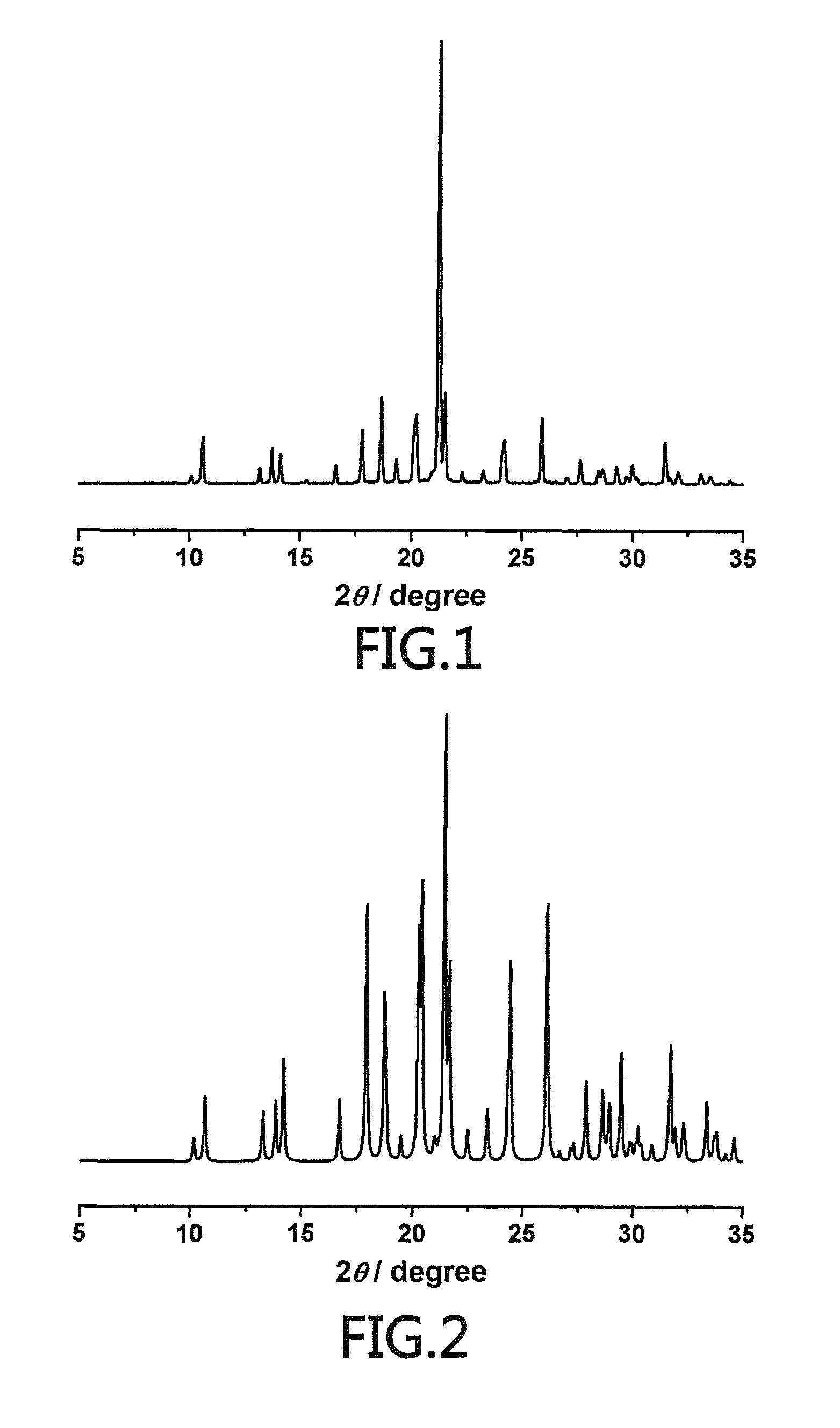
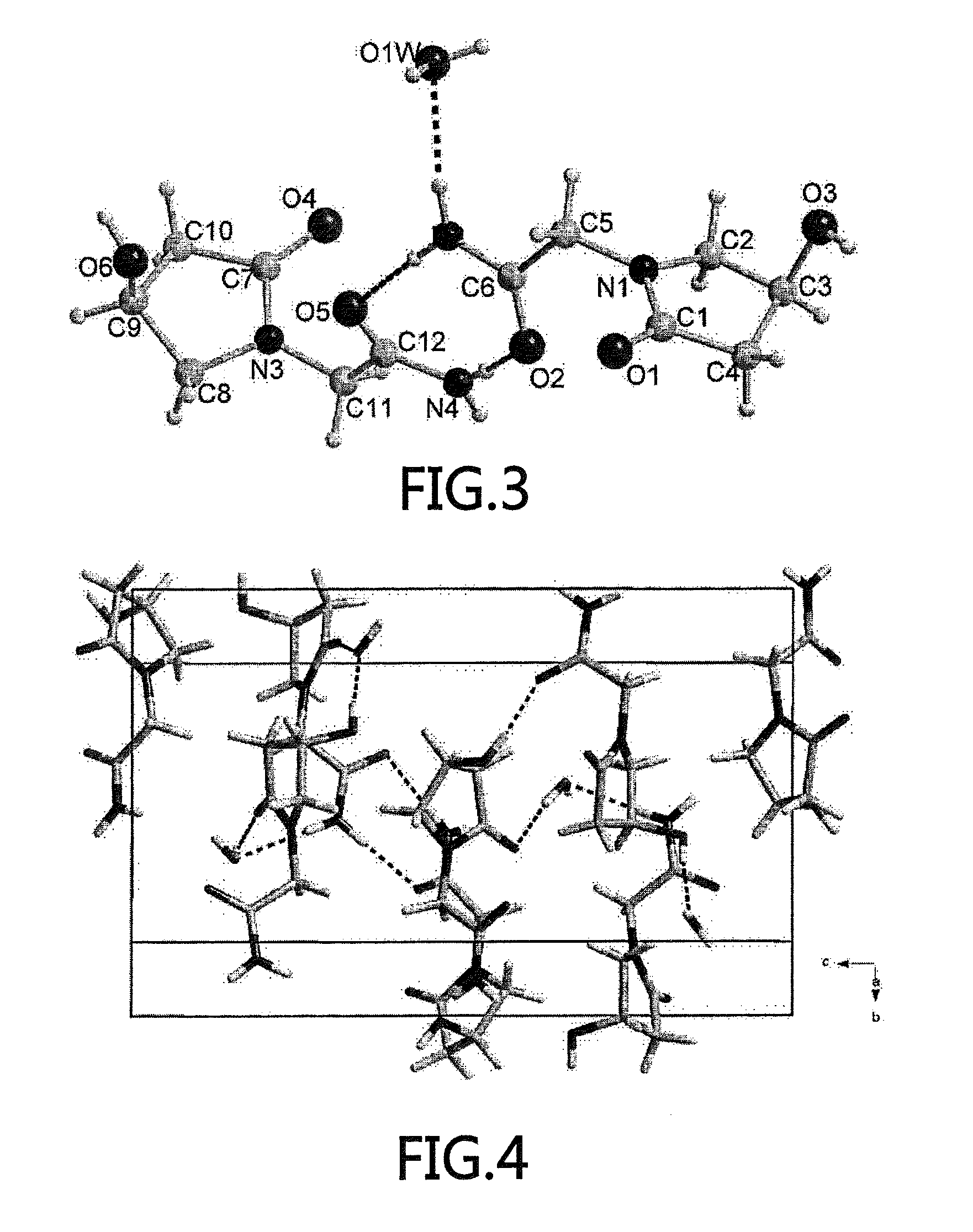
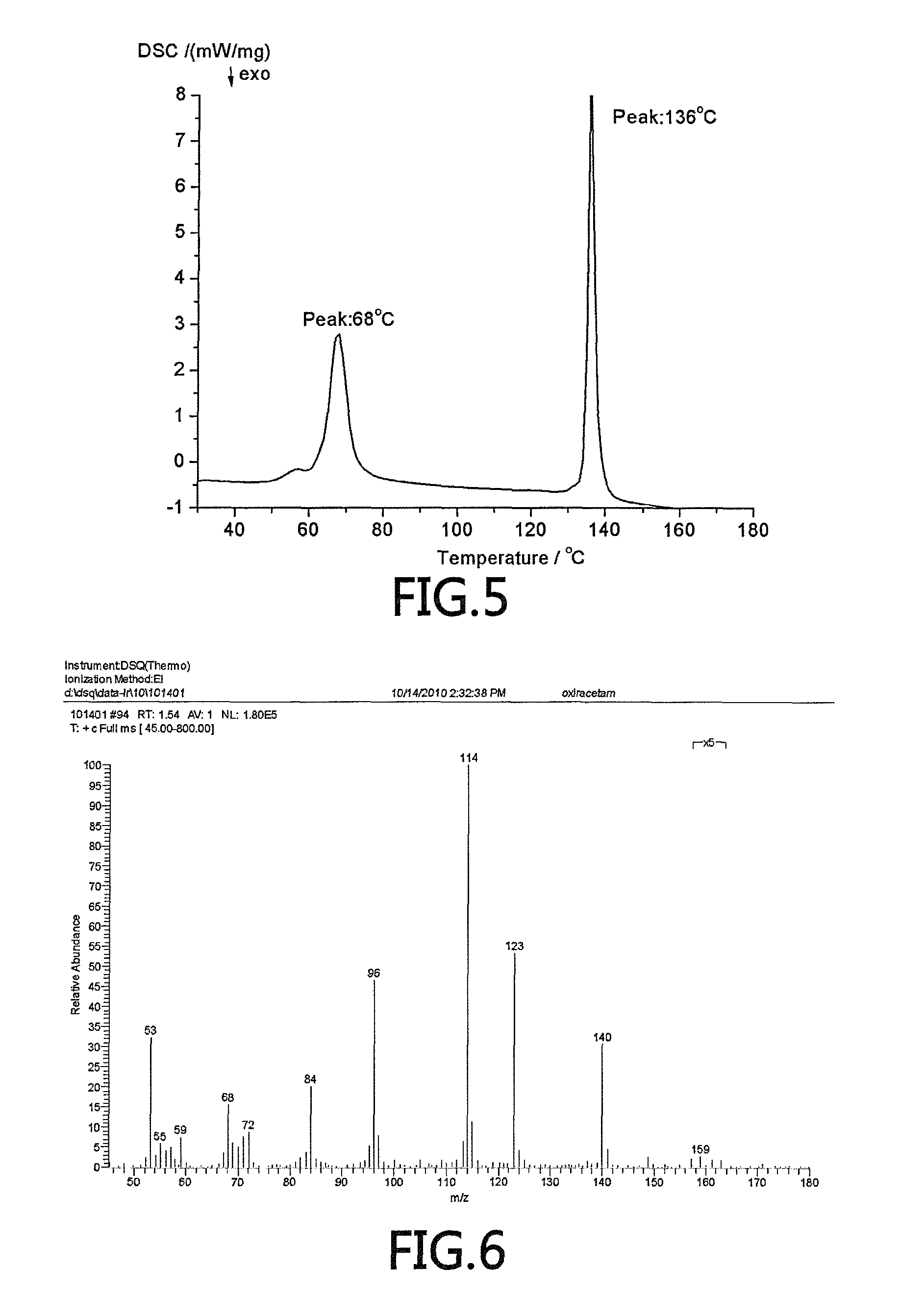
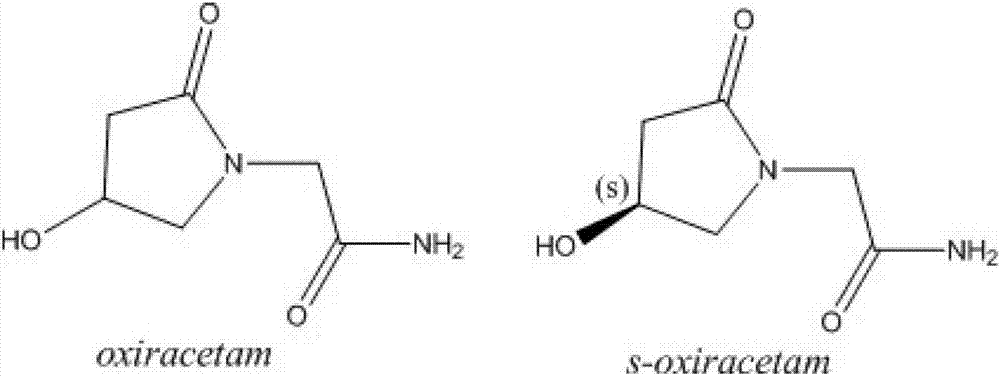
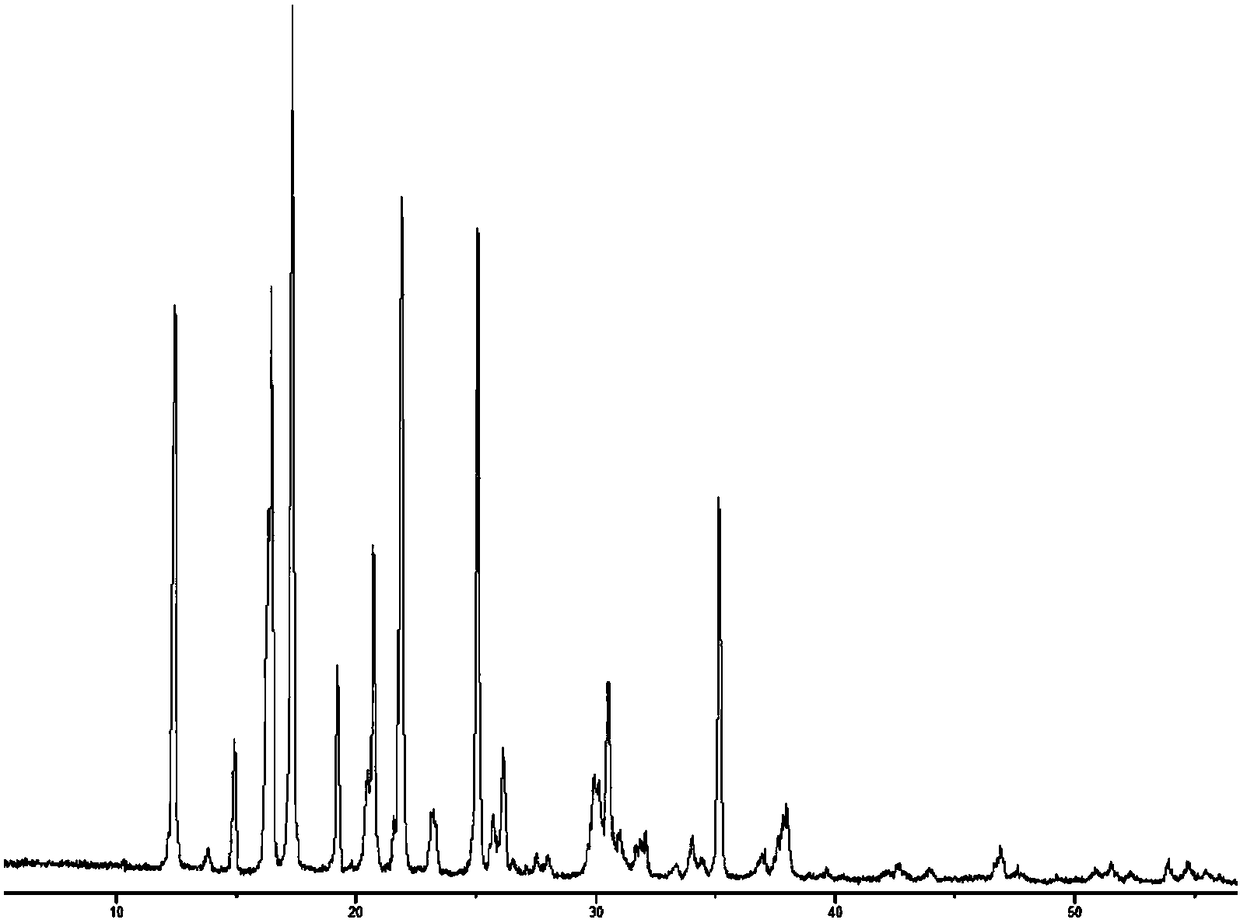
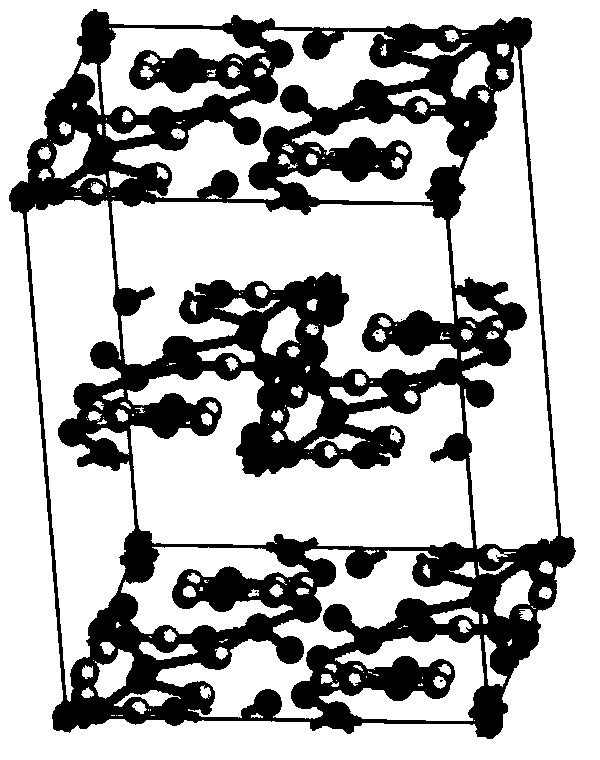
(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide racemate crystal form II and preparation method therefor
ActiveUS9126929B2High purityConvenient medical treatmentOrganic chemistry1-pyrrolidineacetamidePyrrolidine
An (S)-4-hydroxy-2-oxo-1-pyrrolidine acetamide racemate referred to as (S)-oxiracetam crystal form II has a diffraction peak at a diffraction angle 2θ of 10.669, 13.25, 13.847, 14.198, 16.729, 17.934, 18.746, 18.816, 20.273, 20.413, 21.431, 21.617, 21.663, 23.38, 24.324, 24.415, 26.069, 26.107, 27.901, 28.621, 28.925, 29.449, 29.484, 31.702, 36.516, 37.685, or 39.721 degrees. The purity of the (S)-oxiracetam crystal form II can be up to 98.5%, and the (S)-oxiracetam crystal form II has the advantages of simple preparation method, mild control condition, low production cost, and the produced oxiracetam hydrate crystal form II has a high purity (the oxiracetam hydrate crystal form having a purity of 8%˜98.5% can be prepared by a crude levo-oxiracetam having a purity of 92%, and thus having a good reproducibility in production.
Owner:CHONGQING RUNZE PHARM CO LTD
Process for Preparing Levetiracetam and Racemization of (R)- and (S)-2-Amino Butynamide and the Corresponding Acid Derivatives
Process for the preparation of (S)-(−)-α-ethyl-2-oxo-1-pyrrolidineacetamide of Formula (I), comprising the steps of (i) resolution of racemic 2-amino butynamide with L-(+)-tartaric acid either in alcoholic solvents like methanol, isopropanol, ethanol or in water or mixture of water-alcohol to provide (S)-(+)-2-amino butynamide tartarate salt; and ii) direct conversion of (S)-(+)-2-amino butynamide tartarate salt and 4-halobutryl chloride in presence of inorganic or organic base in suitable solvent and drying agents yielded the desired (S)-(−)-α-ethyl-2-oxo-1-pyrrolidineacetamide (I). Further (S)-(+)-2-amino butynamide tartarate salt is converted to (S)-(+)-2-amino butynamide hydrochloride salt, by reacting with an inorganic or organic base in a suitable solvent followed by reaction with HCl gas in an appropriate solvent. The preparation of (S)-(+)-2-amino butynamide hydrochloride salt, which is an intermediate for Levetiracetam, is prepared from (S)-(+)-2-amino butynamide tartarate salt in presence of inorganic base selected from potassium carbonate or hydroxide, sodium carbonate or hydroxide, ammonia gas, and organic base selected from triethyl amine, DMAP, and the like and a suitable solvent selected from methanol, isopropanol, ethanol or in water or mixture of water-alcohol.
Owner:RUBAMIN LAB
Process for the preparation of (S)-alpha-ethyl-2-oxo-1-pyrrolidineacetamide and (R)-alpha-ethyl-2-oxo-pyrrolidineacetamide
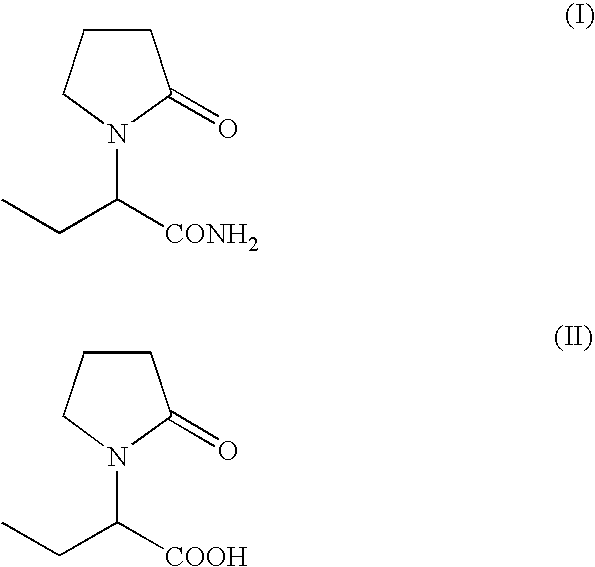
A process provided for the preparation of the (S)- and (R)- alpha- ethyl-2-oxo-1-pyrrolidineacetamide of formula:(1) from (RS)-alpha-ethyl-2-oxo-1-pyrrolidineacetic acid of formula:(2) comprising: i) combining the (RS)-2 with a chiral base (resolving agent) in a resolution solvent and crystallizing from the said mixture the diastereomeric salt of (S)- or (R)-2 and chiral base; ii) regenerating (S)- or (R)-2 from the crystallized diastereomeric salt by treating with a suitable acid or acidic ion-exchange resin; iii) optionally regenerating (R)- or (S)-2 or their mixture (predominantly one enantiomer) from the crystallization mother liquor by treating with a suitable acid or acidic ion-exchange resin; iv) optionally epimerizing (RS)-2 by treating (R)- or (S)-2 or their mixture (predominantly one enantiomer) of step iii with an acid anhydride; V) optionally converting (RS)-2 of step iv into enantiomerically enriched (S)- or (R)-2 through steps i and ii; vi) formation of the mixed anhydride by reacting (R)- or (S)-2 with an alkyl or aryl sulfonyl halogen compound RSO2X in the presence of a suitable base; and vii) reacting the mixed anhydride with ammonia; wherein R represents C 1 to C 15 alkyl or aryl groups such as methyl, ethyl, p-toluenyl, 2,4,6-trimethylbenzyl, 2,4,6-trichloribenzyl, and X represents a halogen atom such as F, Cl and Br atoms.
Owner:APOTEX PHARMACHEN INC
Agent or method for treating severe aphasia in cerebrovascular accident chronic stage
InactiveUS20070185190A1Good conditionNo side effectBiocideNervous disorder1-pyrrolidineacetamideChronic stage
This relates to an agent or method for treating severe aphasia in cerebrovascular accident chronic stage, which comprises 2-oxo-1-pyrrolidineacetamide as an active ingredient.
Owner:OHYAMA HIDEKI
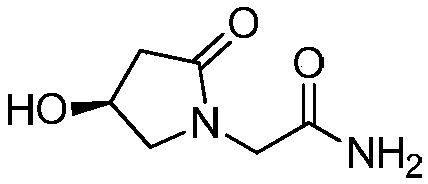
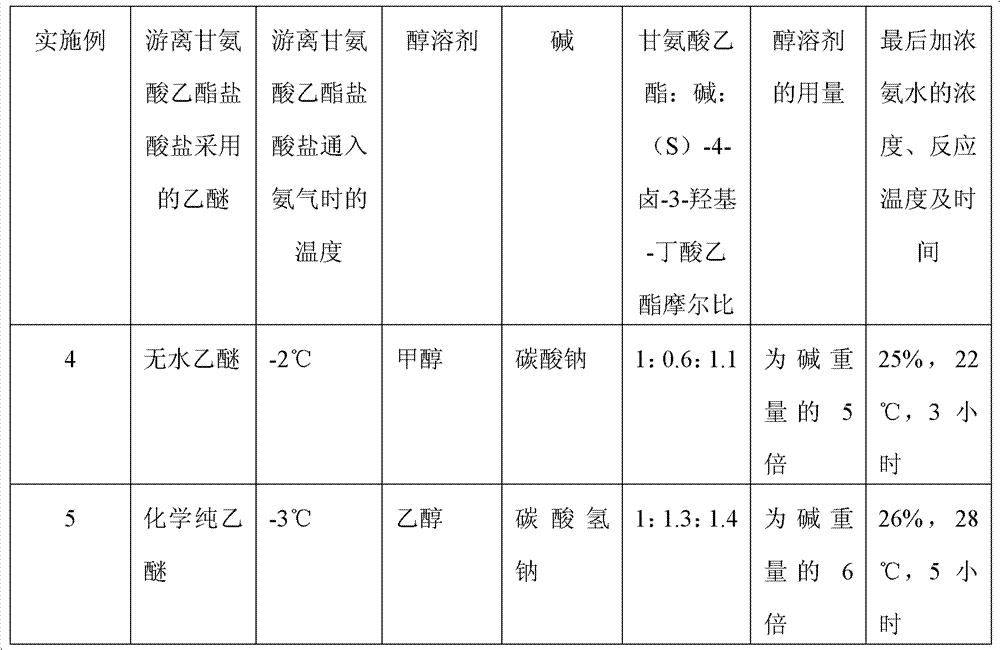
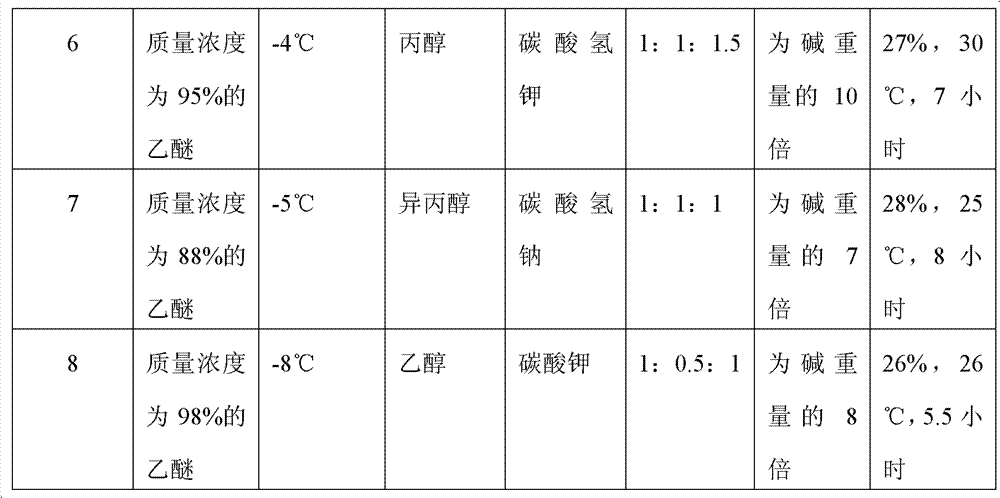
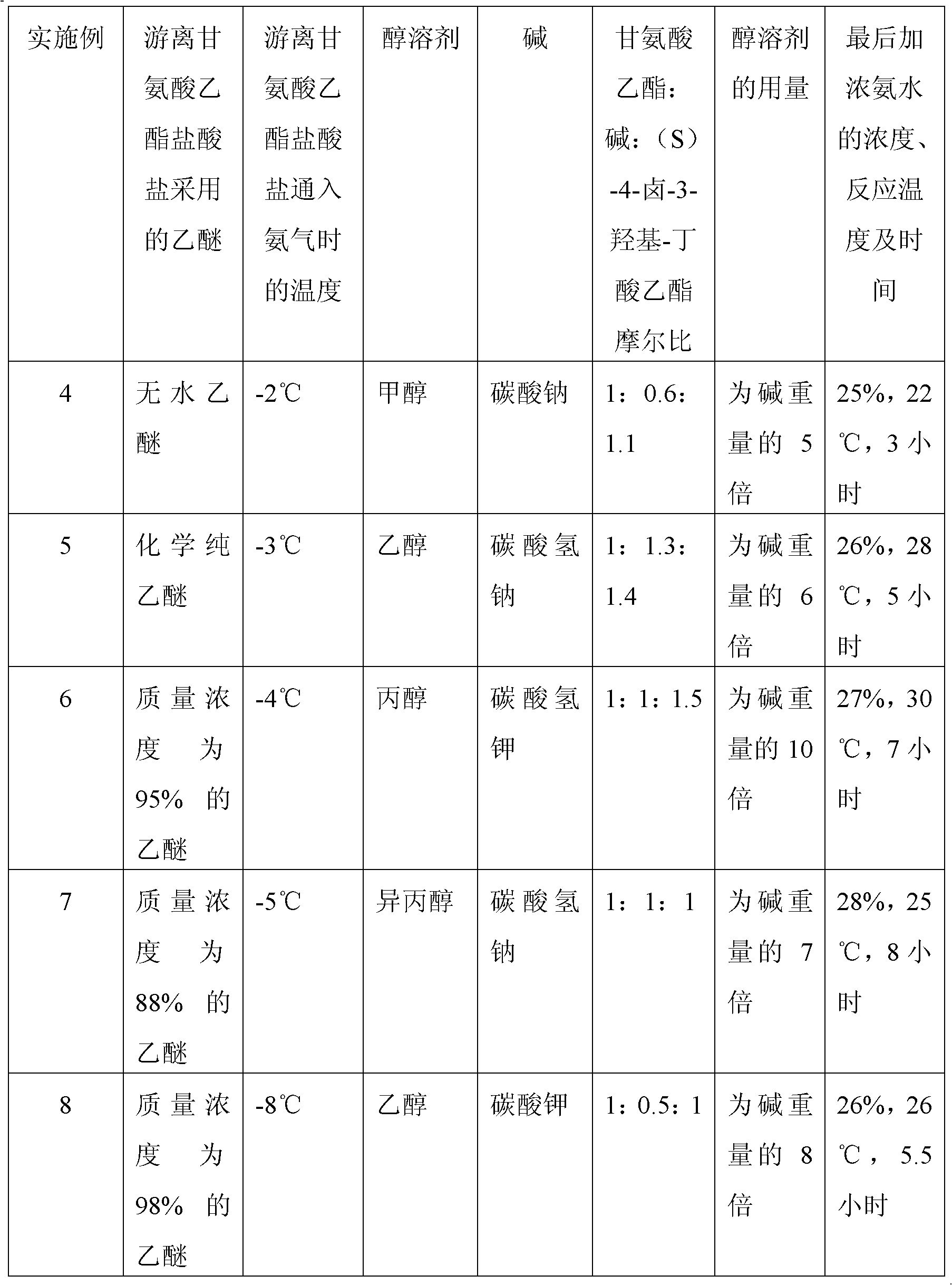
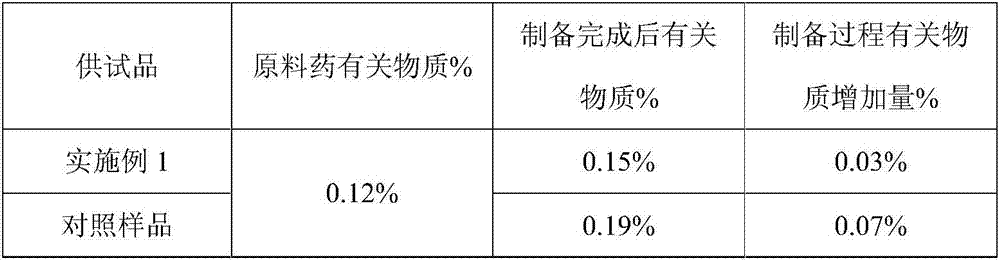
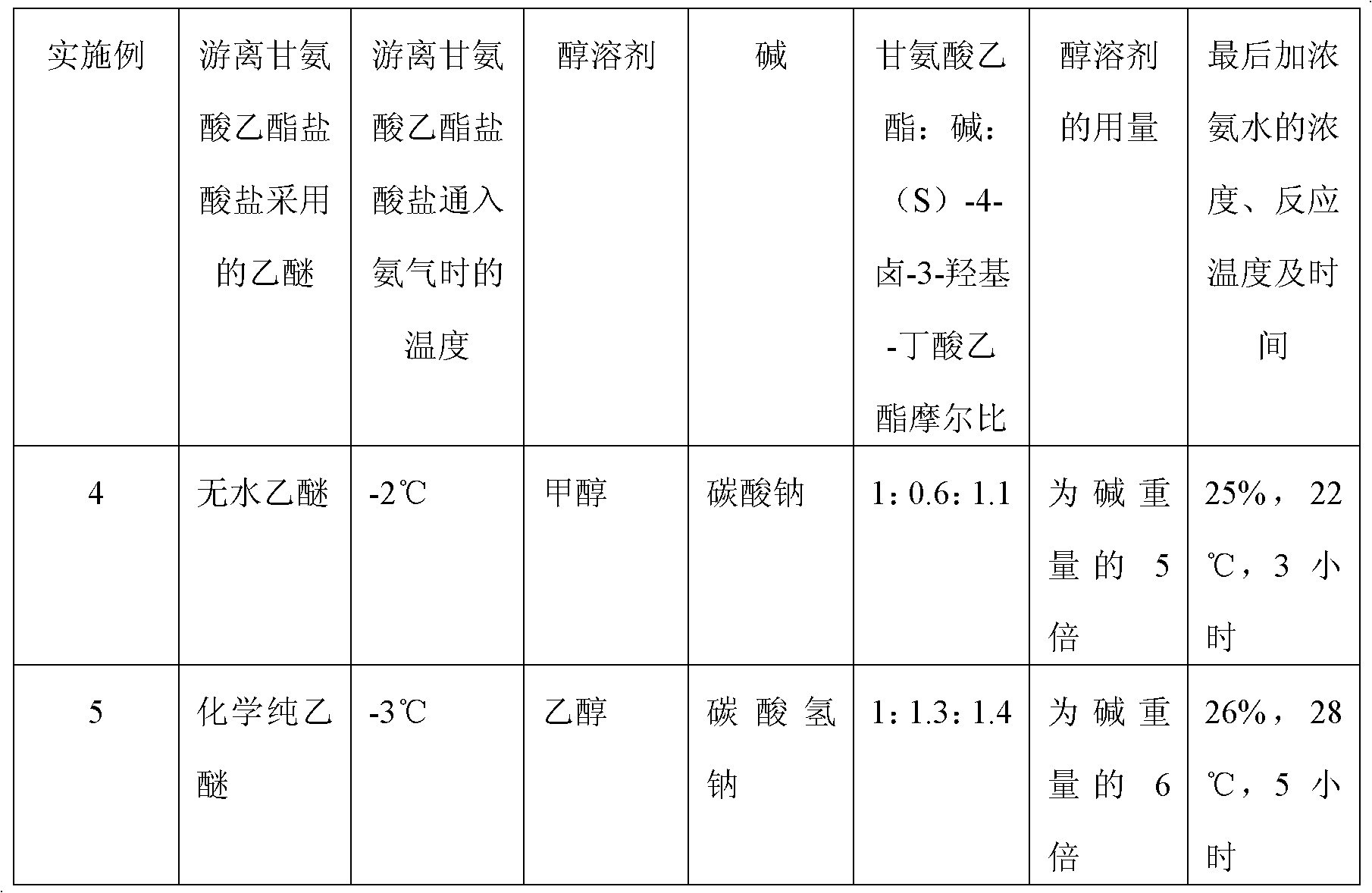
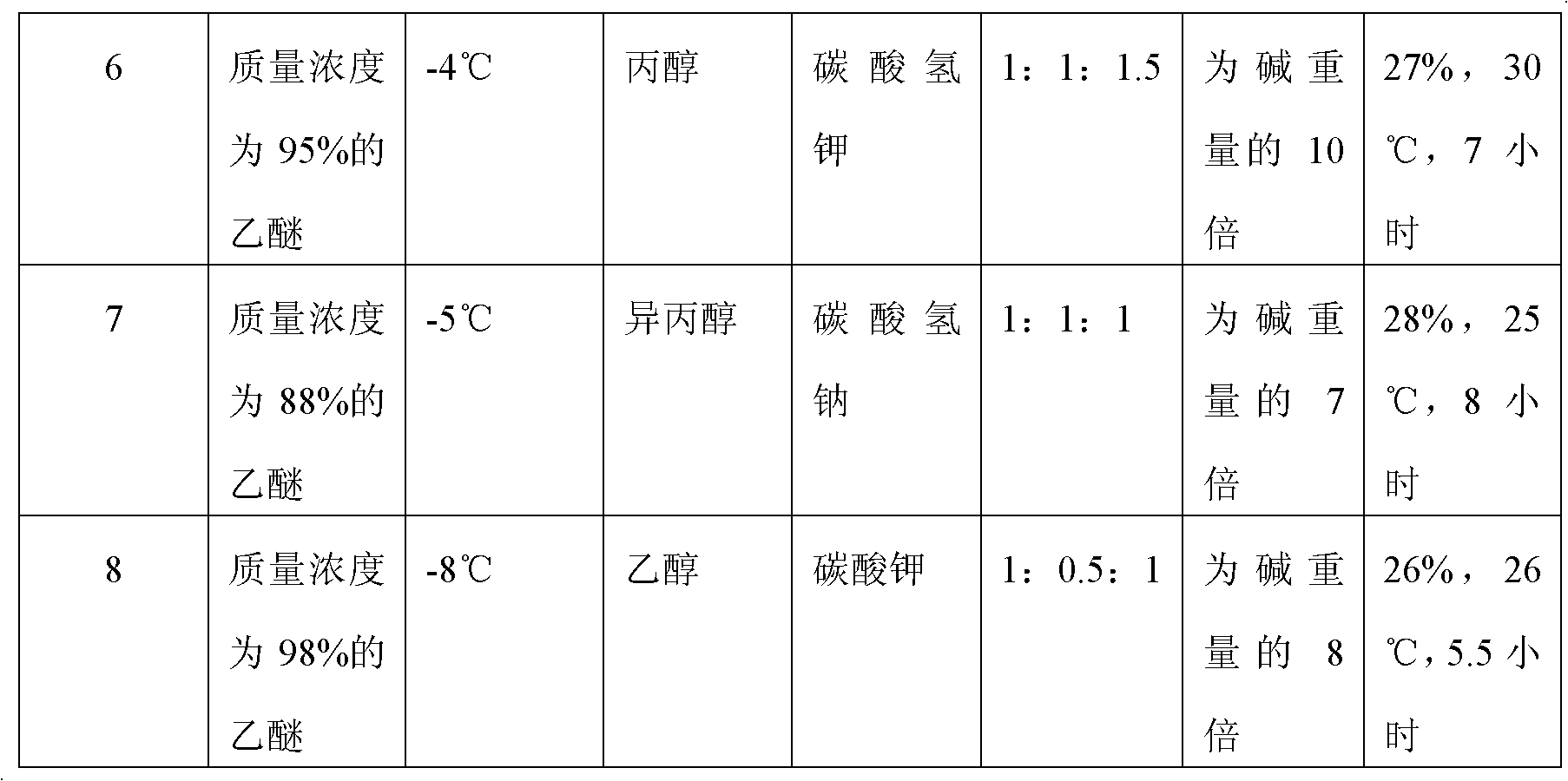
Preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
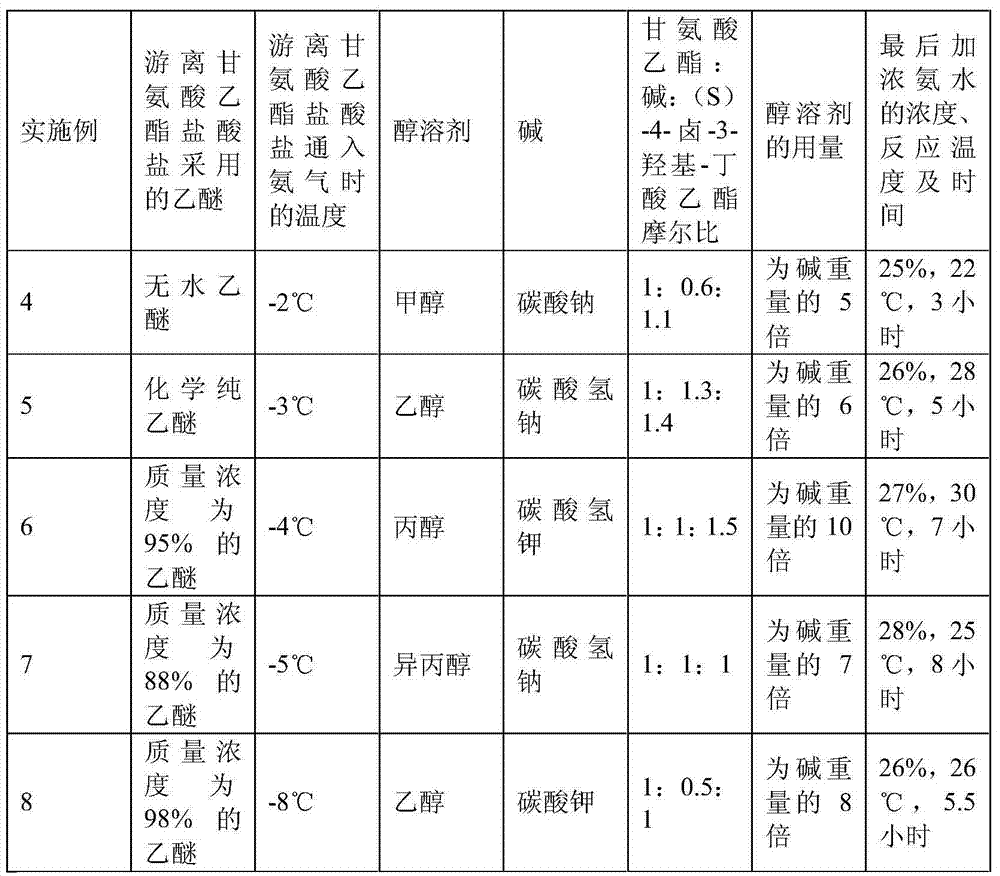
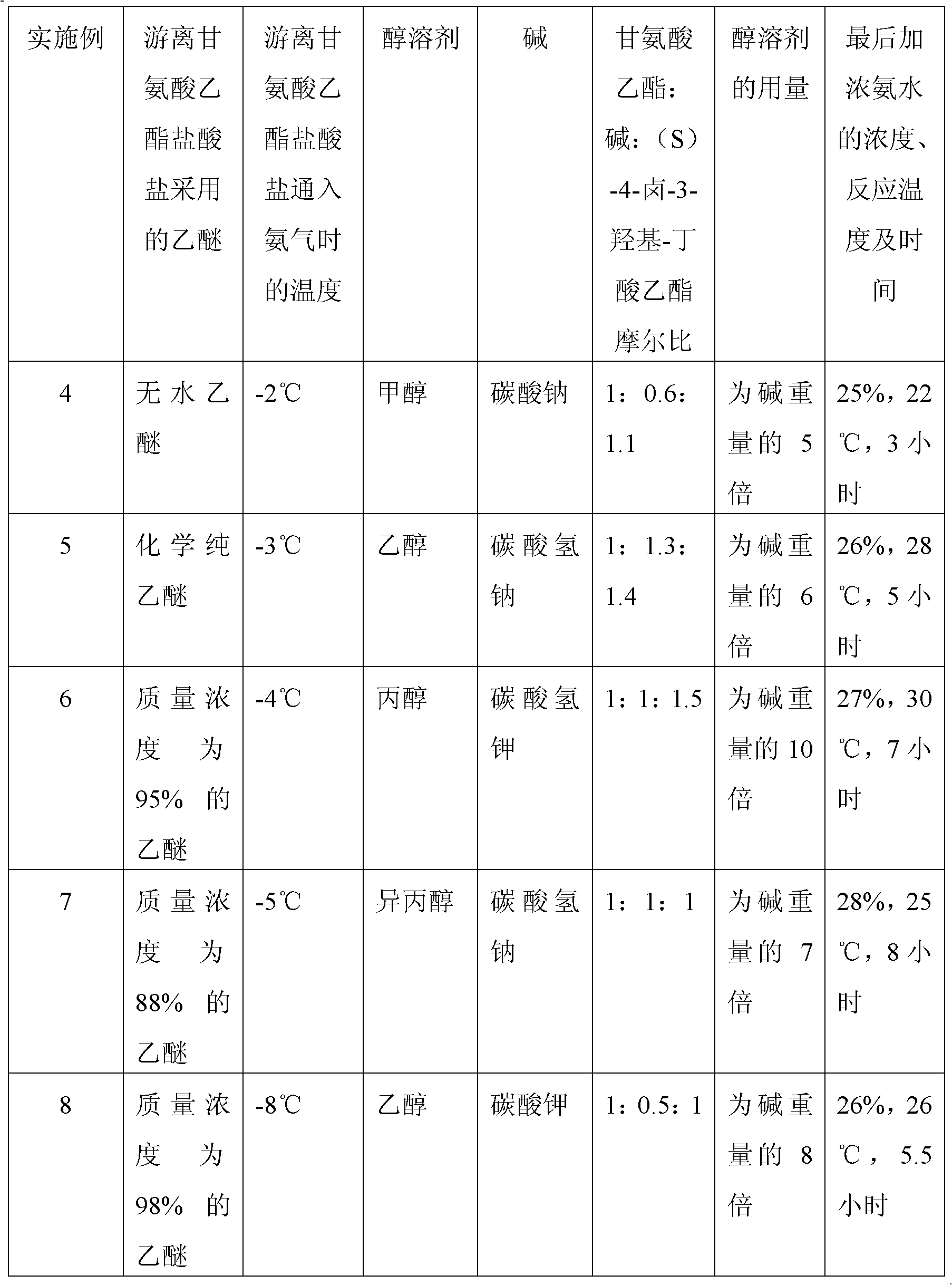
The invention discloses a preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide. The method comprises the following steps: reacting glycine ethyl ester hydrochloride and (S)-4-halo-3-hydroxy-ethyl butyrate serving as raw materials in the presence of an alcohol solvent under an alkaline condition; filtering, washing, concentrating, extracting, separating, introducing ammonia water to obtain a crude product, and purifying the crude product, wherein the glycine ethyl ester hydrochloride is dissociated into glycine ethyl ester by using diethyl ether and ammonia gas, the alcohol solvent is absolute methanol or absolute ethyl alcohol, and the alkali is sodium carbonate or sodium bicarbonate. The (S)-4-halo-3-hydroxy-ethyl butyrate and the glycine ethyl ester hydrochloride are taken as major raw materials, so that the raw materials are cheap, readily available and environmentally friendly; the glycine ethyl ester hydrochloride is dissociated, so that the using quantities of the materials in reaction are reduced, the cost is reduced, and meanwhile the reaction yield is increased. The preparation method is low in preparation cost, the yield can be up to 36 percent, the reaction conditions are mild, the industrial mass production is facilitated, and the HPLC (High Performance Liquid Chromatography) purity of an obtained (S)-oxiracetam product is over 98.5 percent.
Owner:CHONGQING RUNZE PHARM CO LTD
Preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide lyophilized powder
InactiveCN107536809AShorten the production cycleLittle painOrganic active ingredientsPowder deliveryMass ratio1-pyrrolidineacetamide
The invention discloses a preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide lyophilized powder. The lyophilized powder contains (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, methionine, lactose, triethanolamine and benzyl alcohol according to a mass ratio of 1:(0.4-0.6):(0.5-0.9):(0.2-0.4):(0.05-0.1). A specific excipient combination and a lyophilizing curve are used, so the sublimation temperature in a secondary drying stage is improved, the production cycle of the above preparation is shortened, the scattering phenomenon of the powder is avoided, the content and other indexes meet requirements, the aching feeling of patients in the injecting process is slight, the patients' compliance is good, and the untoward effects of the medicine are reduced.
Owner:CHONGQING RUNZE PHARM CO LTD
A kind of preparation method of (s)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide
ActiveCN106366031BHigh ee valuePromote environmental protectionOptically-active compound separationOrganic racemisation1-pyrrolidineacetamidePyridine
Owner:山东默得森生物制药有限公司
Preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
A preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide comprises allowing reaction of ethyl glycinate hydrochloride and ethyl (S)-4-halo-3-hydroxybutyrate in alcohol solvent and alkaline conditions, filtering, washing, concentrating, extracting, separating, introducing ammonia water to obtain a crude product, and purifying the crude product, wherein the ethyl glycinate hydrochloride is dissociated by using diethyl ether and ammonia gas to obtain ethyl glycinate; the alcohol solvent is anhydrous methanol or anhydrous ethanol; and the alkaline condition is provided by sodium carbonate or sodium bicarbonate. The method adopts ethyl (S)-4-halo-3-hydroxybutyrate and ethyl glycinate hydrochloride as main raw materials, which has the characteristics of cheap and easily-accessible raw material, environment friendliness and no pollution; and the method firstly carries out dissociation on the ethyl glycinate hydrochloride, which effectively reduces the material consumption in reaction and lowers the cost, and simultaneously plays a positive role in the reaction yield. The method has the characteristics of low preparation cost, high yield up to 36% and mild reaction conditions, and is suitable for industrial production. The prepared (S)-oxiracetam product has high performance liquid chromatography (HPLC) purity up to above 98.5%.
Owner:CHONGQING RUNZE PHARM CO LTD
Agent or method for treating severe aphasia in cerebrovascular accident chronic stage
The present invention relates to a method for treating a patient having suffered from severe aphasia associated with cerebrovascular accident chronic stage for at least three years, wherein said treatment consists essentially of administering a composition comprising 2-oxo-1-pyrrolidineacetamide as an active ingredient and a pharmaceutically acceptable carrier.
Owner:OHYAMA HIDEKI
(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide sterile powder for injection
ActiveCN107536810AImprove securityShorten the production cyclePowder deliveryOrganic active ingredientsFreeze-drying1-pyrrolidineacetamide
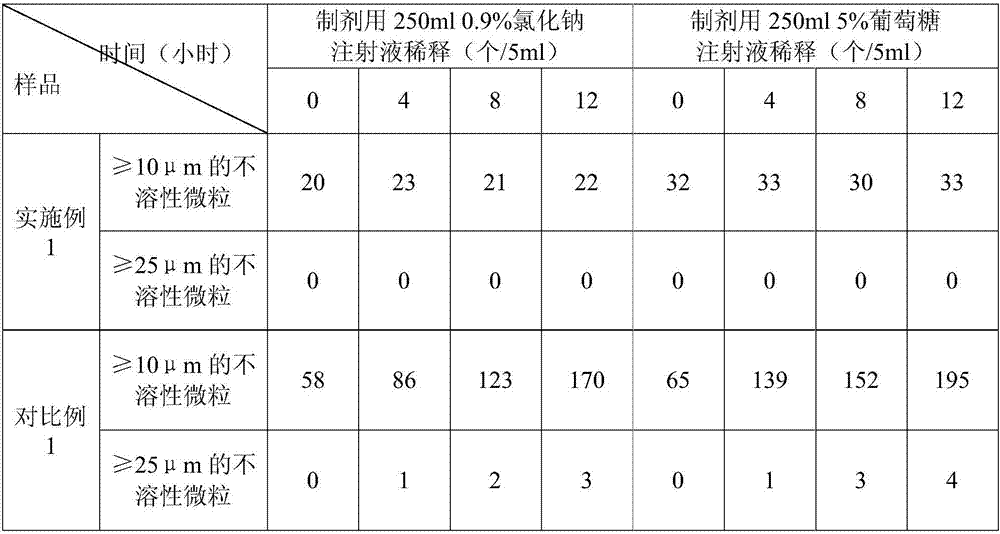
The invention discloses an (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide sterile powder for injection. The sterile powder contains (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, methionine, lactose, triethanolamine and sodium bisulfite according to a mass ratio of 1:(0.4-0.6):(0.5-0.9):(0.2-0.4):(0.1-0.2). A specific excipient combination and a freeze-drying curve are used to improve the sublimation temperature of a secondary drying stage, shorten the production period of the above preparation, avoid the scattering phenomenon of the powder and makes the content and other indexes meet regulations; and the above product has a good stability, and can be dissolved in a glucose injection or a sodium chloride injection to prepare an intravenous drip solution, so insoluble particles are remarkably reduced, the improvement of the drug use safety is facilitated, and the untoward effects of the drug are reduced.
Owner:CHONGQING RUNZE PHARM CO LTD
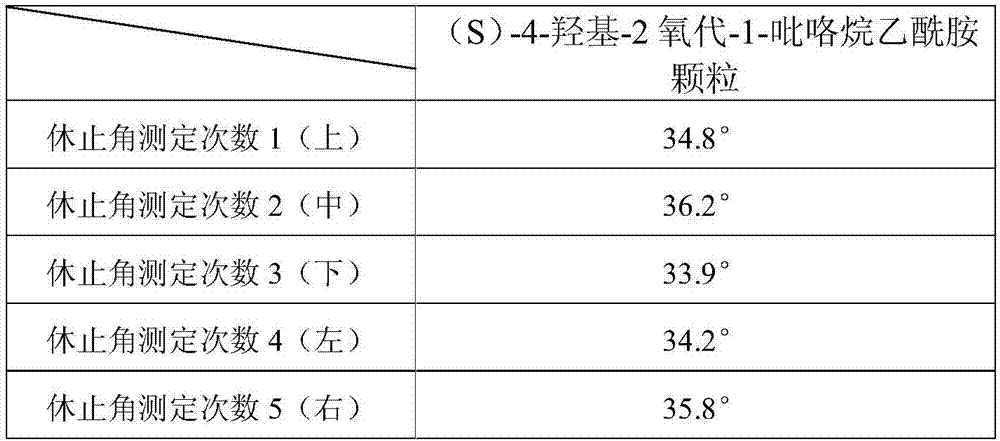
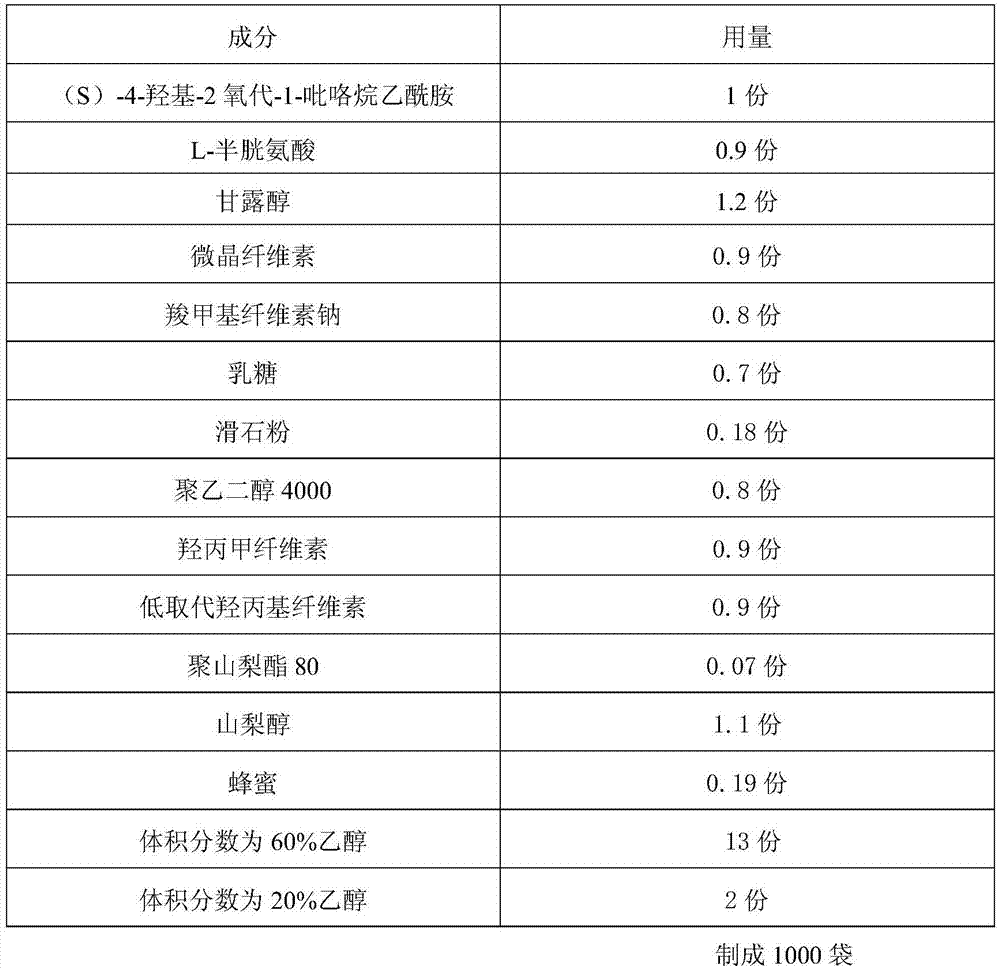
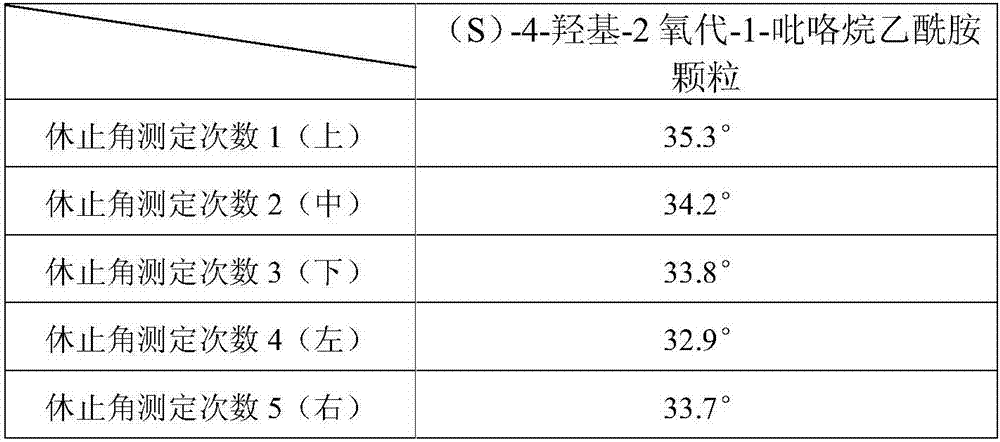
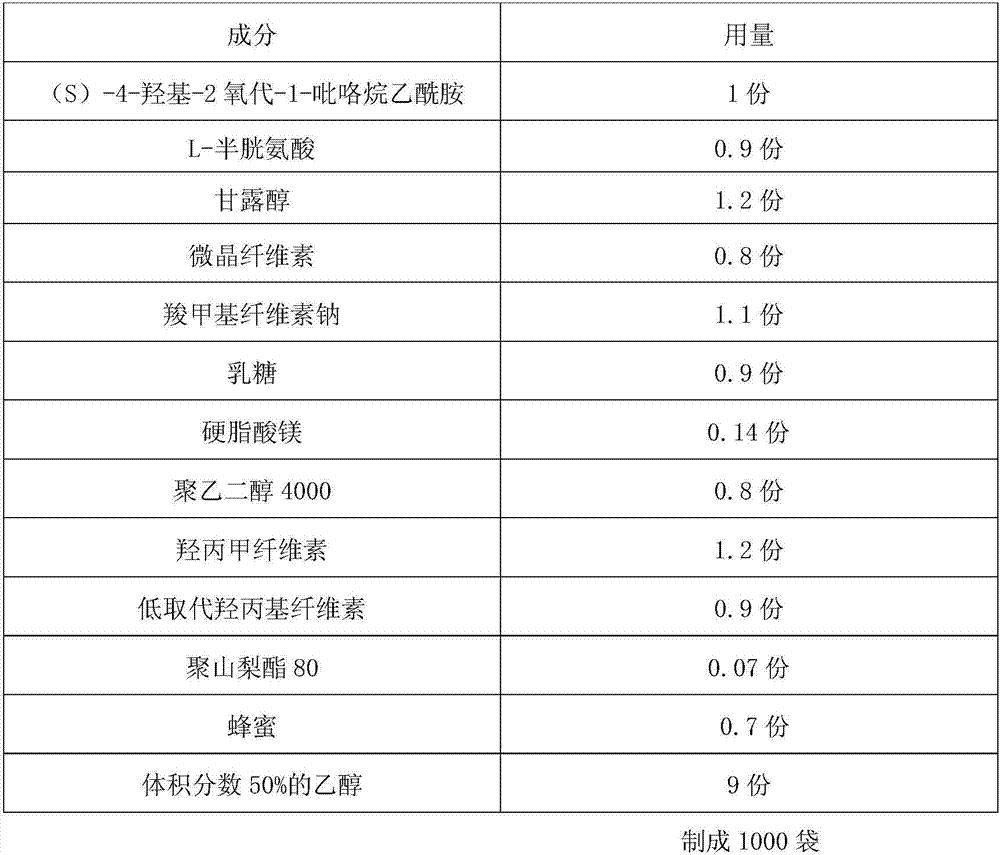
Uniform-content (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule wand preparation method thereof
ActiveCN107510685AFast dissolutionWon't stickOrganic active ingredientsNervous disorderPolyethylene glycol1-pyrrolidineacetamide
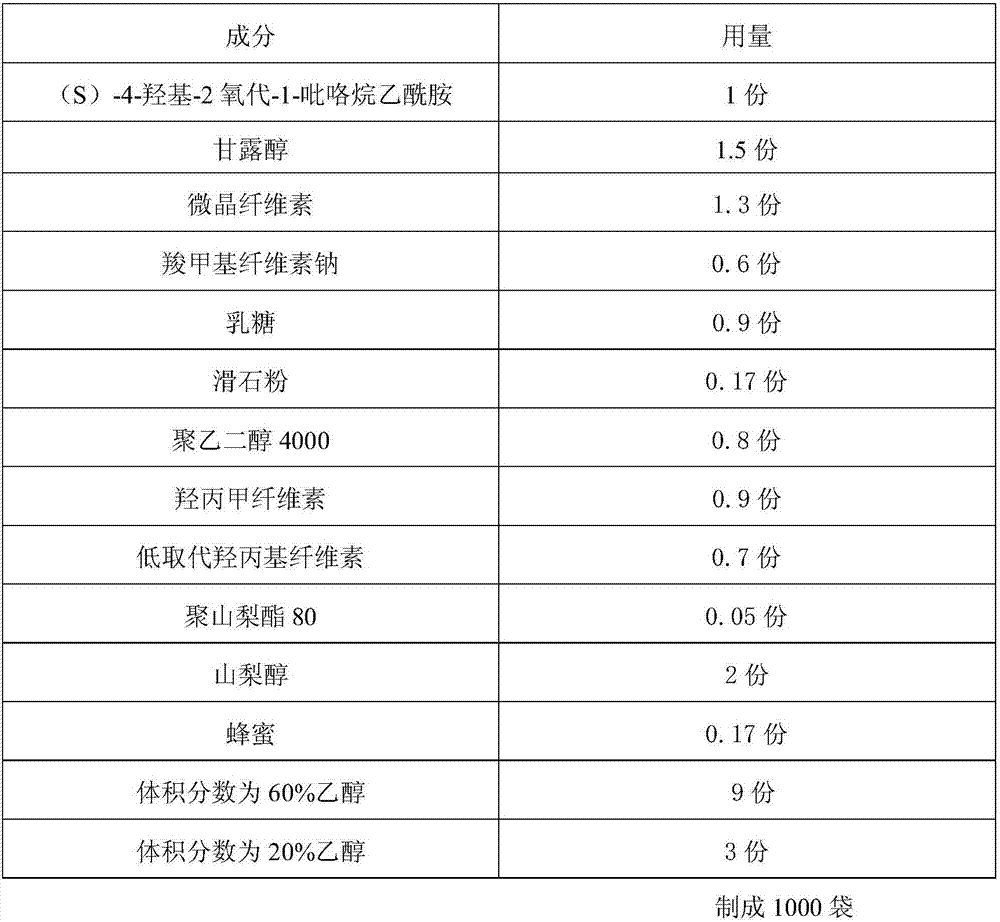
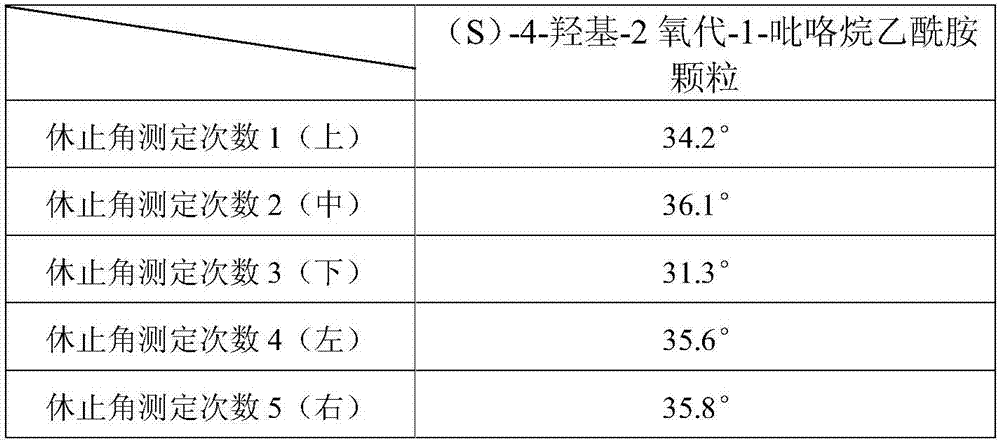
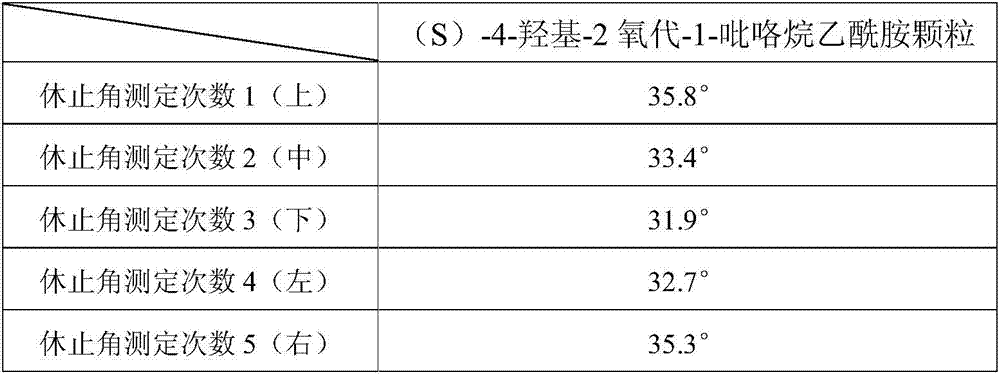
An (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule is prepared from, by weight, 1 part of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, 1.3-1.9 parts of mannitol, 1.1-1.6 parts of microcrystalline cellulose, 0.5-1.0 part of carboxymethylcellulose sodium, 0.8-1.3 parts of lactose, 0.15-0.21 part of talcum powder, 0.7-1.3 parts of polyethylene glycol 4000, 0.8-1.5 parts of hydroxypropyl methylcellulose, 0.5-1.2 parts of low-substituted hydroxypropyl cellulose, 0.03-0.09 part of polysorbate 80, 1-5 parts of sorbitol, 0.15-0.21 part of honey, 8-13 parts of a 60-80% ethanol solution and 2-6 parts of a 20-30% ethanol solution. The (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule prepared in the invention has the advantages of easiness in granulation, small quantity of powder layers, uniform particle sizes, good fluidity, realization of the Xiuzi angle being less than 38 DEG and the loading difference being less than 5%, fast dissolution speed, short complete-dissolution time being less than 30 s, good content uniformity, realization of the determination RSD of the content in multiple points being less than 2%, good stability, no moisture absorbing caking, and long shelf life reaching up to 24 months.
Owner:武汉恒信源药业有限公司
(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide sterile powder and preparation method thereof
InactiveCN107432864AImprove complianceReduce adverse reactionsPowder deliveryOrganic active ingredientsFreeze-dryingPatient compliance
The present invention discloses (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide sterile powder and a preparation method thereof, wherein the sterile powder contains the following raw materials and the auxiliary materials by weight: 50-59% of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, 20-25% of L-serine, 10-17% of mannitol, 5-7% of sodium glutamate, 5-10% of sodium hydrogen sulfite, and 1-3% of benzyl alcohol. According to the present invention, by using the specific excipient combination, the prepared (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide sterile powder has the fixed shape, and cannot be sprayed out of the bottle during the freeze drying, the stability of the product is good, the insoluble particles are obviously reduced and the size of the insoluble particles is obviously reduced, the pain of patients during the injection is less, the patient compliance is good, the drug use safety can be easily improved, and the adverse reaction of the drug can be reduced.
Owner:CHONGQING RUNZE PHARM CO LTD
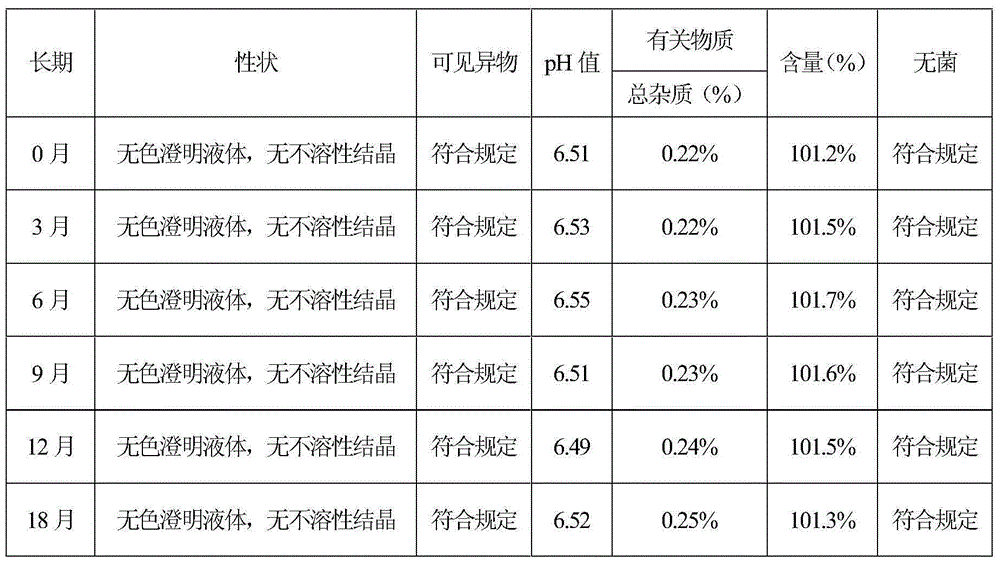
Pharmaceutical compositions comprising (s)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
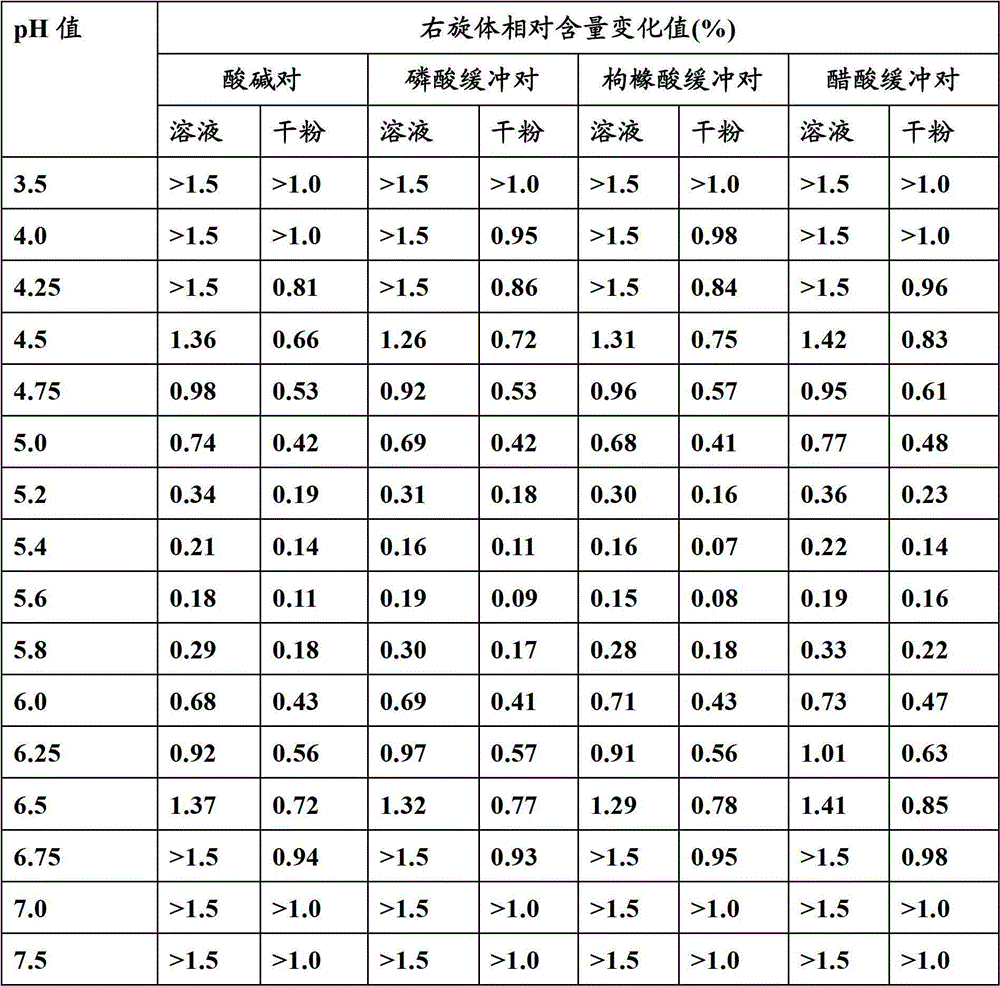
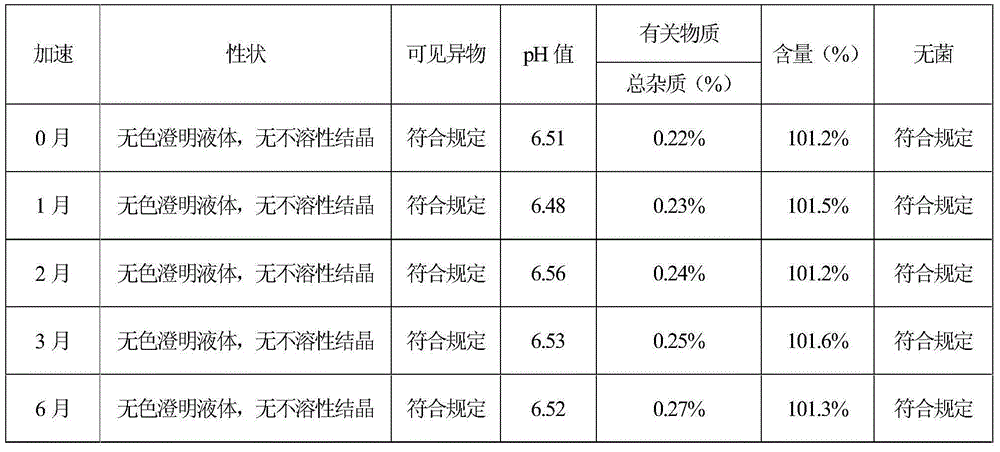
The invention relates to a stable preparation for injection by taking S-oxiracetam as active ingredient. The preparation is a composition for injection, and is formed by the S-oxiracetam or salts thereof serving as active ingredient and pharmaceutically acceptable auxiliary material. To restrain the racemization of the S-oxiracetam, when the powder injection with the active ingredient is prepared, and only the pH value of the S-oxiracetam medicament solution ranges from 4.5 to 7.0, the pH value is between 5.4 to 6.6 preferably, so that the S-oxiracetam medicament solution can be produced, and the final freeze-dried product has acceptable long stability; and moreover, when injection is produced, a rotary steam sterilization method needs to be adopted, terminal rotating sterilization is performed for 15 to 45 minutes under the temperature of 121 DEG C, and the terminal rotating sterilization is performed for 15 to 20 minutes preferably, so that the S-oxiracetam injection with high purity can be obtained and has acceptable long stability.
Owner:FUKANGREN BIO PHARMA
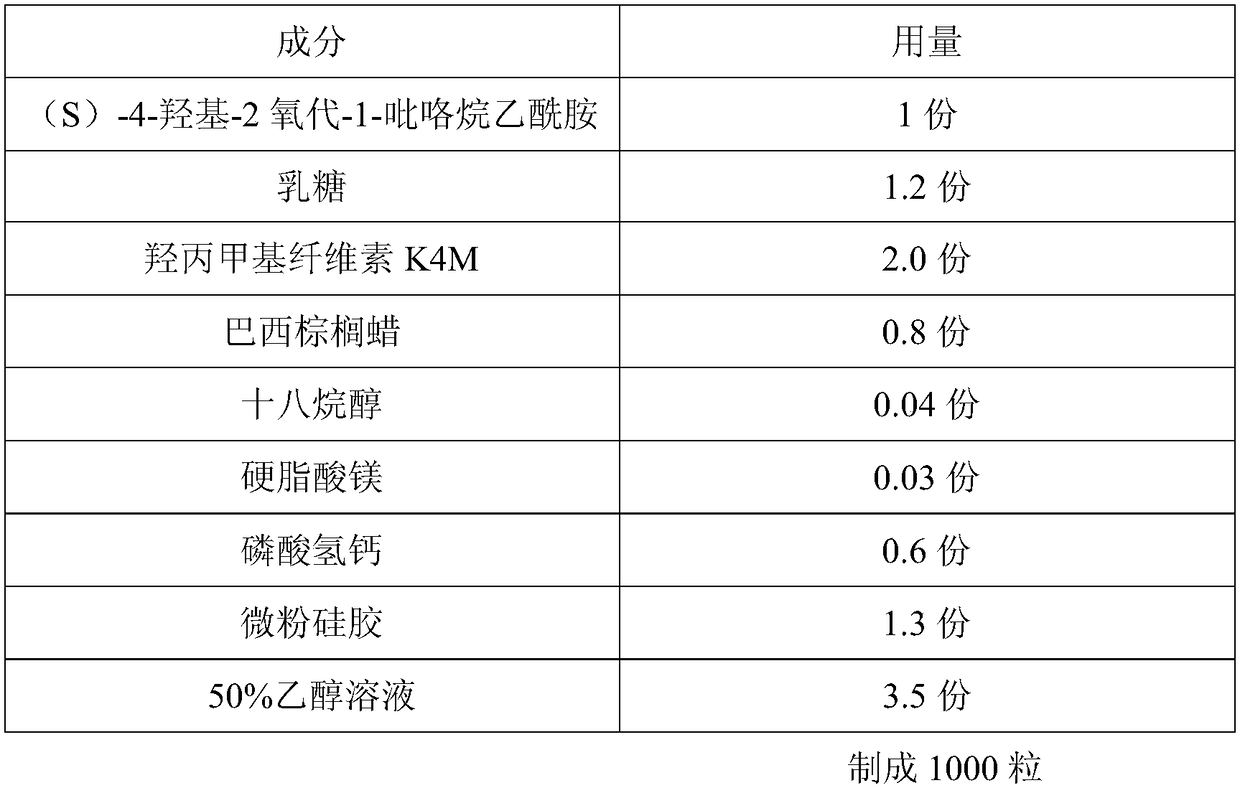
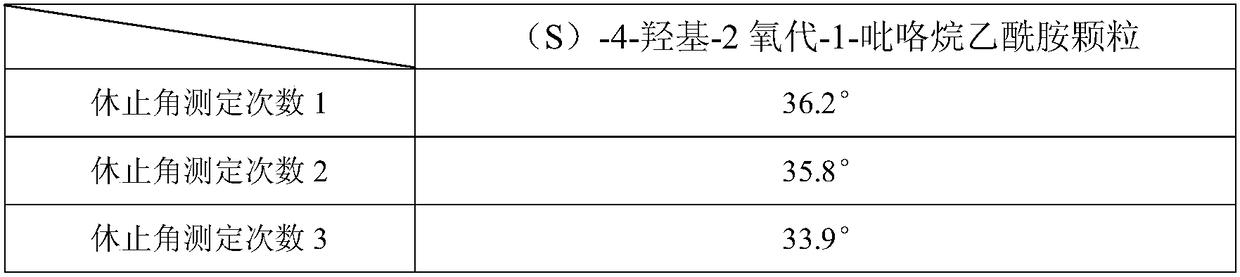
A kind of (s)-4-hydroxy-2-oxo-1-pyrrolidineacetamide sustained-release capsule and its preparation method
ActiveCN106955274BNo stickingParticles have good fluidityOrganic active ingredientsNervous disorderSustained Release CapsulePhosphoric acid
The invention discloses a sustained-release capsule with (S)-4-hydroxyl-2-oxo-1-pyrrolidine acetamide. The sustained-release capsule is prepared by the following raw materials and auxiliary materials in parts by weight: 1 part of (S)-4-hydroxyl-2-oxo-1-pyrrolidine acetamide, 1.1-1.6 parts of lactose, 1.8-2.5 parts of hydroxypropyl methyl cellulose K4M, 0.7-1.1 parts of carnauba wax, 0.03-0.07 part of stearyl alcohol, 0.02-0.08 part of magnesium stearate, 0.5-1.1 parts of calcium hydrophosphate, 1.0-1.7 parts of micro-powder silica gel and 3.2-4.7 parts of alcohol solution with the concentration of 50%-70%. The sustained-release capsule disclosed by the invention has the advantages that the particle fluidity is good, the repose angles of the particles are less than 37 degrees, the release speed is slow, and the release period is 12 hours, therefore, compared with the traditional preparation, the taking times can be reduced and the sustained-release capsule only needs to be taken once a day; simultaneously, the stability is good, the capsule cannot be adhered in the storage process, and the shelf life can reach 24 months.
Owner:CHONGQING RUNZE PHARM CO LTD
Process for Preparing Levetiracetam
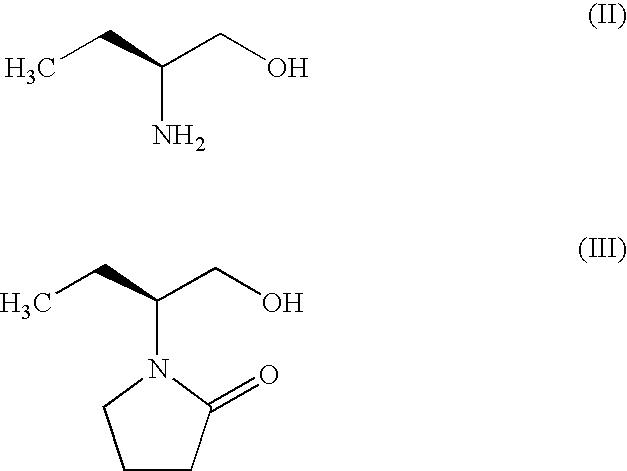
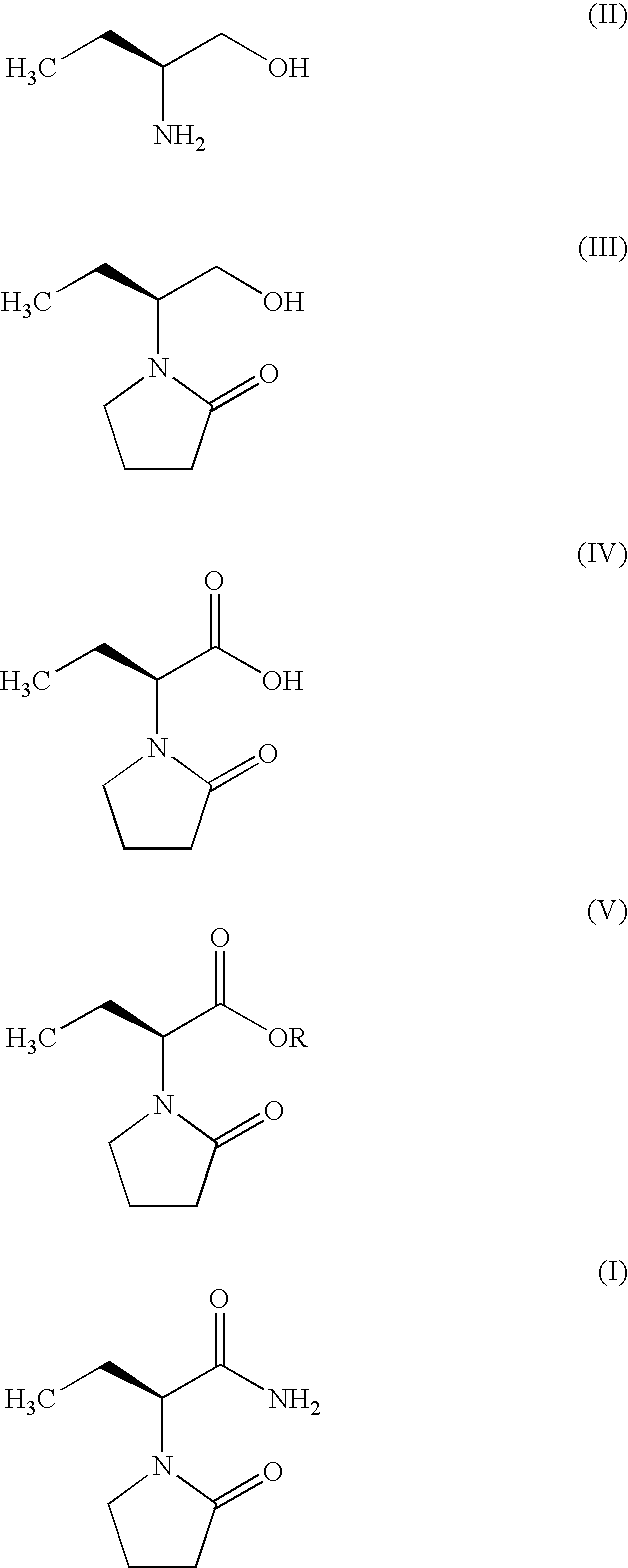
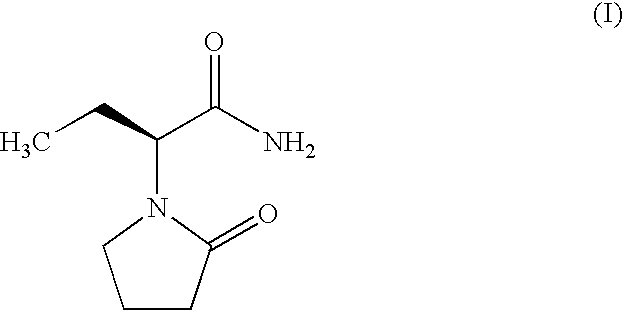
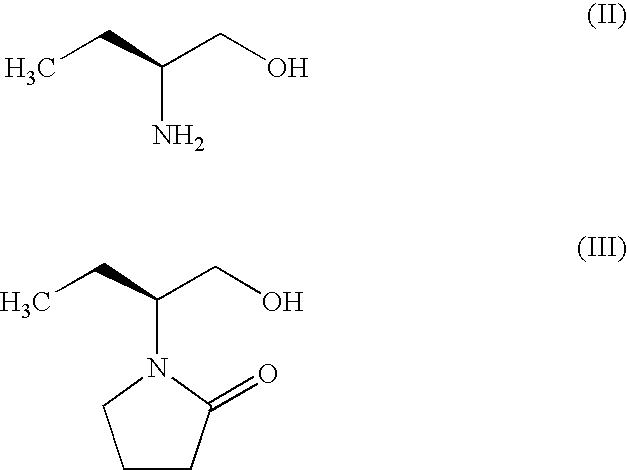
Process for the preparation of (S)-(−)-α-ethyl-2-oxo-1-pyrrolidineacetamide of Formula (I) by the steps of condensation of (S)-2-amino butanol of Formula (II) and 4-halobutryl chloride, where halo group can be chloro, bromo or iodo in solvents to form α-ethyl-2-oxo pyrrolidine ethanol of Formula (III); oxidation of (S)-α-ethyl-2-oxo pyrrolidine ethanol to yield (S)-α-ethyl-2-oxo pyrrolidine acetic acid having the formula (IV); esterification of (S)-α-ethyl-2-oxo pyrrolidine acetic acid (IV) with an alcohol to provide alkyl ester of Formula (V) wherein, R is 14 Carbon atom; ammonolysis of alkyl esters of formula (V) with ammonia to provide (S)-(−)—α-ethyl-2-oxo-1-pyrrolidine acetamide of formula (1).
Owner:RUBAMIN
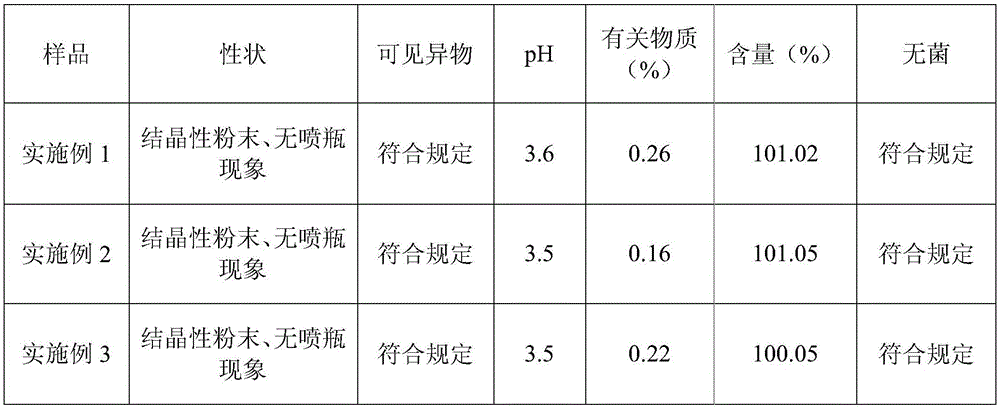
A stable (s)-4-hydroxy-2-oxo-1-pyrrolidineacetamide injection and its preparation method
ActiveCN106943344BImprove compliancePain reliefOrganic active ingredientsNervous disorderPatient compliance1-pyrrolidineacetamide
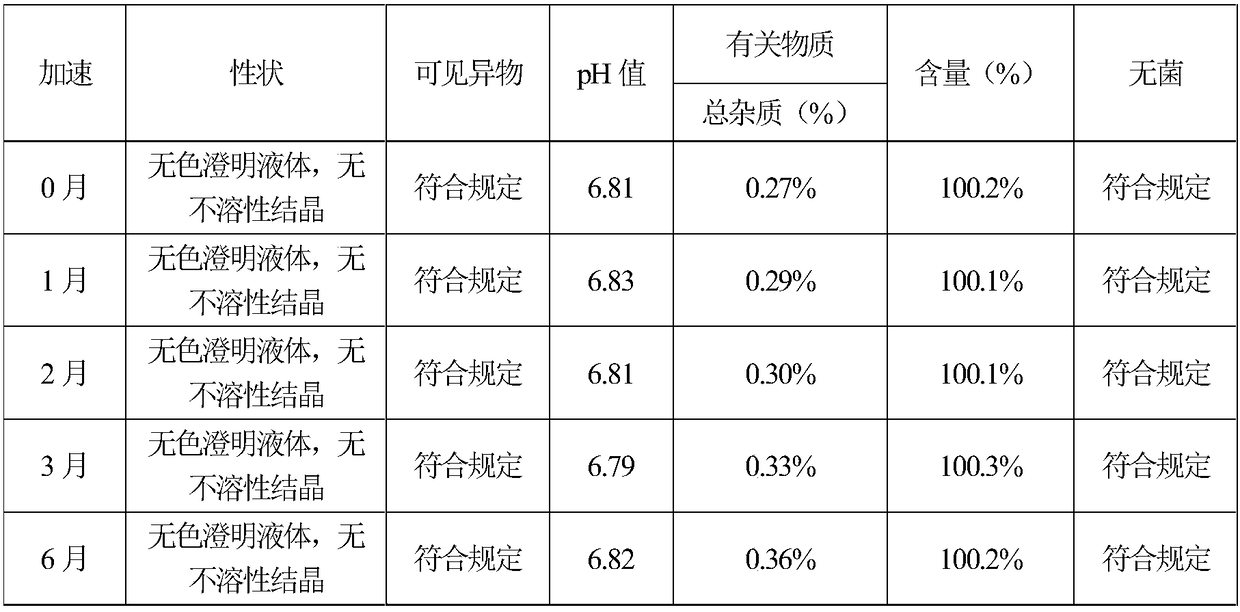
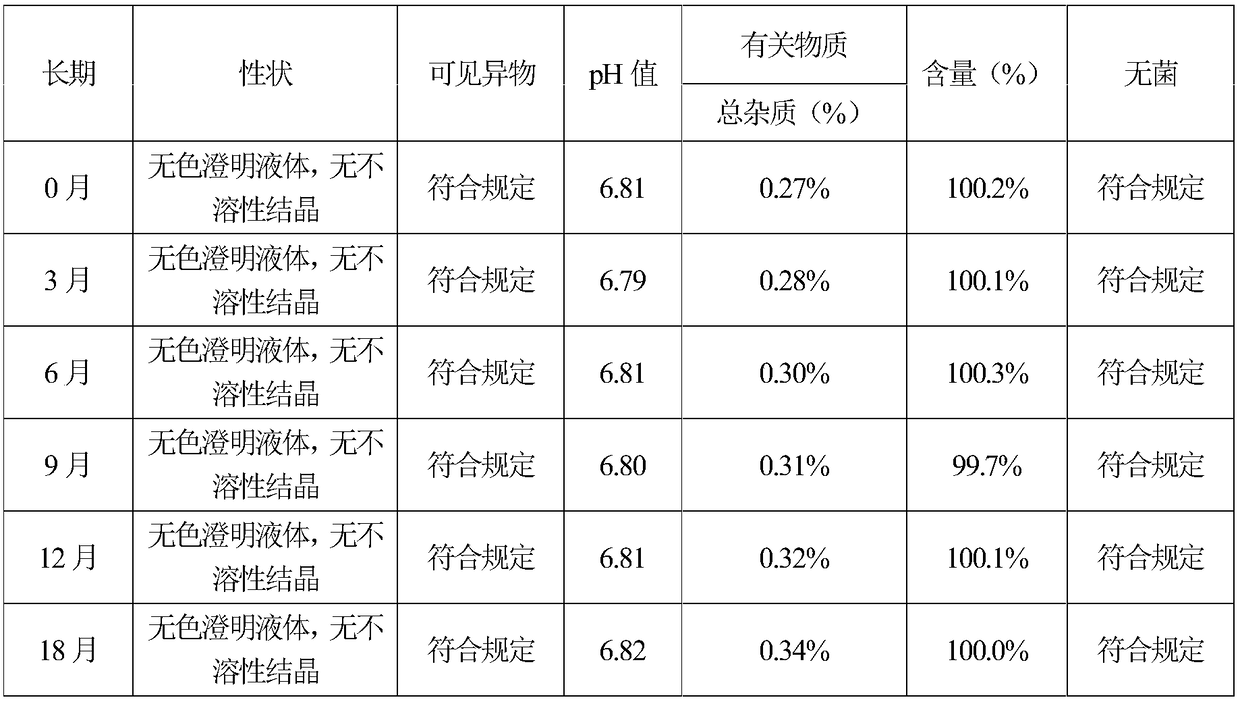
The invention provides (S)-4-hydroxyl-2-oxo-1-pyrrolidine acetamide for injection, which is prepared from the following raw and auxiliary materials: 65-85% of (S)-4-hydroxyl-2-oxo-1-pyrrolidine acetamide, 8-25% of propylene glycol, 5-25% of lecithin and 1-5% of benzyl alcohol. The pH value of the (S)-4-hydroxyl-2-oxo-1-pyrrolidine acetamide injection during sterilization is basically not changed, and the product has good stability, is free of crystallization during storage, has long effective period which can reach more than 18 months, is low in impurities during effective period with total impurity being lower than 0.36%, can reduce pain during injection to patients and has good patient compliance.
Owner:CHONGQING RUNZE PHARM CO LTD
(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule having good stability, and preparation method thereof
InactiveCN107510667AUniform particle sizeDecrease increaseOrganic active ingredientsNervous disorderSucrose1-pyrrolidineacetamide
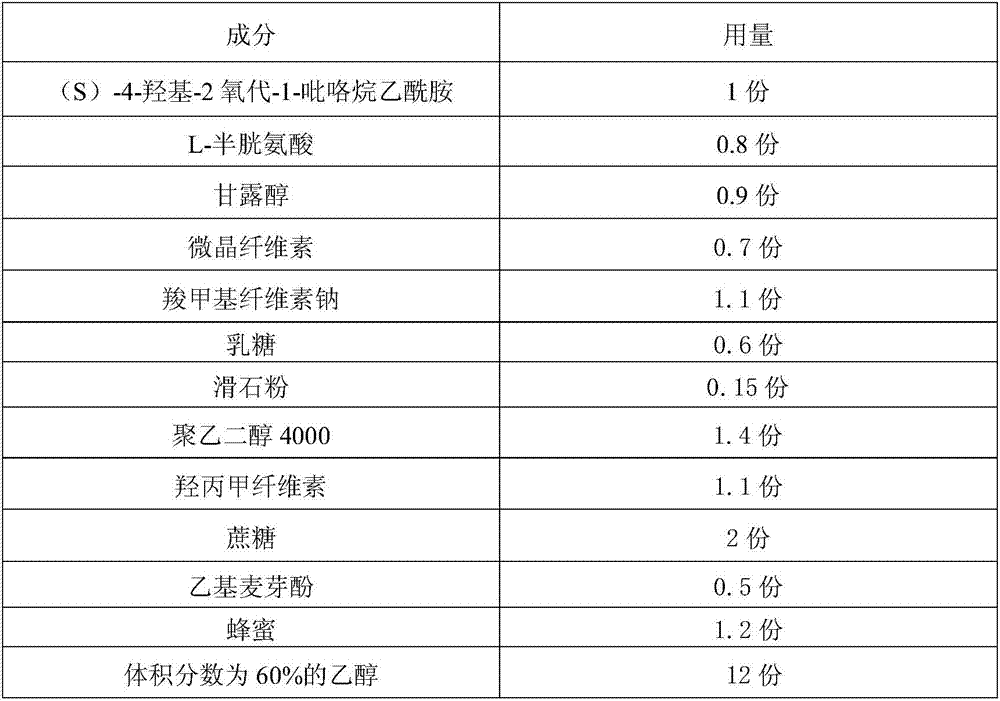
An (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide having a good stability is prepared from, by weight, 1 part of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, 0.6-1.1 parts of L-cysteine, 0.7-1.3 parts of mannitol, 0.5-1.1 parts of microcrystalline cellulose, 0.9-1.5 parts of carboxymethylcellulose sodium, 0.5-1.0 part of lactose, 0.13-0.18 part of talcum powder, 1.3-1.8 parts of polyethylene glycol 4000, 1.0-1.5 parts of hydroxypropyl methylcellulose, 1-5 parts of sucrose, 0.3-0.9 part of ethyl maltol, 1.0-1.5 parts of honey and 10-16 parts of ethanol with the volume fraction being 55-70%. The (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule prepared in the invention has the advantages of small impurity increasing amount being only 0.03% in the preparation process, no adhesion to a screen and easiness in granulation during granulation, small quantity of powder layers, uniform particle size, good fluidity, realization of the Xiuzi angle being less than 37 DEG and the loading difference being less than 5%, good stability during storage, no moisture absorption caking, long shelf life reaching up to 24 months, and good mouthfeel, and can be received by most patients.
Owner:CHONGQING RUNZE PHARM CO LTD
Preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
Owner:CHONGQING RUNZE PHARM CO LTD
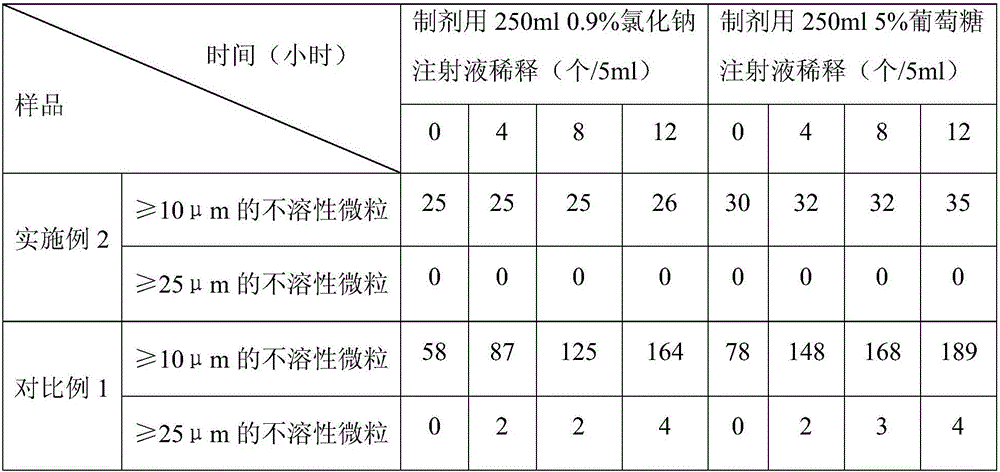
(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide injection and preparation method of (S)-4-hydroxyl-2oxo-1-pyrrolidineacetamide injection
InactiveCN106692133AWon't crystallizeNot easily oxidizedOrganic active ingredientsNervous disorderAcetic acidVitamin C
The invention provides a (S)-4-hydroxyl-2oxo-1-pyrrolidineacetamide injection. The (S)-4-hydroxyl-2oxo-1-pyrrolidineacetamide injection is characterized by being prepared by taking (S)-4-hydroxyl-2oxo-1-pyrrolidineacetamide, propylene glycol, lecithin, vitamin c and ethylenediamine tetraacetic acid as raw and auxiliary materials through steps of concentrating, diluting, filling and sealing, sterilizing, checking and packaging, wherein the use amount of the raw and auxiliary materials is as follows in percentage by weight: 68 percent to 76 percent of the (S)-4-hydroxyl-2oxo-1-pyrrolidineacetamide, 10 percent to 18 percent of the propylene glycol, 10 percent to 17 percent of the lecithin, 1 percent to 3 percent of the vitamin c and 2 percent to 5 percent of the ethylenediamine tetraacetic acid. The (S)-4-hydroxyl-2oxo-1-pyrrolidineacetamide injection prepared by the invention has the advantages that a product is not crystallized and not easily oxidized in a storage process, the increasing amount of impurities in a sterilization process is only 0.03 percent and the stability is good; the validity period reaches 18 months or more, the product contains few impurities during the validity period and the content of the total impurities is lower than 0.27 percent; a preparation process is simple and feasible and is worthy of being popularized in the market.
Owner:CHONGQING RUNZE PHARM CO LTD
Preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
The invention relates to a preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide. The preparation method comprises the following steps of: taking glycine ethyl ester hydrochloride and ethyl-(S)-4-halo-3-hydroxy-butanoate as raw materials, reacting in an alcohol solvent under alkaline conditions, washing with an inorganic alcohol, concentrating, extracting, separating, and introducing ammonia water to get a crude product of the (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide; and performing purification treatment on the crude product, wherein the glycine ethyl ester hydrochloride firstly needs to adopt ethyl ether and ammonia gas to dissociate so as to get glycine ethyl ester. The main raw materials adopted in the preparation method disclosed by the invention are the ethyl-(S)-4-halo-3-hydroxy-butanoate and the glycine ethyl ester hydrochloride, and the raw materials are low in price, easy to obtain, environment-friendly and pollution-free; and according to the preparation method disclosed by the invention, dissociation treatment is firstly performed on the glycine ethyl ester hydrochloride, thus the using quantity of the materials in the reaction is effectively reduced, the cost is lowered, and a positive role is simultaneously played for the reaction yield. (S)-oxiracetam prepared by the preparation method disclosed by the invention has low cost, high yield which is as high as 36% and mild reaction conditions, and is conductive to industrialized large-scale production, and the HPLC (high-performance liquid chromatography) purity of the prepared (S)-oxiracetam product is above 98.5%.
Owner:CHONGQING RUNZE PHARM CO LTD
Uniform-content (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule wand preparation method thereof
InactiveCN107510676AFast dissolutionDecrease increaseOrganic active ingredientsNervous disorder1-pyrrolidineacetamidePolyethylene glycol
An (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule is prepared from, by weight, 1 part of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, 0.7-1.2 parts of L-cysteine, 0.6-1.0 part of mannitol, 1.1-1.6 parts of microcrystalline cellulose, 0.5-1.2 parts of carboxymethylcellulose sodium, 0.8-1.3 parts of lactose, 0.13-0.18 part of talcum powder, 1.1-1.7 parts of polyethylene glycol 4000, 0.9-1.5 parts of hydroxypropyl methylcellulose, 0.6-1.3 parts of low-substituted hydroxypropyl cellulose, 0.05-0.11 part of polysorbate 80, 1-5 parts of sorbitol, 10-16 parts of a 6-8% starch slurry and 2-5 parts of a 20-30% ethanol solution. The (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule prepared in the invention has the advantages of small impurity increasing amount only being 0.03%, small quantity of powder layers, uniform particle sizes, good fluidity, realization of the Xiuzi angle being less than 38 DEG and the loading difference being less than 5%, fast dissolution speed, short dissolution time being less than 30 s, good content uniformity, realization of the determination RSD of the content in multiple points being less than 2%, good stability during storage, no moisture absorbing caking, and long shelf life reaching up to 24 months.
Owner:CHONGQING RUNZE PHARM CO LTD
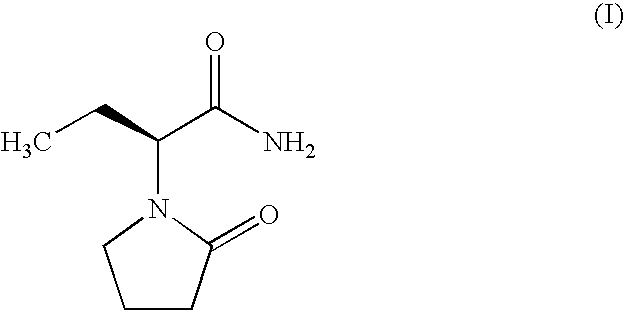
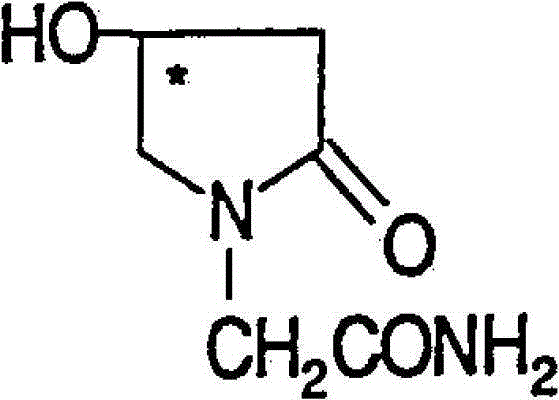
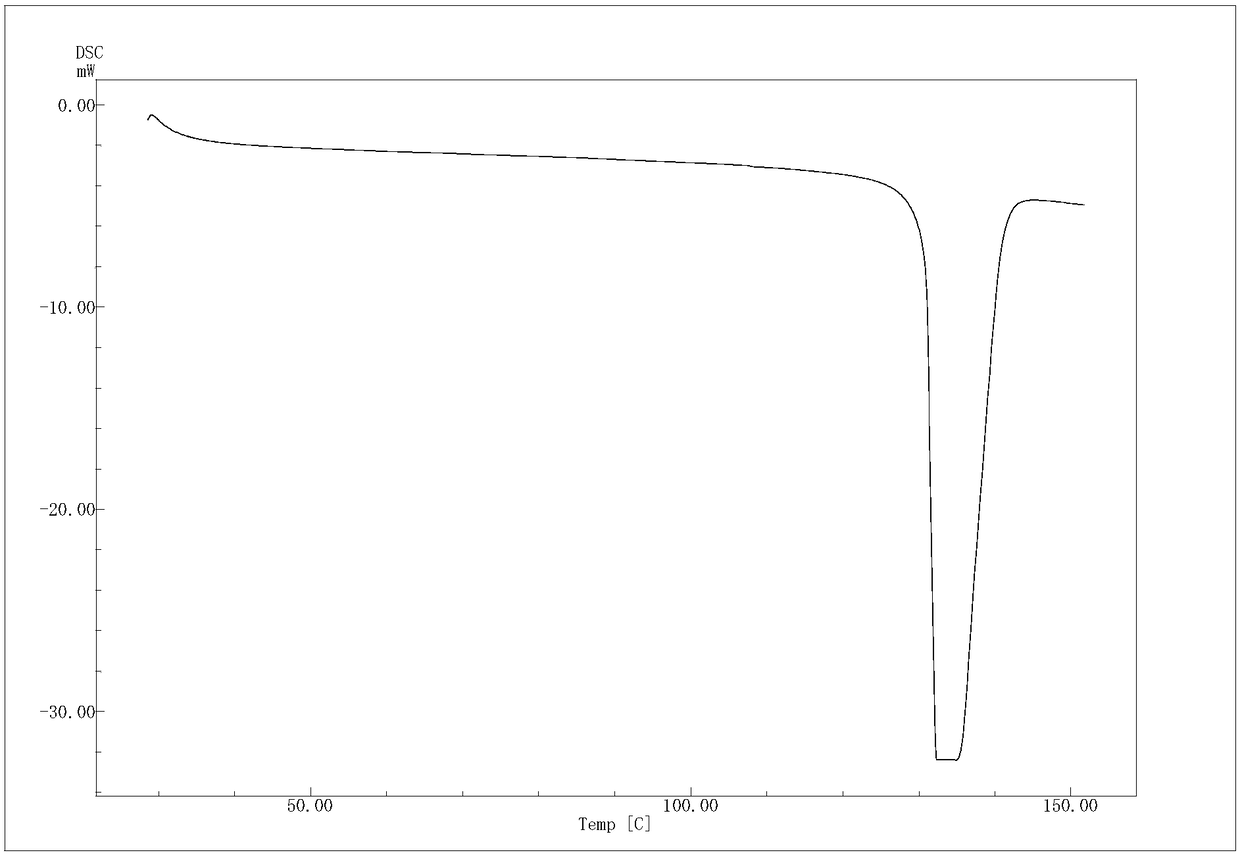
(R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form, and preparation method and use thereof
InactiveCN108299267AEasy to synthesizePromote brain metabolismOrganic active ingredientsNervous disorderSolubilityPhosphorylcholine
The invention provides an (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form. The (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form has diffraction peaks at 2theta of 12.423 + / - 0.2 DEG, 16.465 + / - 0.2 DEG, 17.344 + / - 0.2 DEG, 21.889 + / - 0.2 DEG and 25.054 + / - 0.2 DEG. The (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form can promote the synthesis of phosphorylcholine and o-acylethanolamine, promote brain metabolism, has a stimulation effect on the specific central nervous pathway through blood brain barrier, and has special bioactivity in calming and anti-epilepsy fields. Thepeak melting temperature of the (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form is 133.1 + / - 2 DEG C, and the (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form has a fast dissolution rate in water, has a solubility in water of 100 mg / mL or more, has a high bioavailability, a good stability and a good particle fluidity, and is suitable for being applied to the production of medicinalpreparations, storage and transportation; and the (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form is suitable for preparing various medicinal compositions, and also can be processed to prepare various preparations, such as a tablet, a capsule, a dripping pill, a sustained release and controlled release preparation and a lyophilized powder injection. The preparation method is simple, and is suitable for industrial production.
Owner:CHONGQING RUNZE PHARM CO LTD
Uniform-content (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule wand preparation method thereof
InactiveCN107510679AFast dissolutionDecrease increaseOrganic active ingredientsNervous disorderPolyethylene glycol1-pyrrolidineacetamide
An (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule is prepared from, by weight, 1 part of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, 0.8-1.3 parts of L-cysteine, 1.1-1.6 parts of mannitol, 0.8-1.2 parts of microcrystalline cellulose, 0.8-1.2 parts of carboxymethylcellulose sodium, 0.5-1.1 parts of lactose, 0.17-0.22 part of talcum powder, 0.6-1.1 parts of polyethylene glycol 4000, 0.7-1.3 parts of hydroxypropyl methylcellulose, 0.8-1.3 part of low-substituted hydroxypropyl cellulose, 0.05-0.10 part of polysorbate 80, 0.8-1.8 parts of sorbitol, 0.18-0.27 part of honey, 12-18 parts of 60-70% ethanol and 1-5 parts of 20-30% ethanol. The (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule prepared in the invention has the advantages of small impurity increasing amount only being 0.04%, no adhesion to a screen during granulation, small quantity of powder layers, uniform particle size, good fluidity, realization of the Xiuzi angle being less than 38 DEG, small loading difference being less than 5%, short complete-dissolution time being less than 30 s, good content uniformity, realization of the content RSD of multiple points being less than 2%, good stability during storage, and long shelf life reaching up to 24 months.
Owner:CHONGQING RUNZE PHARM CO LTD
Method for preparing (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form
InactiveCN108299268AEasy to synthesizePromote brain metabolismOrganic chemistry methodsSolubilityPhosphorylcholine
The invention provides a method for preparing an (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form. The (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form has diffraction peaks at 2theta of12.423 + / - 0.2 DEG, 16.465 + / - 0.2 DEG, 17.344 + / - 0.2 DEG, 21.889 + / - 0.2 DEG and 25.054 + / - 0.2 DEG. The (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form can promote the synthesis of phosphorylcholine and o-acylethanolamine, promote brain metabolism, has a stimulation effect on the specific central nervous pathway through blood brain barrier, and has special bioactivity in calming and anti-epilepsy fields. The peak melting temperature of the (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form is 133.1 + / - 2 DEG C, and the (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form has a fast dissolution rate in water, has a solubility in water of 100 mg / mL or more, has a high bioavailability, a good stability and a good particle fluidity, and is suitable for being applied to theproduction of medicinal preparations, storage and transportation. The preparation method is simple, and is suitable for industrial production.
Owner:CHONGQING RUNZE PHARM CO LTD
(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule and preparation method thereof
ActiveCN107510681ADecrease increasePromote increaseOrganic active ingredientsNervous disorder1-pyrrolidineacetamidePolyethylene glycol
An (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule is prepared from, by weight, 1 part of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, 0.7-1.3 parts of L-cysteine, 1.0-1.5 parts of mannitol, 0.7-1.2 parts of microcrystalline cellulose, 0.9-1.5 parts of carboxymethylcellulose sodium, 0.7-1.3 parts of lactose, 0.13-0.18 part of magnesium stearate, 0.6-1.1 parts of polyethylene glycol 4000, 0.9-1.7 parts of hydroxypropyl methylcellulose, 0.8-1.3 part of low-substituted hydroxypropyl cellulose, 0.06-0.11 part of polysorbate 80, 0.5-1.2 parts of honey and 8-13 parts of an ethanol solution with the volume fraction being 50-70%. The (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide prepared in the invention has the advantages of small impurity increasing amount only being 0.03%, no adhesion to a screen and easy granulation during granulation, fast dissolution speed, short complete-dissolution time not exceeding 30 s, good stability during storage, no moisture absorbing caking, and long shelf life reaching up to 24 months.
Owner:武汉恒信源药业有限公司
Preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
A preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide comprises allowing reaction of ethyl glycinate hydrochloride and ethyl (S)-4-halo-3-hydroxybutyrate in alcohol solvent and alkaline conditions, filtering, washing, concentrating, extracting, separating, introducing ammonia water to obtain a crude product, and purifying the crude product, wherein the ethyl glycinate hydrochloride is dissociated by using diethyl ether and ammonia gas to obtain ethyl glycinate; the alcohol solvent is anhydrous methanol or anhydrous ethanol; and the alkaline condition is provided by sodium carbonate or sodium bicarbonate. The method adopts ethyl (S)-4-halo-3-hydroxybutyrate and ethyl glycinate hydrochloride as main raw materials, which has the characteristics of cheap and easily-accessible raw material, environment friendliness and no pollution; and the method firstly carries out dissociation on the ethyl glycinate hydrochloride, which effectively reduces the material consumption in reaction and lowers the cost, and simultaneously plays a positive role in the reaction yield. The method has the characteristics of low preparation cost, high yield up to 36% and mild reaction conditions, and is suitable for industrial production. The prepared (S)-oxiracetam product has high performance liquid chromatography (HPLC) purity up to above 98.5%.
Owner:CHONGQING RUNZE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com