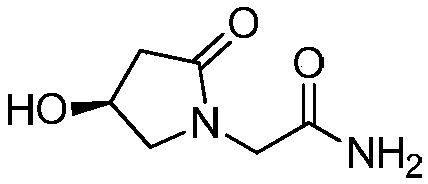
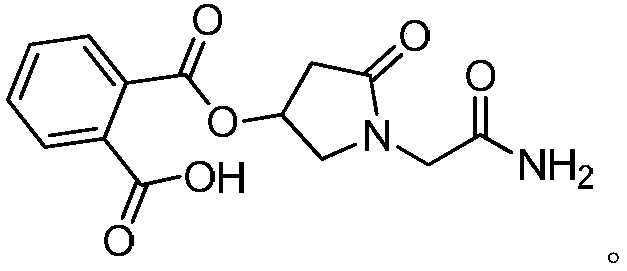
A kind of preparation method of (s)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide
A technology of pyrrolidine acetamide and oxopyrrolidine, which is applied in the field of preparing -4-hydroxy-2-oxo-1-pyrrolidine acetamide and oxiracetam derivatives, can solve the problem of large amount of solvent and pollution large, time-consuming and other problems, to achieve the effect of easy preparation, cheap and easy to obtain acid anhydrides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of racemic 2-(((1-(2-amino-2-oxoethyl)-5-oxopyrrolidinyl-3-yl) oxygen) carbonyl) benzoic acid
[0034] Add 15.7g phthalic anhydride, 15.8g (0.1mol) racemic oxiracetam, 8.2g pyridine to the reactor, stir the mixture at 77°C for 1.5 hours, cool to room temperature, and acidify with 10% hydrochloric acid to pH to 1.0, extracted with ethyl acetate, the organic phase was washed successively with dilute hydrochloric acid, pure water, and saturated brine, dried, and concentrated to give 30 g of a white solid, with a yield of 98% (based on oxiracetam); elemental analysis: C , 55.03; H, 4.62; N, 9.14; O, 31.38. HRMS (C 14 h 14 N 2 o 6 ): calculated 306.2708, measured 306.2736.
Embodiment 2
[0035] Example 2 Preparation of racemic 2-(((1-(2-amino-2-oxoethyl)-5-oxopyrrolidinyl-3-yl)oxy)carbonyl)benzoic acid
[0036] Add 158g of phthalic anhydride, 158g (1mol) of racemic oxiracetam, and 83g of pyridine into the reactor, stir the mixture at 85°C for 1.5 hours, cool to room temperature, and acidify to pH 1.5 with 10% hydrochloric acid, After extraction with ethyl acetate, the organic phase was washed successively with dilute hydrochloric acid, pure water, and saturated brine, dried, and concentrated to obtain 302 g of a white solid, with a yield of 98.7% (based on oxiracetam).
Embodiment 3
[0037] Example 3 Preparation of racemic 2-(((1-(2-amino-2-oxoethyl)-5-oxopyrrolidinyl-3-yl)oxy)carbonyl)benzoic acid
[0038] Add 1.57kg of phthalic anhydride, 1.58kg of racemic oxiracetam, and 830g of pyridine into the reactor, stir the mixture at 80°C for 2.0 hours, cool to room temperature, acidify to pH 2.0 with 10% hydrochloric acid, and acetic acid After extraction with ethyl ester, the organic phase was washed successively with dilute hydrochloric acid, pure water, and saturated brine, dried, and concentrated to obtain 3.0 kg of a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com