Avalopam intermediate as well as preparation method and application thereof
A technology for avacopam and uses, applied in the field of avacopam intermediates and their preparation, can solve the problems of long preparation steps, low yield of final products, etc., and achieves cheap and easy-to-obtain raw materials and high yield of final products , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
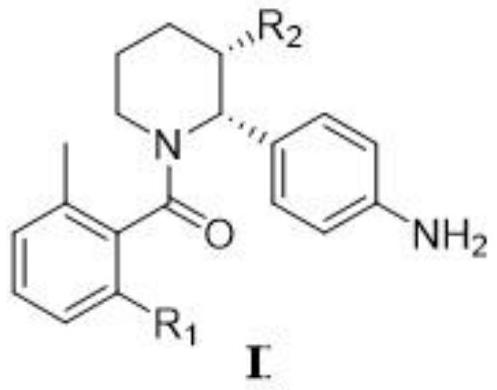
[0046] The synthesis of embodiment 1 compound I
[0047] Formula II compound (R 1 is fluorine; R 2 for COR 4 ; 3 is nitro; R 4 For methoxy) reduction preparation formula I compound (R 1 is fluorine; R 2 for COR 4 ; 4 For methoxy) reaction scheme is:
[0048]
[0049] In containing bis(1,5-cyclooctadiene) rhodium(I) tetrafluoroborate (Rh(COD) 2 BF 4, 81mg, 0.2mmol) and ligand (R)-Ligand 1 (174mg, 0.4mmol) in dichloromethane (20mL) reaction flask, was added compound II (4g, 10mmol) isopropanol solution (40mL). It was then stirred at room temperature under 1 atm of hydrogen for 18 hours, and TLC showed that the reaction was complete. The reaction solution was diluted with 200mL ethyl acetate, and then saturated NaHCO 3 aqueous solution (100mL X2), saturated NaCl aqueous solution (100mL X2), and the organic layer was washed with anhydrous NaCl 2 SO 4 After drying, it was concentrated under reduced pressure, and the residue was separated through a silica gel column...
Embodiment 2
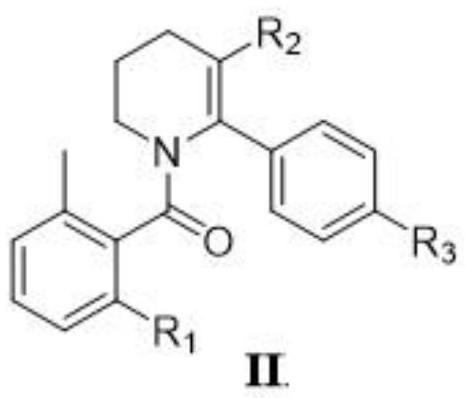
[0050] The synthesis of embodiment 2 compound II
[0051] Formula II compound (R 1 is fluorine; R 2 for COR 4 ; 3 is nitro; R 4 is methoxy) by formula III compound (R 2 for COR 4 ; 3 is nitro; R 4 For methoxy) and formula IV compound (R 1 It is prepared by fluorine) coupling, and the reaction formula is as follows:
[0052]
[0053] Dissolve 4.9g (22mmol) of compound III in 100mL of anhydrous acetone, then add 4.2g (30mmol) of K 2 CO 3 , and 5.5g (20mmol) of compound IV in 10mL of DMF, stirred at room temperature for 36 hours, added 5g of anhydrous Na 2 SO 4 , and then continued to stir at 50° C. for 3 hours, TLC showed that the reaction was complete. The residue after the reaction solution was concentrated under reduced pressure was added to 200 mL ethyl acetate, washed with saturated NaCl (100 mL X2) aqueous solution, and the organic layer was washed with anhydrous NaCl 2 SO 4 After drying, the concentrated crude product was filtered and separated through a...
Embodiment 3
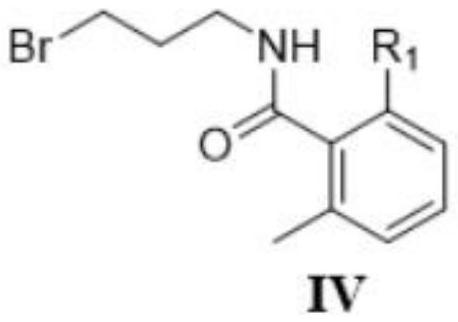
[0054] The synthesis of embodiment 3 compound IV
[0055] IV compound (R 1 is fluorine) by formula VI compound and formula V compound (R 1 It is prepared by fluorine) reaction, and the reaction formula is as follows:
[0056]
[0057] Add 50mL of anhydrous THF and 0.2mL of anhydrous DMF, and 3.1g (20mmol) of compound V (2-fluoro-6-methylbenzoic acid) into a 250mL dry three-necked flask with magnetic stirring, and cool down in a low-temperature reaction tank After reaching 0°C, a THF (15 mL) solution containing 1.7 mL (20 mmol) oxalyl chloride was added dropwise, and after the addition was completed, it was moved to room temperature and stirred for 2 hours. After the reaction system was cooled to 0°C, a THF (20 mL) solution containing 1.7 mL (40 mmol) of pyridine and 2.8 g (20 mmol) of compound VI was added dropwise, and after the addition was completed, it was moved to room temperature and continued to stir for 4 hours. TLC showed that the reaction was complete. Add 200m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com