Preparation method for synthesizing chiral nicotine from chiral tert-butyl sulfinamide
A technology of tert-butylsulfinamide and sulfenamide, which is applied in the direction of organic chemistry and organic chemistry, can solve the problems of complex preparation methods of organometallic catalysts, multiple reaction steps in the synthesis of chiral nicotine, and low yield of chiral nicotine. problem, to achieve the effect of short reaction route, high ee value, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Examples 1-15 provide a preparation method for synthesizing chiral nicotine from chiral tert-butylsulfinamide, and the following uses Example 1 as an example to illustrate.
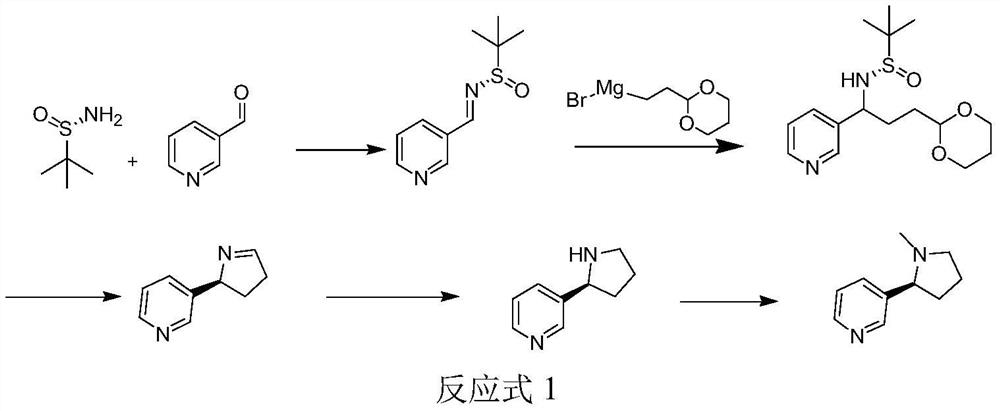
[0044] The preparation method for synthesizing chiral nicotine with chiral tert-butyl sulfinamide provided in Example 1, wherein the chiral tert-butyl sulfinamide is S-tert-butyl sulfinamide, and the chiral nicotine is S- -chiral nicotine, its synthetic route is as shown in Reaction Formula 1:
[0045]
[0046] Concrete preparation steps are:
[0047] S1. Under a nitrogen atmosphere, 106.7g (1mol, 1eq) 3-pyridinecarbaldehyde, 121.7g (1mol, 1eq) (S)-tert-butylsulfinamide and 455.5g (2mol, 2eq) tetraethyl titanate Dissolve in 6L of anhydrous tetrahydrofuran and react at 70°C for 2h. After the reaction, pour the reaction solution into 10L of saturated saline solution, stir at 1000rpm for 15min, filter, take the filtrate, wash the filter cake with 3L of ethyl acetate, and collect The filtrate, the...
Embodiment 2-3
[0051] The only difference between Examples 2-3 and Example 1 is that in the S1 step reaction, the amount of titanate is adjusted, as shown in Table 1 for details.
[0052] The impact of the amount of titanate in table 1 on (S)-nicotine yield
[0053] Numbering Equivalent number of titanate (eq) (S)-nicotine yield (%) Example 1 2 72 Example 2 1 43 Example 3 3 68
Embodiment 4
[0054] Example 4 differs from Example 1 only in that in the S1 step reaction, the type of titanate is adjusted, as shown in Table 2 for details.
[0055] The selection of table 2 titanate affects the yield of (S)-nicotine
[0056] Numbering titanate selection (S)-nicotine yield (%) Example 1 Tetraethyl titanate 72 Example 4 Tetrabutyl titanate 70
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com