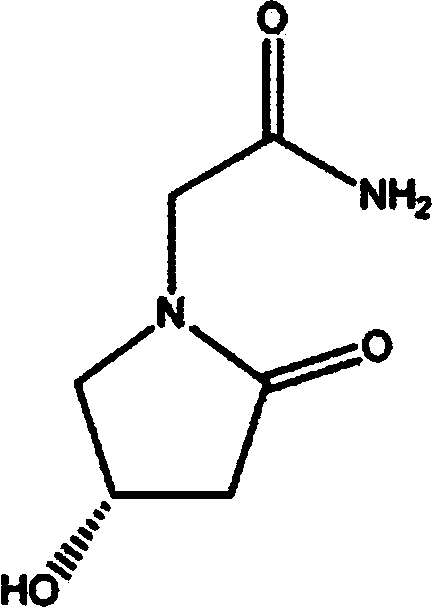
A kind of (s)-4-hydroxy-2-oxo-1-pyrrolidineacetamide sustained-release capsule and its preparation method
A technology of pyrrolidine acetamide and sustained-release capsules is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. The problems of slow-release preparations cannot be achieved, and the preparation process is simple and feasible, the number of doses is reduced, and the granule fluidity is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
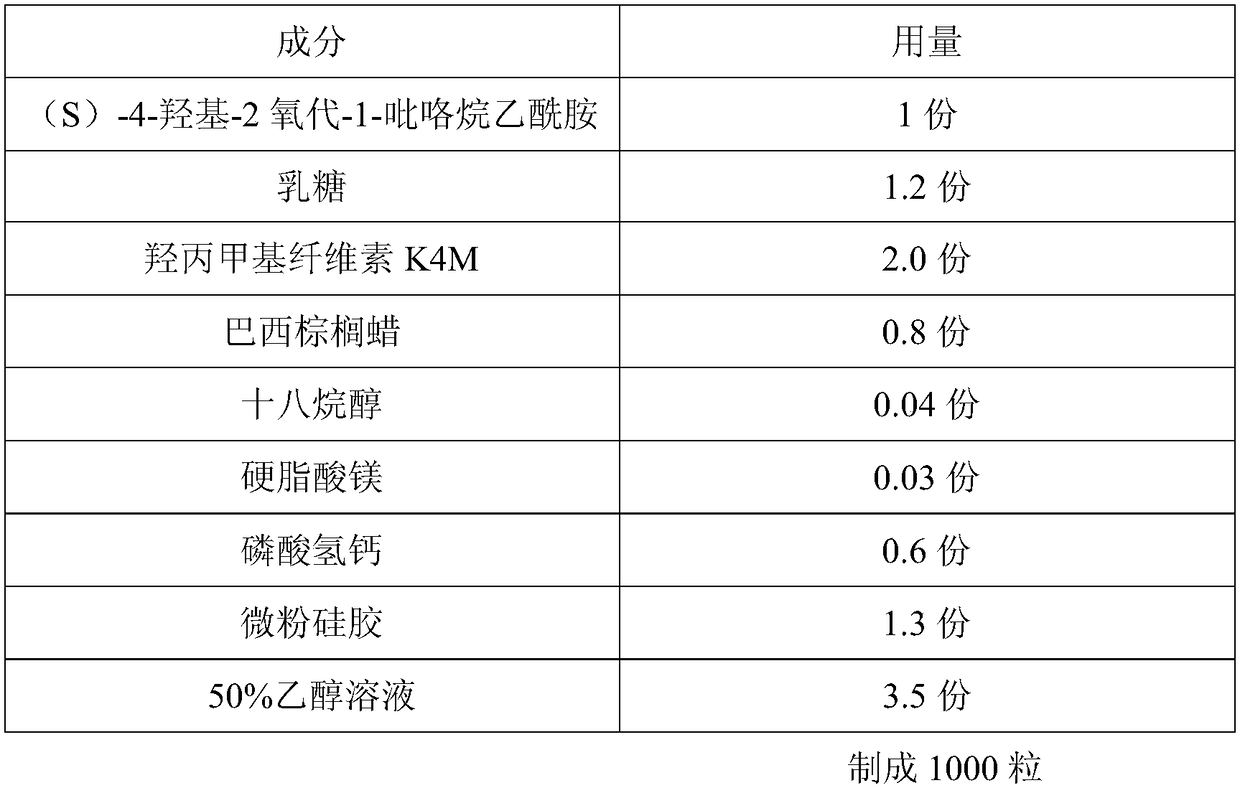
[0023] A kind of (S)-4-hydroxyl-2 oxo-1-pyrrolidineacetamide slow-release capsules, prepared according to the following steps:
[0024]
[0025] Preparation process:
[0026] 1. Pretreatment of raw and auxiliary materials: take the prescribed amount of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, lactose, hydroxypropylmethylcellulose, carnauba wax, calcium hydrogen phosphate, and micronized silica gel Mix and pulverize into fine powder in the mixing pulverizer (the amount that can pass through No. 5 sieve and No. 6 sieve must not be less than 95% of the total amount), and sieve;
[0027] 2. Granulation: Add ethanol solution, mix and granulate with 18-mesh sieve, place the wet granules in a hot air oven, set the temperature at 40-60°C, dry until the moisture content of the granules is ≤3%, and granulate (over 24 Mesh sieve), spare;
[0028] 3. Total blending: crush stearyl alcohol and magnesium stearate through a 100-mesh sieve, add to the granulated granules, and mix with ...
Embodiment 2
[0067] A kind of (S)-4-hydroxyl-2 oxo-1-pyrrolidineacetamide slow-release capsules, prepared according to the following steps:
[0068]
[0069]
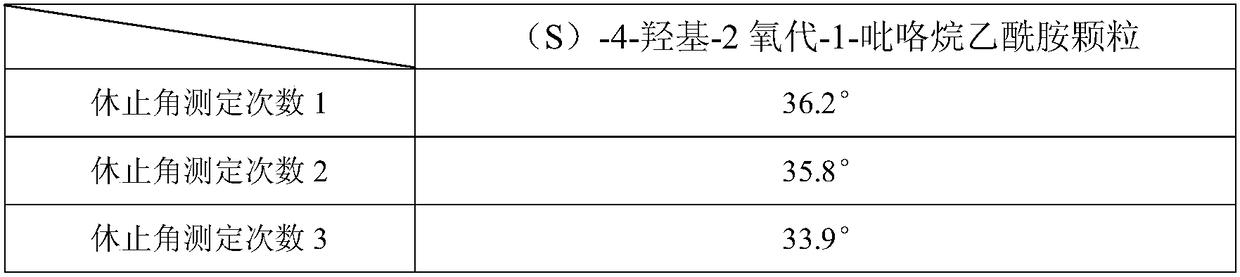
[0070] Preparation process: prepared according to the preparation process of Example 1. Tested by the test method of Example 1, the angle of repose result shows that this product has good fluidity, and the angle of repose is less than 36 °, and the release measurement test result shows that (S)-4-hydroxyl-2 oxo-1-pyrrolidine acetamide Sustained-release capsules are released slowly, with a release time of up to 12 hours, which can meet the requirements of sustained-release preparations. The results of the stability test show that the sample quality is stable in six months of acceleration, and no capsule adhesion occurs. The quality is stable for a long period of 24 months, and no capsules Adhesion phenomenon, so this product is valid for at least 24 months.
Embodiment 3
[0072] A kind of (S)-4-hydroxyl-2 oxo-1-pyrrolidineacetamide slow-release capsules, prepared according to the following steps:
[0073]
[0074] Preparation process: prepared according to the preparation process of Example 1. Tested by the test method of Example 1, the angle of repose result shows that this product has good fluidity, and the angle of repose is less than 37 °, and the release measurement test result shows that (S)-4-hydroxyl-2 oxo-1-pyrrolidine acetamide Sustained-release capsules are released slowly, with a release time of up to 12 hours, which can meet the requirements of sustained-release preparations. The results of the stability test show that the sample quality is stable in six months of acceleration, and no capsule adhesion occurs. The quality is stable for a long period of 24 months, and no capsules Adhesion phenomenon, so this product is valid for at least 24 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com