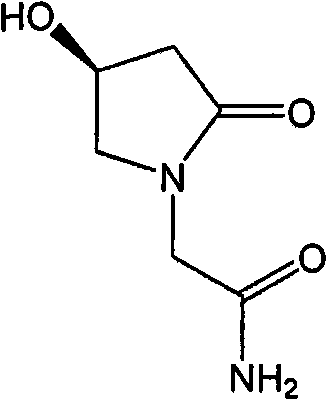
Preparation method of (s)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
A technology of pyrrolidine acetamide and oxo, which is applied in directions such as organic chemistry, can solve the problems of high impurity content of levo-oxiracetam, unfavorable feeding method for industrialized production and the like, and achieves the effects of high purity, easy operation and quality improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation steps of levo-oxiracetam of the present invention are as follows:
[0031] 1. Put 65.0g of glycinamide hydrochloride, 500ml of absolute ethanol and 49.3g of sodium bicarbonate into a three-necked reaction flask, and raise the temperature to reflux under stirring;
[0032] 2. After heating to reflux for 2 hours, add 49.3g of sodium bicarbonate, then dropwise add (S)-4-chloro-3-hydroxybutyric acid ethyl ester 97.7g, continue the reflux reaction for 24h after the dropwise addition, and the reaction solution is slightly cooled After filtering, the filtrate was concentrated to obtain a reddish-brown oil;
[0033] 3. Dissolve the sample with 65ml of water, extract 260ml of dichloromethane four times (65ml each time), concentrate the aqueous solution to remove residual dichloromethane, pass through the 001X7 cation exchange resin after dilution of the aqueous solution, and collect the product containing part Neutralize with anion exchange resin 201X7, remove t...
Embodiment 2
[0037] The preparation steps of levo-oxiracetam are as follows:
[0038] 1. Put 65.0g of glycinamide hydrochloride, 500ml of absolute ethanol and 49.3g of sodium bicarbonate into a three-necked reaction flask, and raise the temperature to reflux under stirring;
[0039]2. After heating to reflux for 2 hours, add 49.3g of sodium bicarbonate, then dropwise add (S)-4-chloro-3-hydroxybutyric acid ethyl ester 97.7g, continue the reflux reaction for 24h after the dropwise addition, and the reaction solution is slightly cooled After filtering, the filtrate was concentrated to obtain a reddish-brown oil;
[0040] 3. Dissolve the sample with 65ml of water, extract with 260ml of dichloromethane, concentrate the aqueous solution to remove residual dichloromethane, pass the aqueous solution through 001X7 cation exchange resin after dilution, and neutralize the product-containing part of the collected liquid with anion exchange resin 201X7, and filter to remove Resin, the solution is conc...
Embodiment 3-6
[0043] Embodiment 3-6: Carry out according to following substance and its amount and process parameter, other are all identical with embodiment 1. The prepared levo-oxiracetam has a purity greater than 99.3%, and the impurity is 0-5%, calculated by mass percentage.
[0044] Reality
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com