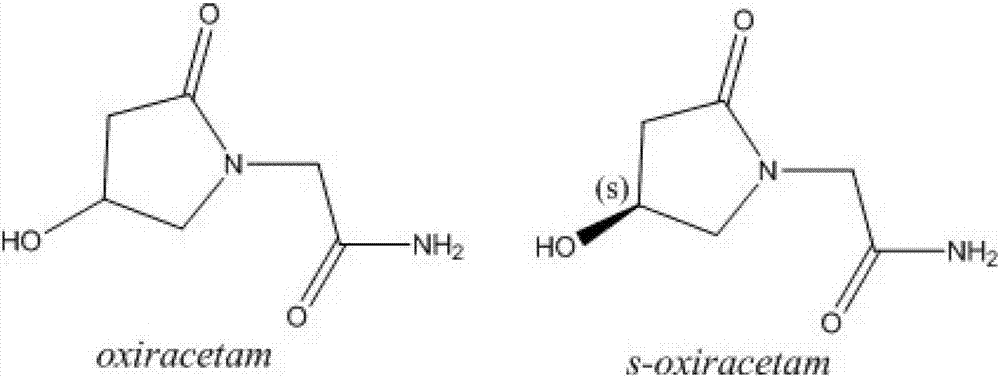
Uniform-content (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule wand preparation method thereof
A technology of pyrrolidine acetamide and hydroxyl group, which is applied in the field of -4-hydroxy-2 oxo-1-pyrrolidine acetamide particles and their preparation, and can solve the problem that the particle size is not easy to control, the particles have strong hygroscopicity and are easy to stick to the block. and other problems, to achieve the effect of simple and feasible preparation process, good content uniformity, and not easy to absorb moisture and agglomerate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
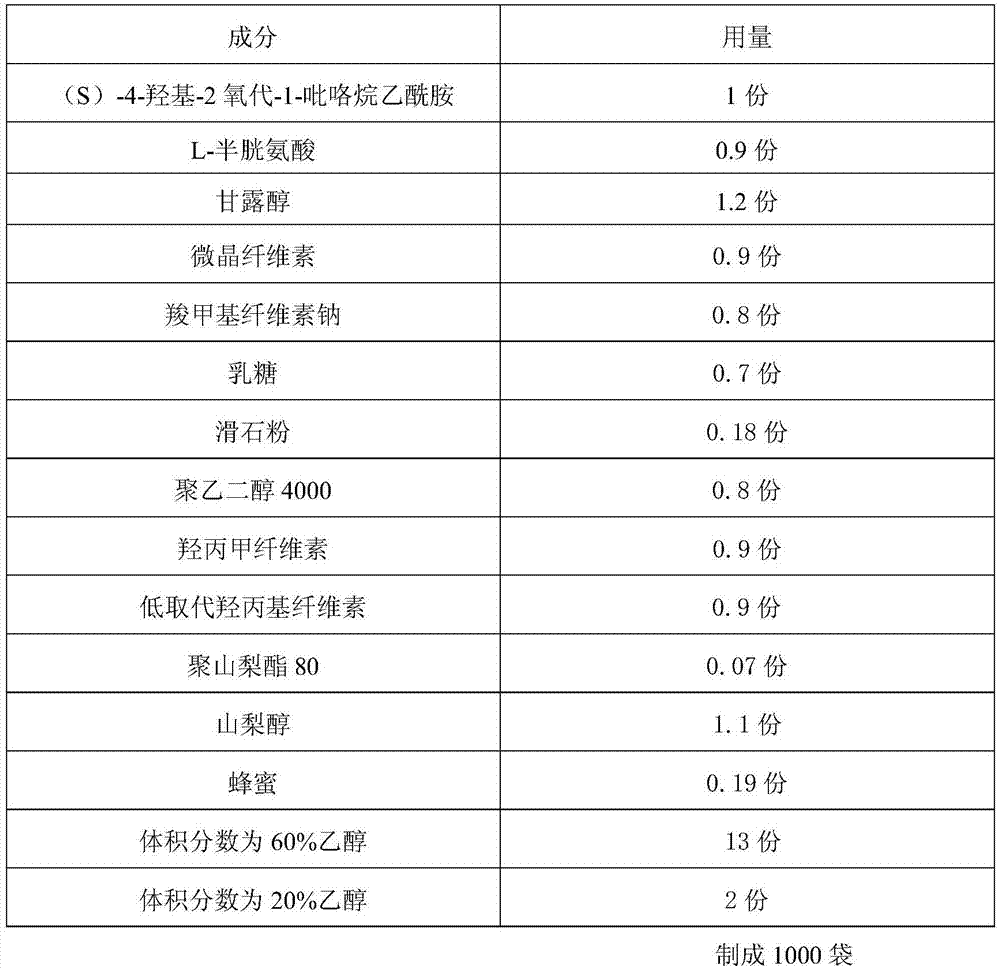
[0027] A kind of (S)-4-hydroxyl-2 oxo-1-pyrrolidineacetamide granule with uniform content is prepared according to the following steps:
[0028]
[0029] Preparation process:
[0030] 1. Preparation of adhesive: Take the prescribed amount of honey, put it in an iron pot, add purified water with 2 times the weight of honey, stir evenly, heat to 100-105°C, keep warm for 20-25 minutes, take it out, and use 80 Filter through a mesh sieve, take the filtrate, let it cool, add ethanol with a volume fraction of 60% to 80% of the prescription amount, stir to dissolve, and set aside;
[0031] 2. Pre-treatment of raw and auxiliary materials: take the prescribed amount of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide and polysorbate 80 and dissolve them in a 20% to 30% ethanol solution with a volume fraction of the prescribed amount , and set aside; another prescription amount of L-cysteine, mannitol, microcrystalline cellulose, sodium carboxymethyl cellulose, lactose, low-substituted hy...
Embodiment 2
[0094] A kind of (S)-4-hydroxyl-2 oxo-1-pyrrolidineacetamide granule with uniform content is prepared according to the following steps:
[0095]
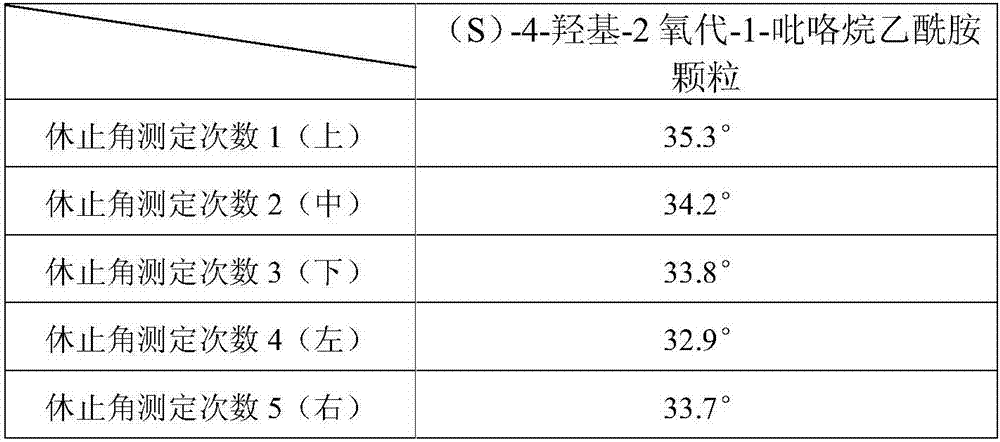
[0096] Preparation process: prepared according to the preparation process of Example 1. Observing the granulation process of the product did not find the phenomenon of sticking to the screen, and the product was easy to granulate. Carry out the test by the test method of embodiment 1, the Hughes angle test measurement result shows that this product granule fluidity is good, and Hughes angle is lower than 38 °, and the content uniformity test result shows that this product content uniformity is good, each after its total mixing The content RSD of point granules is less than 1%, and the difference test of the filling volume shows that the loading volume difference of this product is less than 5%, the loading volume of this product is stable and controllable, and the test results of the influence of product prescription on the incre...
Embodiment 3
[0098] A kind of (S)-4-hydroxyl-2 oxo-1-pyrrolidineacetamide granule with uniform content is prepared according to the following steps:
[0099]
[0100] Preparation process: prepared according to the preparation process of Example 1. Observing the granulation process of the product did not find the phenomenon of sticking to the screen, and the product was easy to granulate. Carry out the test by the test method of embodiment 1, the Hughes angle test measurement result shows that this product granule fluidity is good, and Hughes angle is lower than 36 °, and the content uniformity test result shows that this product content uniformity is good, each after its total mixing The content RSD of point granules is less than 2%. The difference test of the filling volume shows that the filling volume difference of this product is less than 4%. The loading volume of this product is stable and controllable. The test result of the influence of product prescription on the increase of im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com