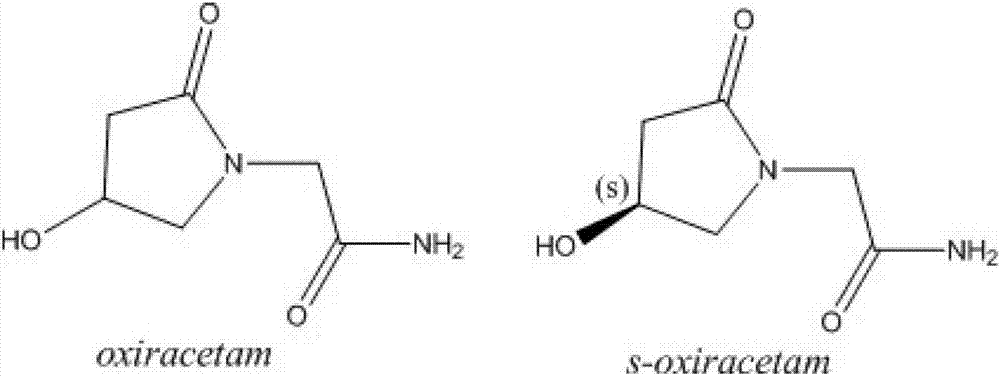
Preparation method of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide lyophilized powder
A technology of pyrrolidine acetamide and freeze-dried powder, which is applied in freeze-dried delivery, powder delivery, nervous system diseases, etc. It can solve the problems of prolonging the production cycle of preparations, the temperature cannot be higher than 20°C, and the sublimation temperature is low, so as to reduce the The effect of adverse drug reactions, shortening the production cycle of preparations, and increasing the sublimation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
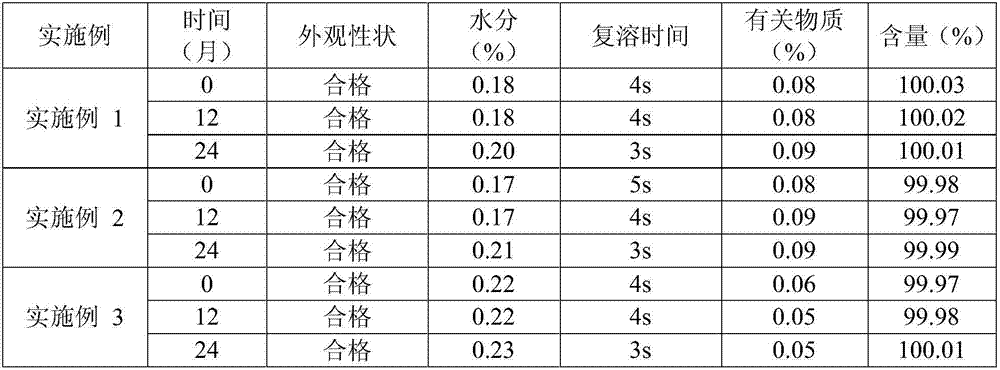
[0018] The prescription of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide freeze-dried powder of embodiment 1 is shown in the table below:
[0019] prescription
weight percentage
(S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide
100g
50g
70g
30g
8g
Water for Injection
Add to 500mL
[0020] The preparation method of the (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide freeze-dried powder of embodiment 1 comprises the following steps:
[0021] (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, methionine, lactose, triethanolamine and benzyl alcohol were dissolved in water for injection, adjusted with 0.1mol / L hydrochloric acid solution pH to 4.5, then lyophilized as follows:
[0022] (1) Pre-freezing stage: Freeze the temperature to -15°C within 5 minutes; then raise the temperature to -9°C for 30 minutes; freeze the temperature to -32°C within 5 minutes again; then raise ...
Embodiment 2
[0026] The prescription of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide freeze-dried powder of embodiment 2 is shown in the table below:
[0027] prescription
weight percentage
(S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide
100g
40g
lactose
50g
20g
5g
Water for Injection
Add to 500mL
[0028] The preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide freeze-dried powder of embodiment 2 comprises the following steps:
[0029] (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, methionine, lactose, triethanolamine and benzyl alcohol were dissolved in water for injection, adjusted with 0.1mol / L hydrochloric acid solution pH to 5.0, then lyophilized as follows:
[0030] (1) Pre-freezing stage: freeze the temperature to -15°C within 10 minutes; then raise the temperature to -10°C, and the heating time is 40 minutes; freeze the temperature to -35°C within 10 minute...
Embodiment 3
[0034] The prescription of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide freeze-dried powder of embodiment 3 is shown in the table below:
[0035] prescription
weight percentage
(S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide
100g
60g
lactose
90g
40g
10g
Water for Injection
Add to 500mL
[0036] The preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide freeze-dried powder of embodiment 3 comprises the following steps:
[0037] (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, methionine, lactose, triethanolamine and benzyl alcohol were dissolved in water for injection, adjusted with 0.1mol / L hydrochloric acid solution pH to 5.0, then lyophilized as follows:
[0038] (1) Pre-freezing stage: freeze the temperature to -18°C within 15 minutes; then raise the temperature to -8°C, and the heating time is 60 minutes; freeze the temperature to -36°C within 15 minute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com