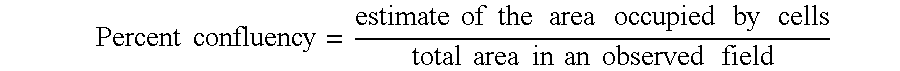Patents
Literature
161 results about "Stage iib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stage IIB. Invasive cancer more extensive than stage I, usually involving local lymph nodes without spread to distant anatomic sites. The definition of stage IIB depends on the particular type of cancer that it refers to; for example, for breast cancer, stage IIB is defined as follows: (T2, N1, MO); (T3, NO, MO).
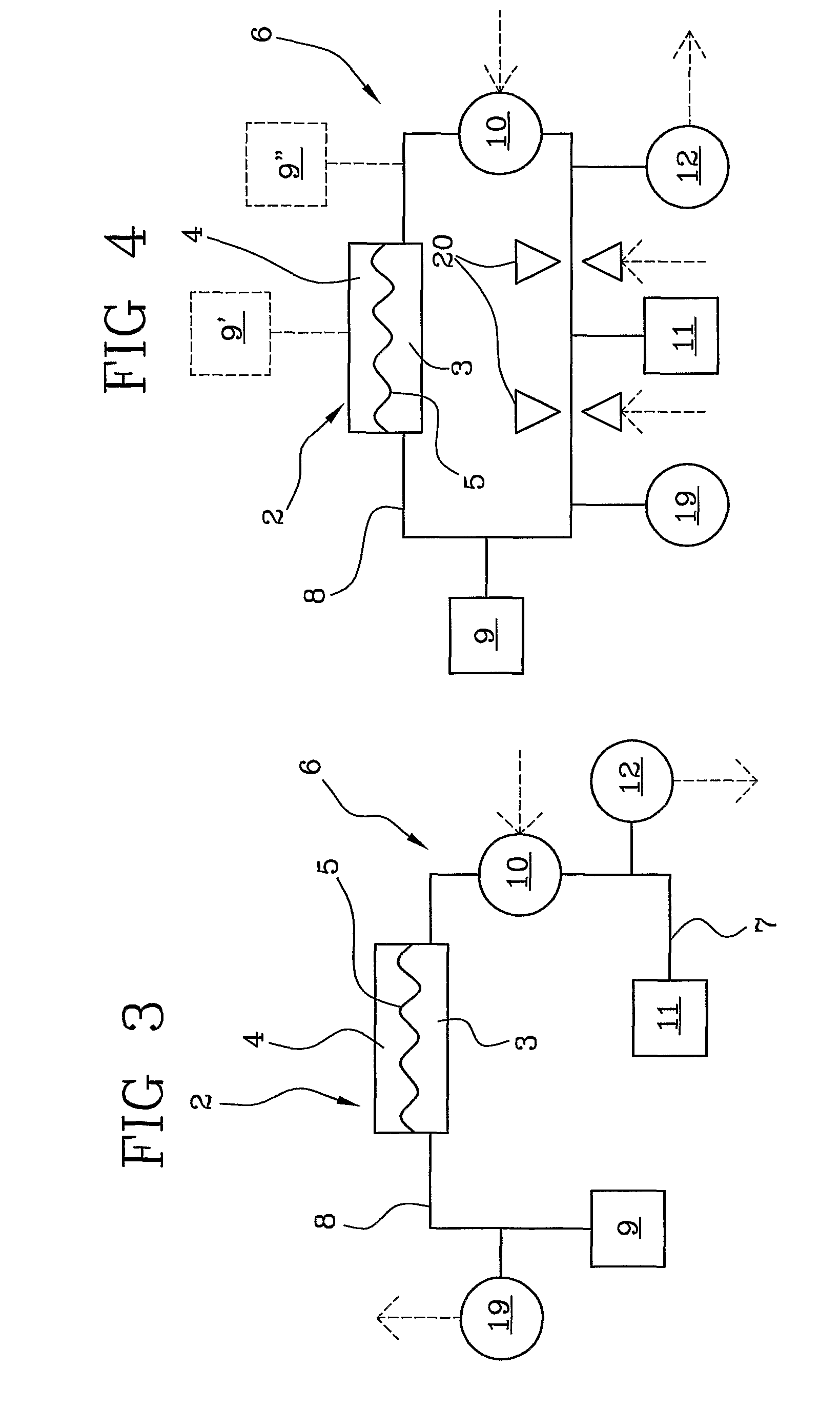
Method for preparing cell cultures from biological specimens for chemotherapeutic and other assays
InactiveUS6887680B2Promote growthMicrobiological testing/measurementMicroorganism separationParticulatesAnticarcinogen
An improved system for screening a multiple of candidate therapeutic or chemotherapeutic agents for efficacy as to a specific patient, in which a tissue sample from the patient is harvested, cultured and separately exposed to a plurality of treatments and / or therapeutic agents for the purpose of objectively identifying the best treatment or agent for the particular patient. Specific method innovations such as tissue sample preparation techniques render this method practically as well as theoretically useful. One particularly important tissue sample preparation technique is the initial preparation of cohesive multicellular particulates of the tissue sample, rather than enzymatically dissociated cell suspensions or preparations, for initial tissue culture monolayer preparation. With respect to the culturing of malignant cells, for example, it is believed (without any intention of being bound by the theory) that by maintaining the malignant cells within a multicellular particulate of the originating tissue, growth of the malignant cells themselves is facilitated versus the overgrowth of fibroblasts or other cells which tends to occur when suspended tumor cells are grown in culture. Practical monolayers of cells may thus be formed to enable meaningful screening of a plurality of treatments and / or agents. Growth of cells is monitored to ascertain the time to initiate the assay and to determine the growth rate of the cultured cells; sequence and timing of drug addition is also monitored and optimized. By subjecting uniform samples of cells to a wide variety of active agents (and concentrations thereof), the most promising agent and concentration for treatment of a particular patient can be determined. For assays concerning cancer treatment, a two-stage evaluation is contemplated in which both acute cytotoxic and longer term inhibitory effect of a given anti-cancer agent are investigated.
Owner:PRECISION THERAPEUTICS
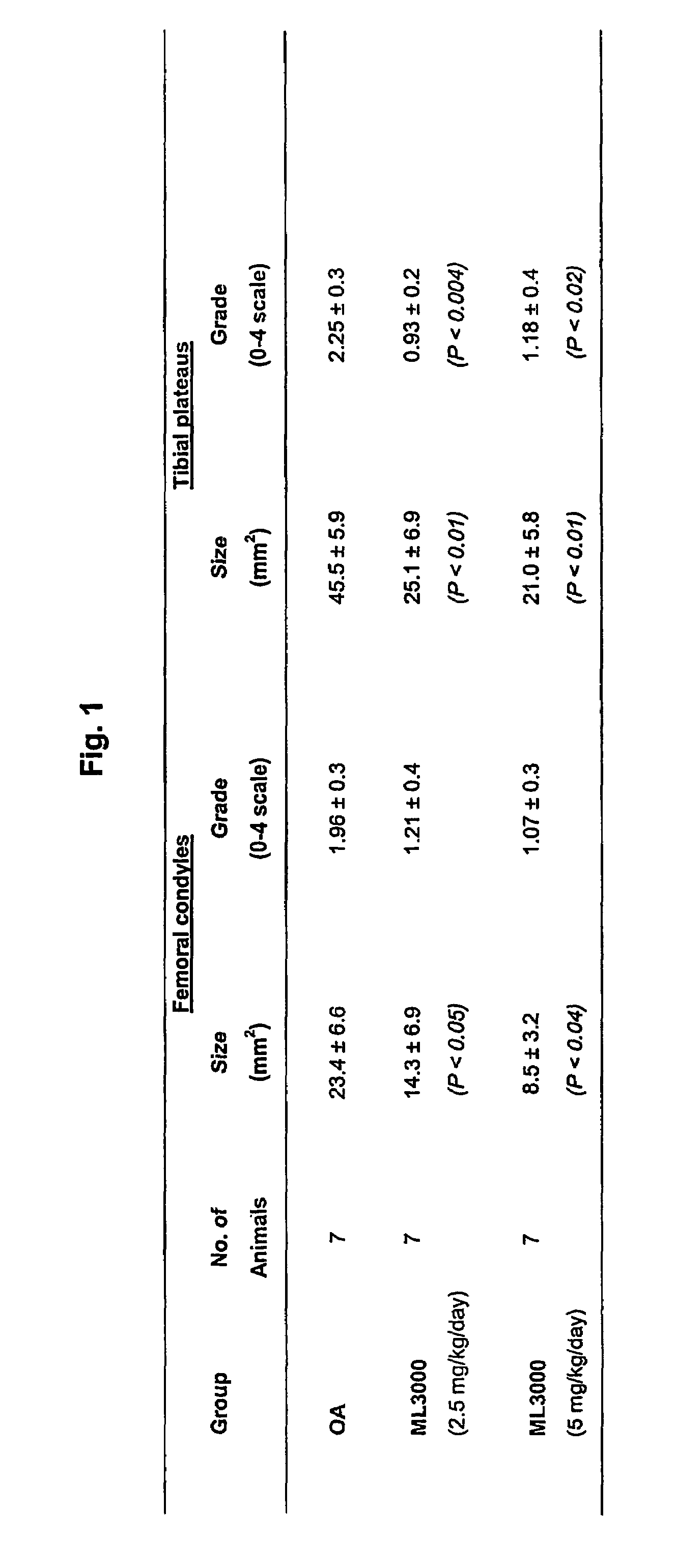
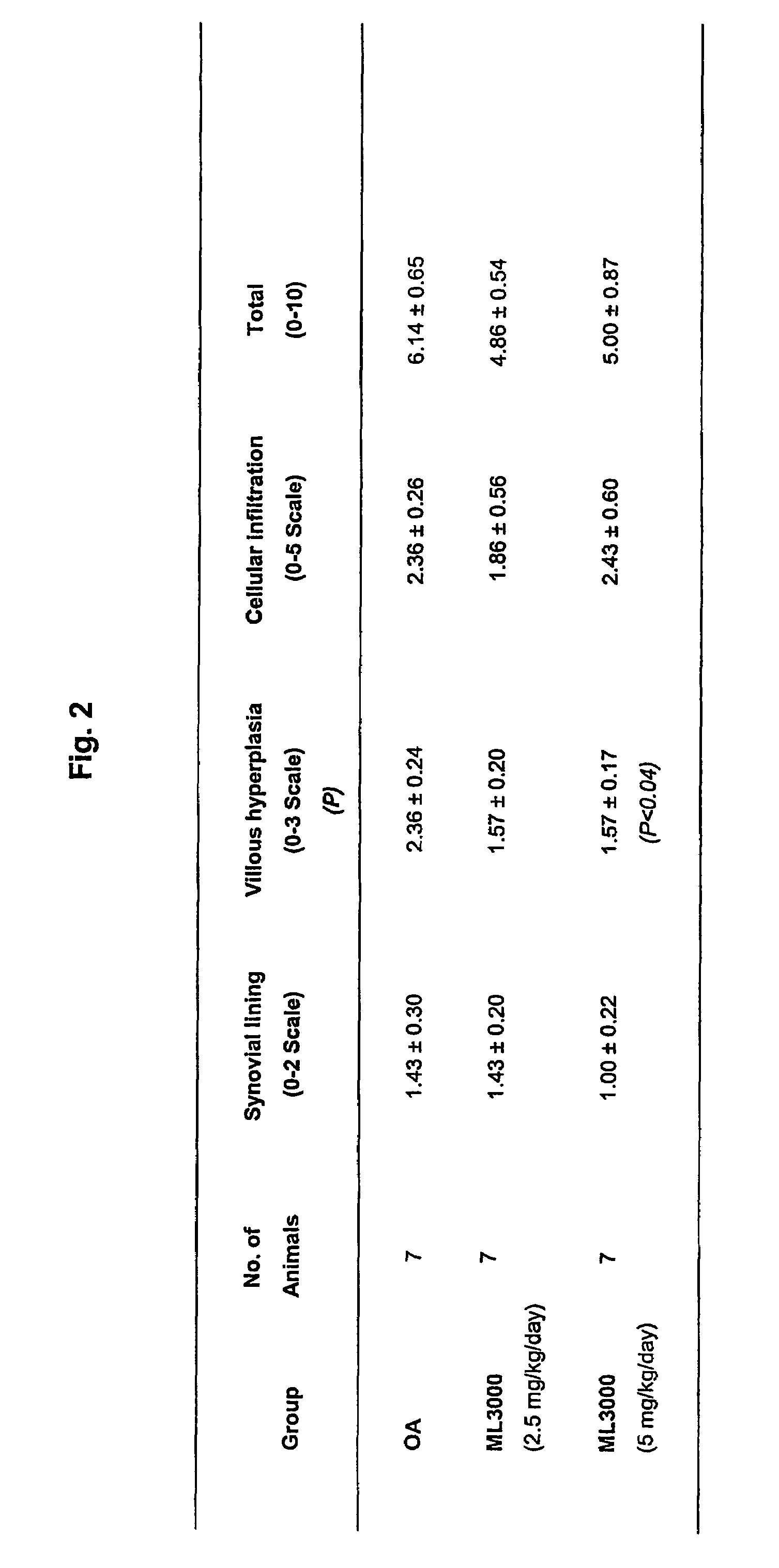
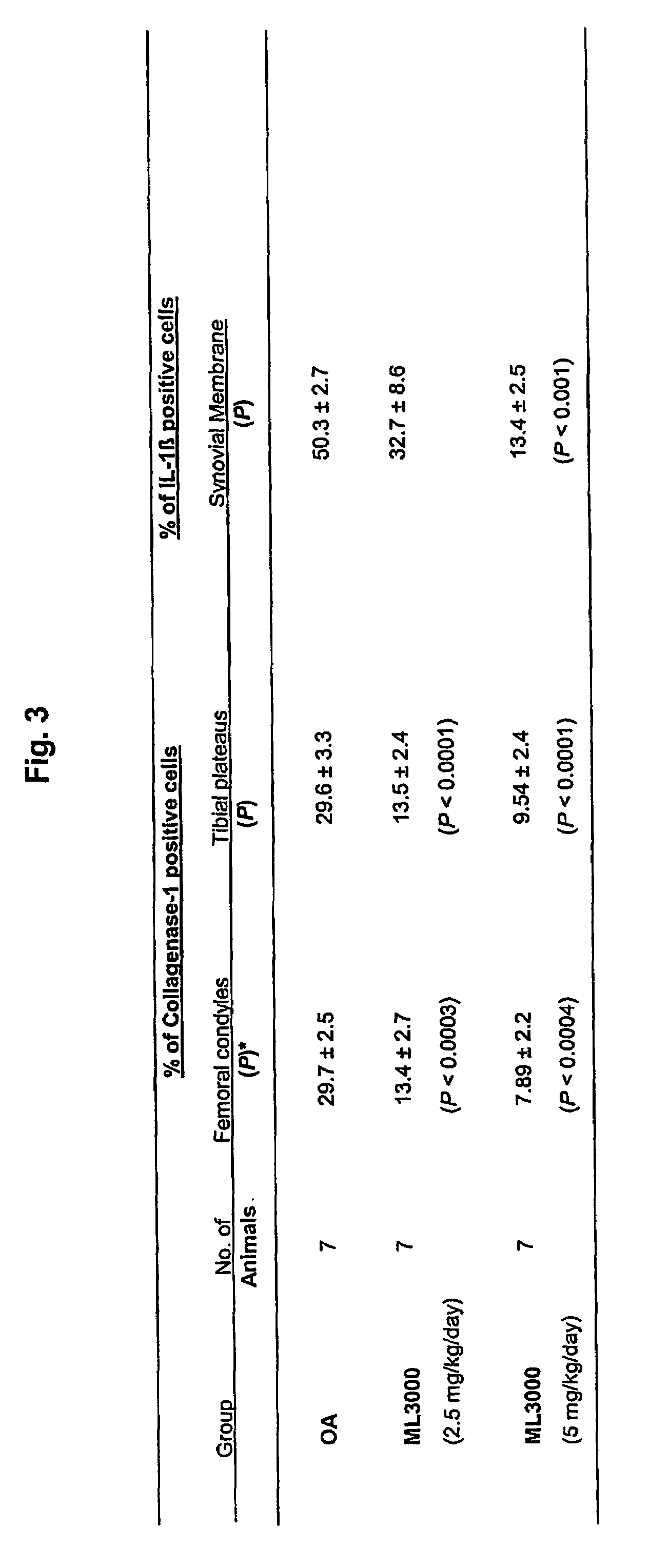
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
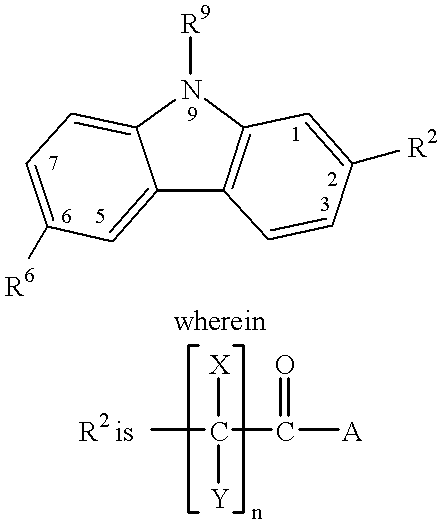
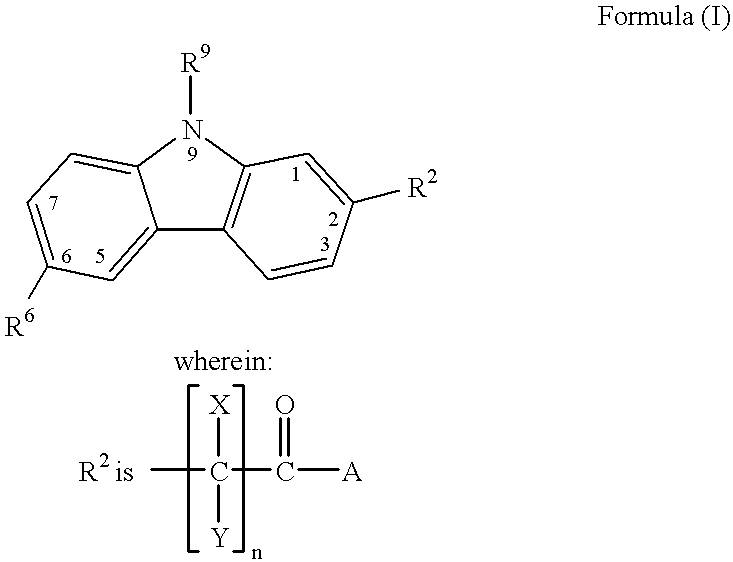
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
Treatment of multiple sclerosis (MS)
The invention relates to the treatment multiple sclerosis, and in particular to the treatment of the inflammatory injury seen in the progressive stages in the disease such as seen with the recurrent upsurges of acute disease, classically known as “relapses” or “exacerbations” or “relapsing / remitting” disease seen in multiple sclerosis. The invention provides a method for modulating a relapsing / remitting disease in a subject suffering therefrom involving providing the subject with a gene-regulatory peptide or functional analogue thereof. Furthermore, the invention provides the use of an NF-κB down-regulating peptide or functional analogue thereof for the production of a pharmaceutical composition for the treatment of relapsing / remitting disease as seen with multiple sclerosis.
Owner:BIOTEMPT
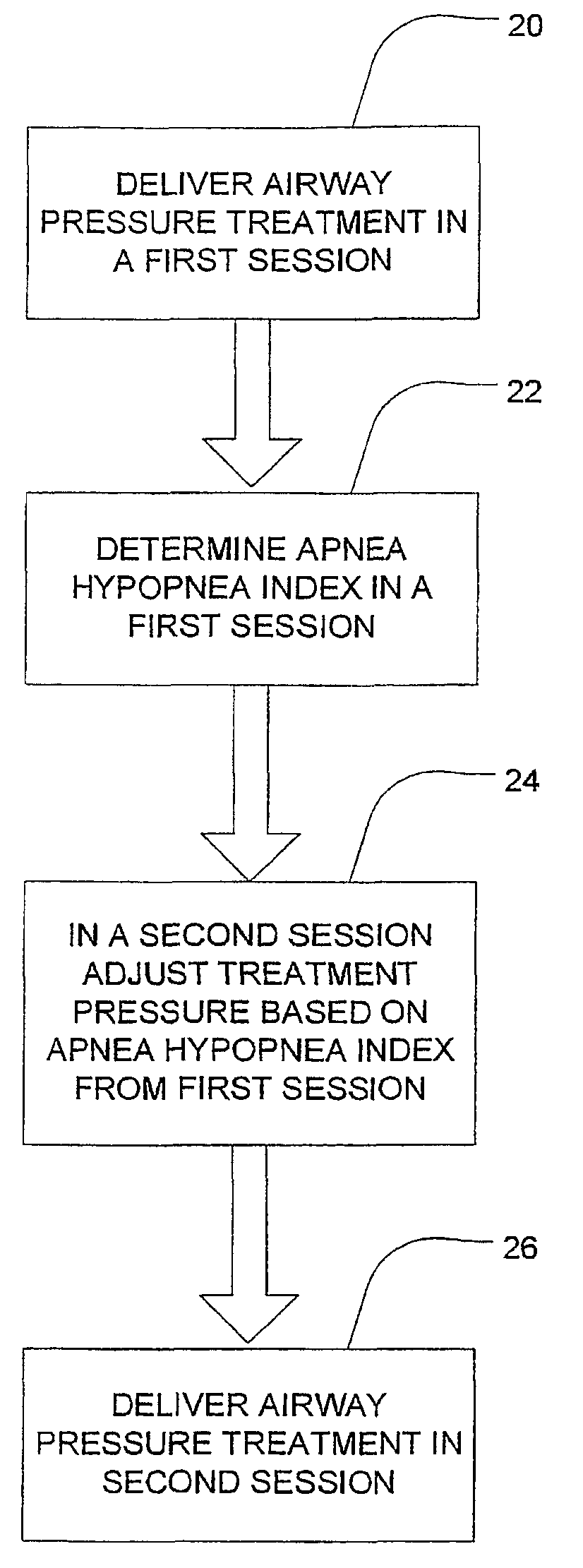
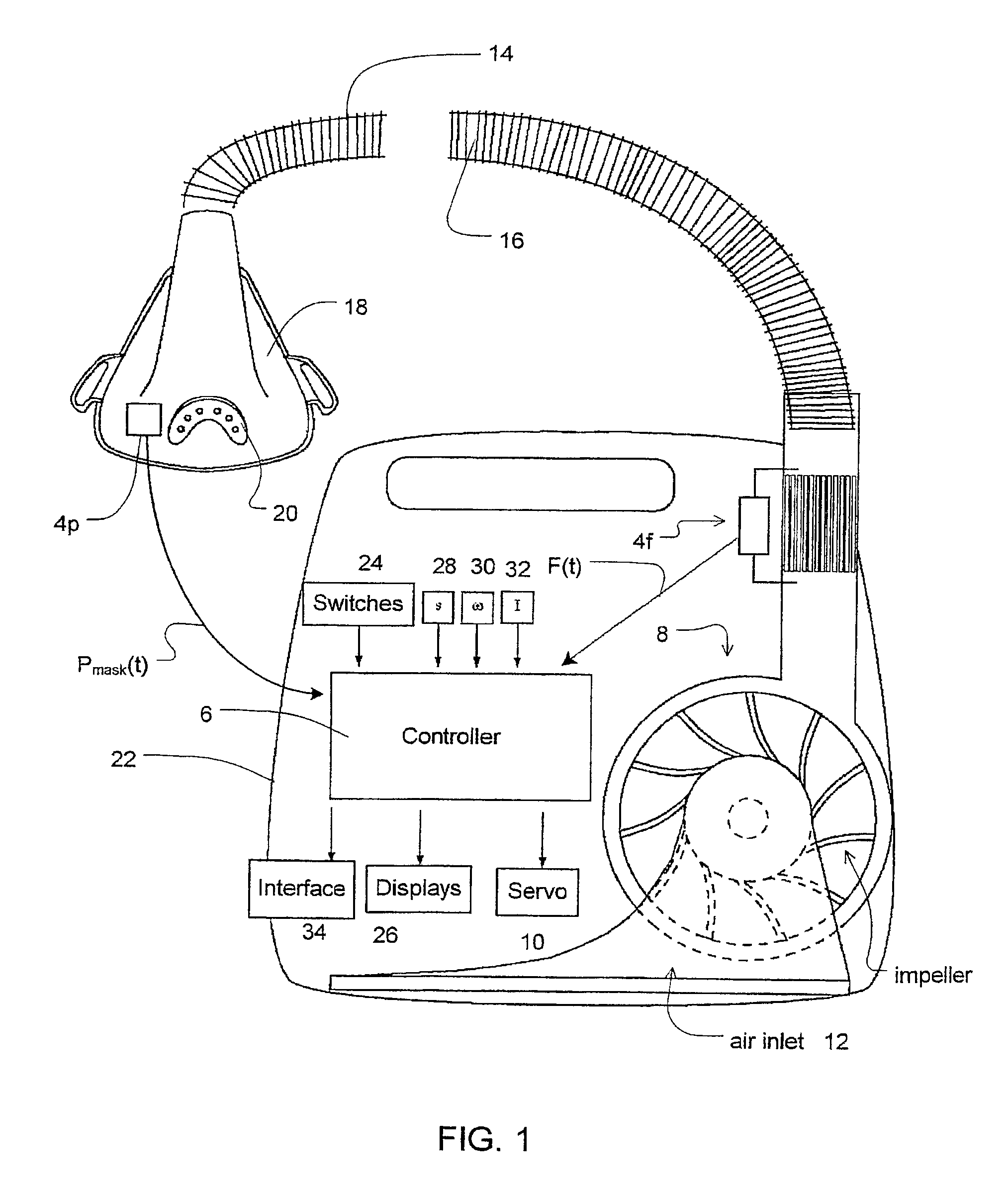
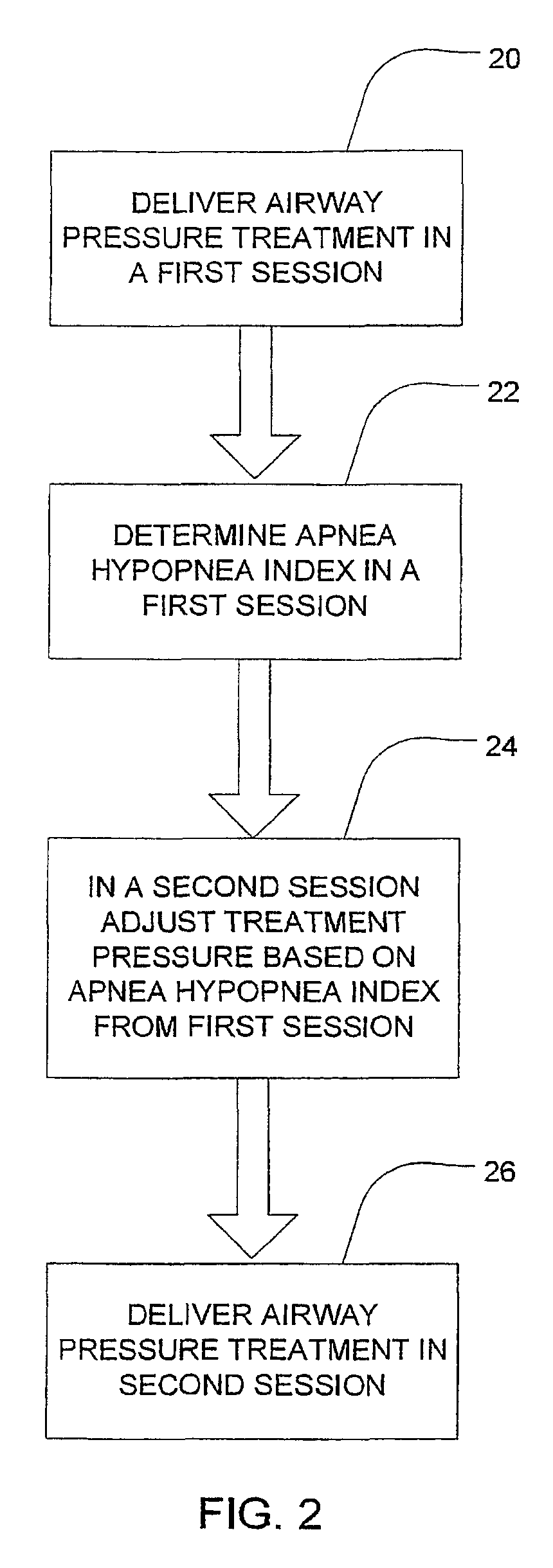
Session-by-session adjustments of a device for treating sleep disordered breathing
InactiveUS7913691B2Effectively determine an appropriate pressure responseMinimizes pressure treatmentRespiratorsOperating means/releasing devices for valvesStage iibRegimen
A method is disclosed for operating a device that treats sleep disordered breathing (SDB) during successive treatment sessions; where the device provides continuous positive airway pressure during sleep. The method comprises the steps of applying a constant treatment pressure during a first session (20) and deriving a sleep disorder index (SDI) (22) representative of the number of SDB episodes that occurred during the first session. If the treatment pressure should be increased based on the SDI, it is increase during a second, subsequent session (24).
Owner:RESMED LTD
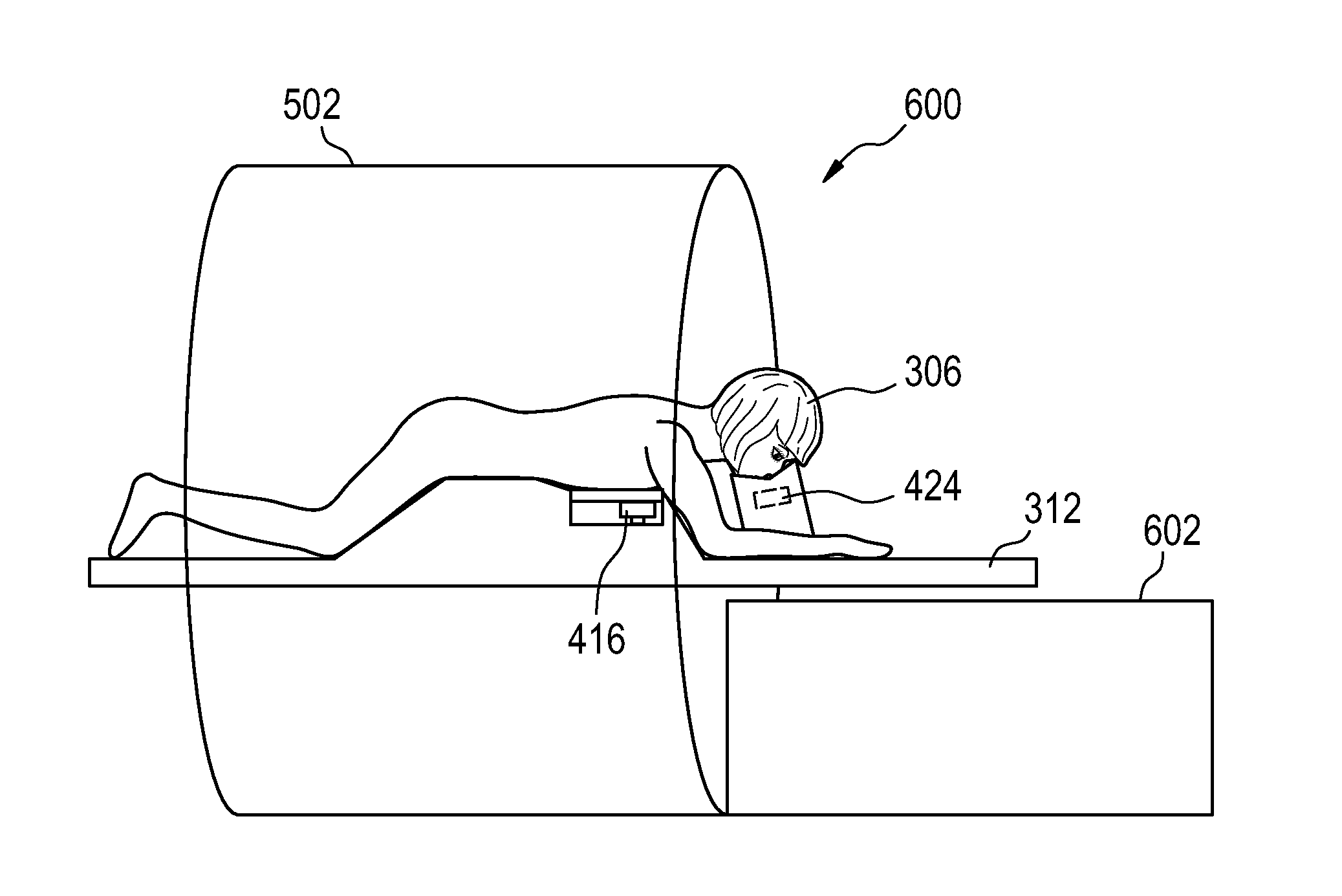
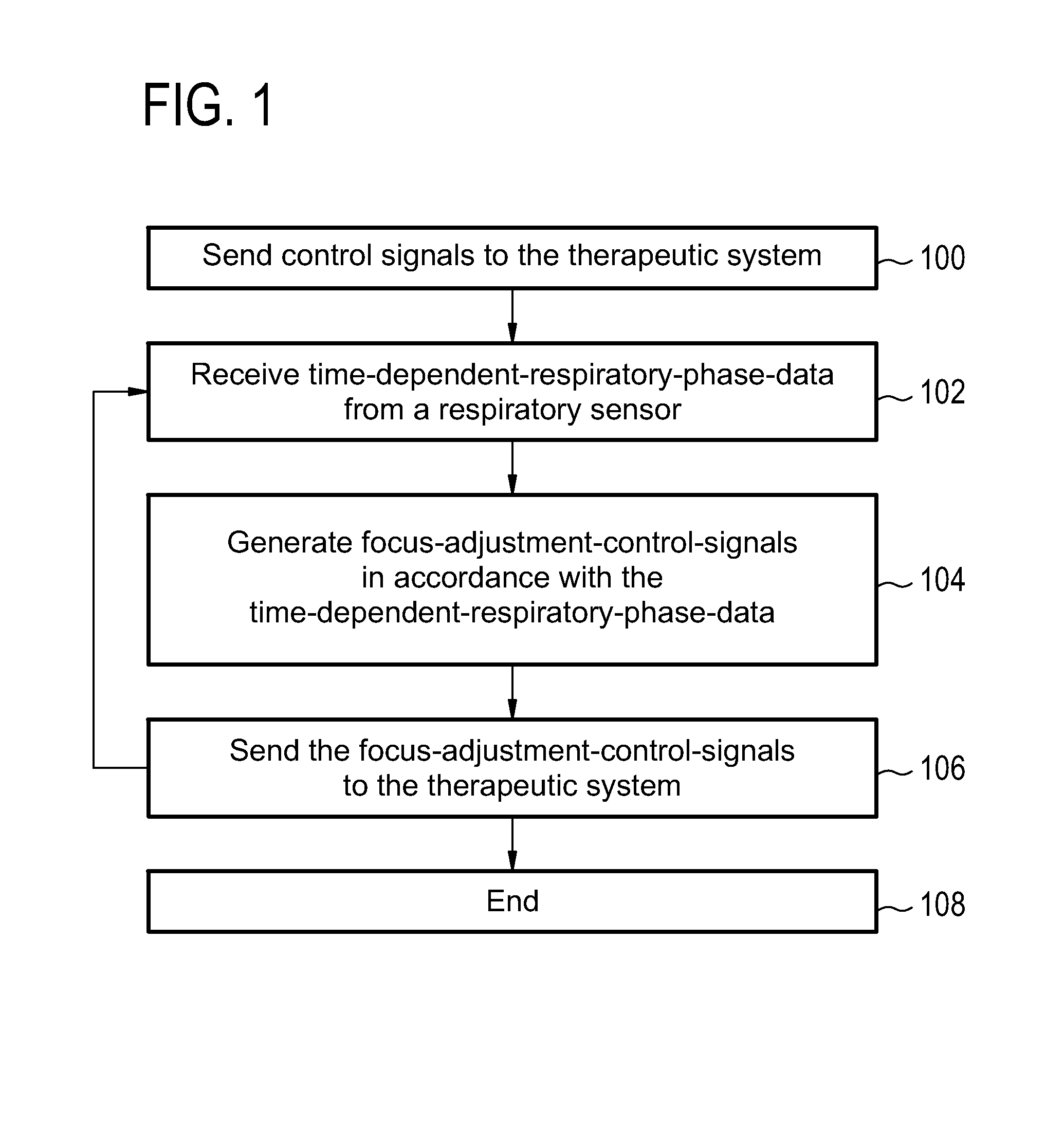
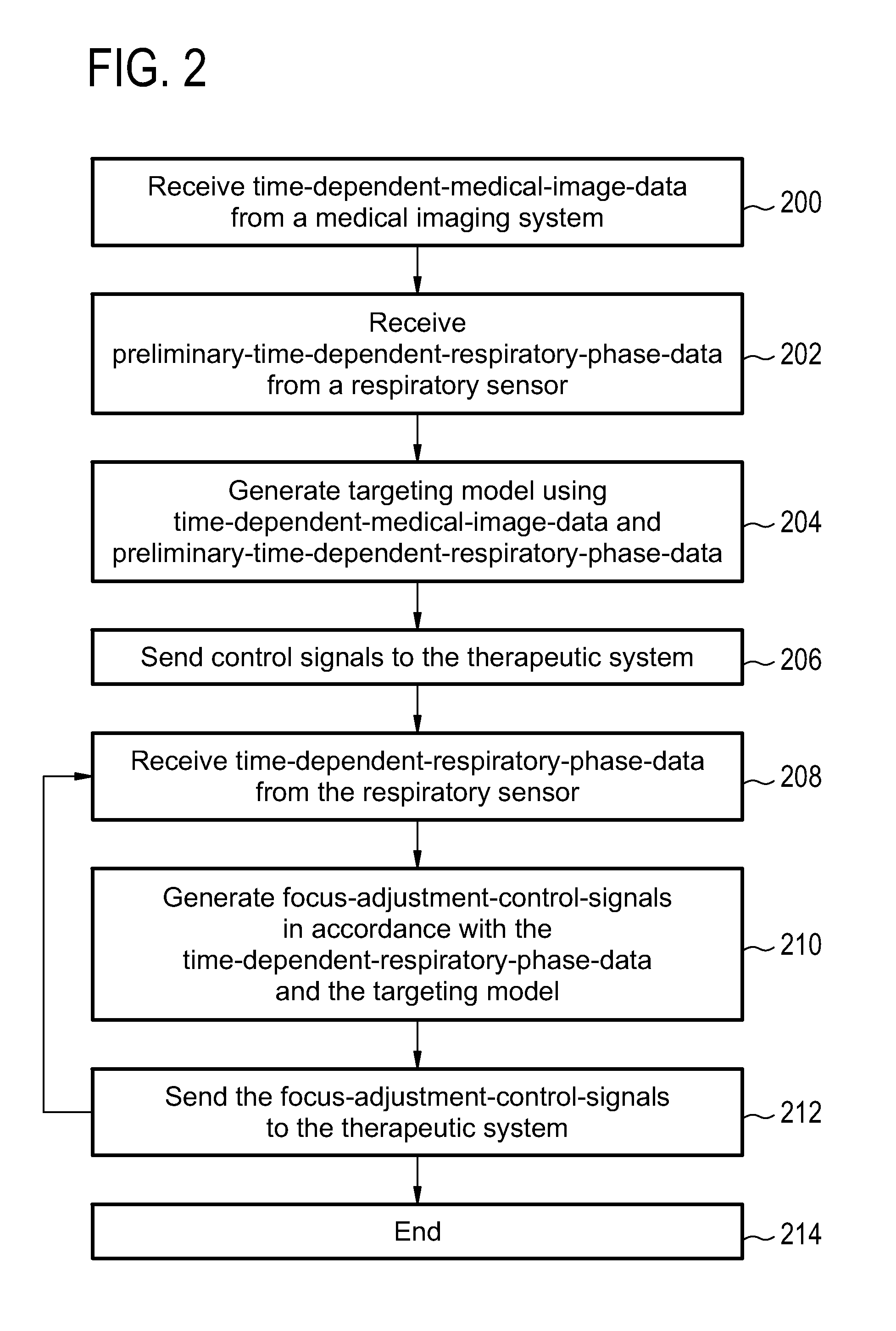
Therapeutic apparatus, computer-implemented method, and computer program product for controlling the focus of radiation into a moving target zone
A therapeutic apparatus (300, 400, 500, 600) comprising: a therapeutic system (302, 402) for treating a target zone (304) of a subject (306), wherein the therapeutic system has an adjustable focus for directing radiation (410) into the target zone; a respiration sensor (308, 310, 416, 424) for measuring a respiratory phase of the subject; a processor (322) for controlling the therapeutic apparatus; and a memory (326, 328) containing machine executable instructions (340, 342, 344, 346, 348, 350, 352) for execution by the processor, wherein execution of the instructions causes the processor to: send (100, 206) control signals to the therapeutic system that cause treatment of the target zone, receive (102, 208) time-dependant-respiratory-phase-data (330) from the respiration sensor, generate (104, 210) focus-adjustment-control-signals in accordance with the time-dependant-respiratory-phase-data, and send (106, 212) the focus-adjustment-control-signals to the therapeutic system.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
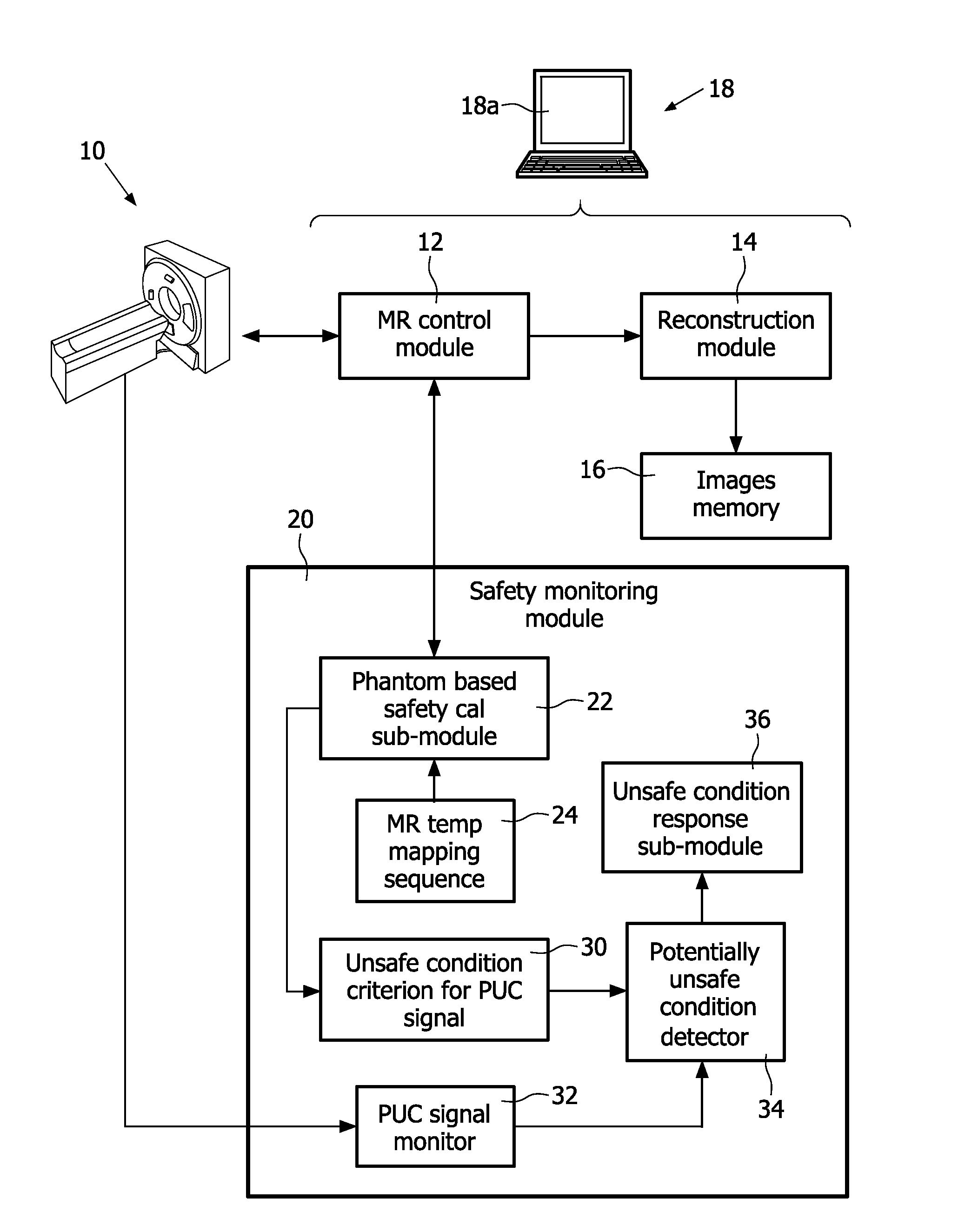
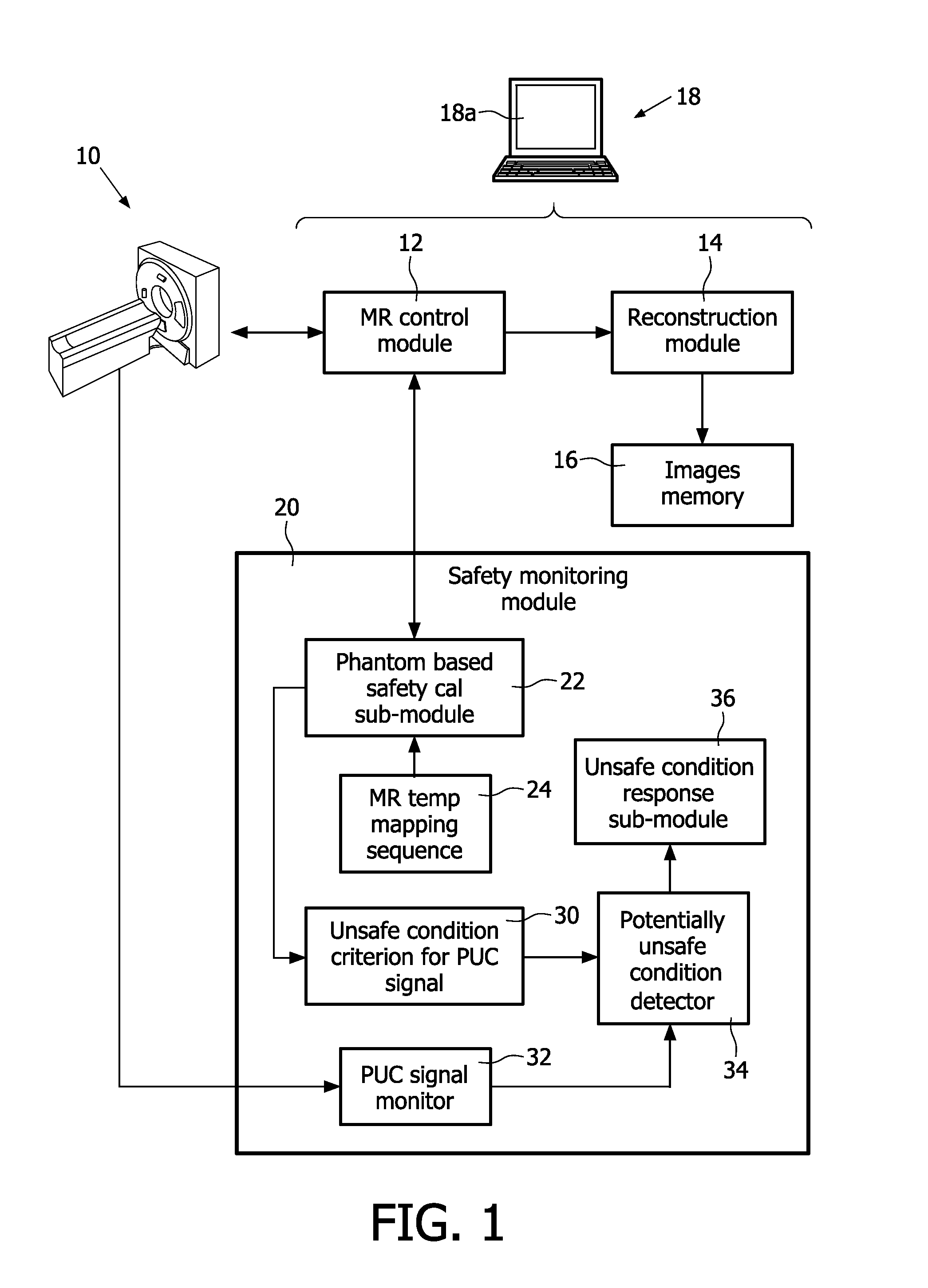
Magnetic resonance system and method for comprehensive implantable device safety tests and patient safety monitoring
InactiveUS20120086449A1Rapid assessmentReduce the possibilityElectric/magnetic detectionMeasurements using NMRStage iibUnsafe condition
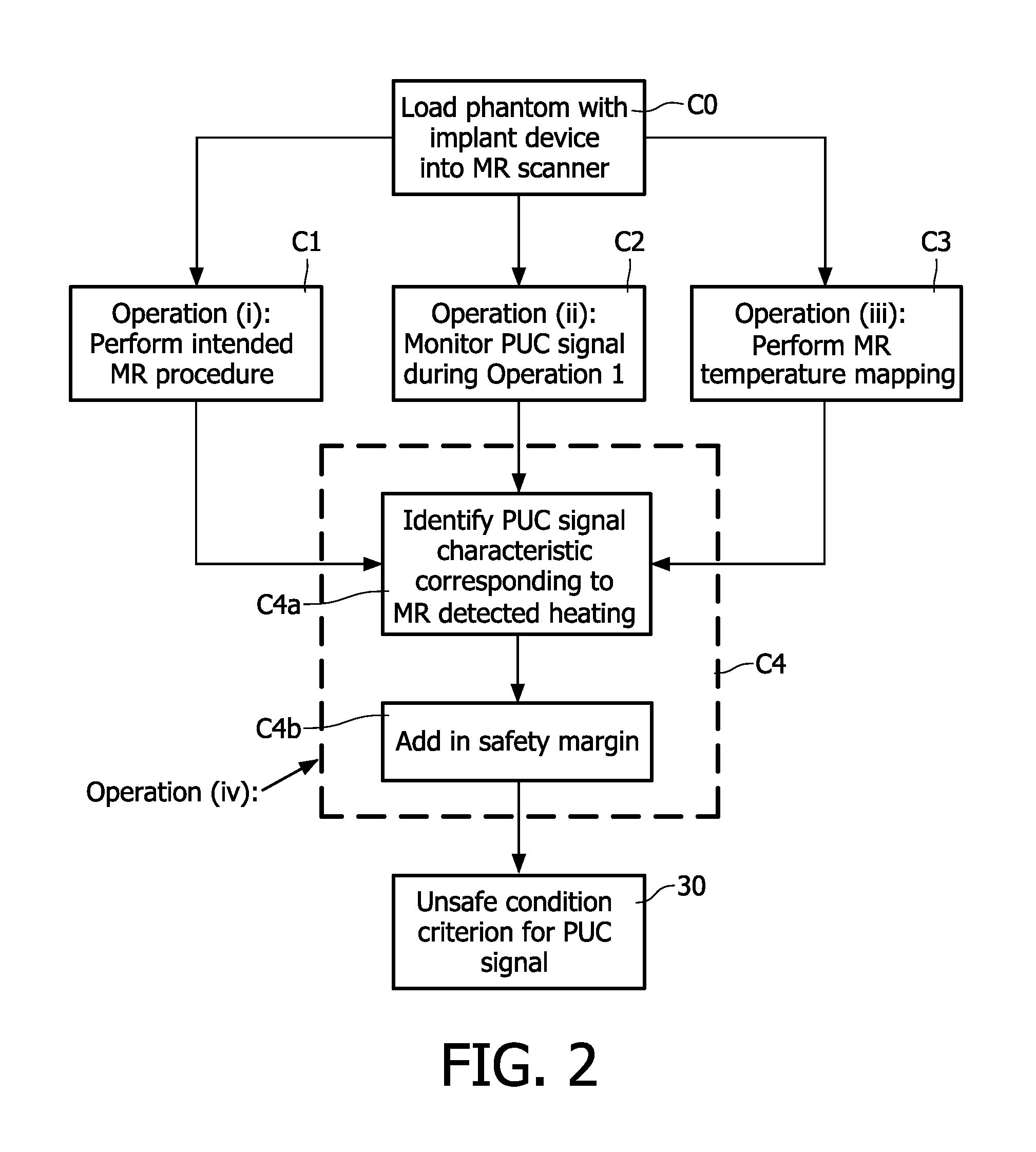
A magnetic resonance method comprises: performing (C1) a magnetic resonance procedure on a calibration subject including an implant device; detecting (C2) a pick-up coil (PUC) signal at least during a radio frequency transmit phase of operation (C1); performing (C3) three dimensional temperature mapping of the calibration subject using a magnetic resonance sequence configured to detect any temperature change induced in any part of the implant device by operation (C1); generating (C4) an unsafe condition criterion (30) for the detected PUC signal based on correlating a PUC signal characteristic detected by operation (C2) with a temperature change detected by operation (C3); performing (M5) the magnetic resonance procedure on a subject containing an implant device; detecting (M6) a PUC signal at least during a radio frequency transmit phase of operation (M5); and monitoring (M7) for an unsafe condition indicated by the PUC signal detected in operation (M6) satisfying the unsafe condition criterion (30).
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Lung simulator
ActiveUS7959443B1Cutting costsEliminate time consumptionEducational modelsLoop controlControl signal
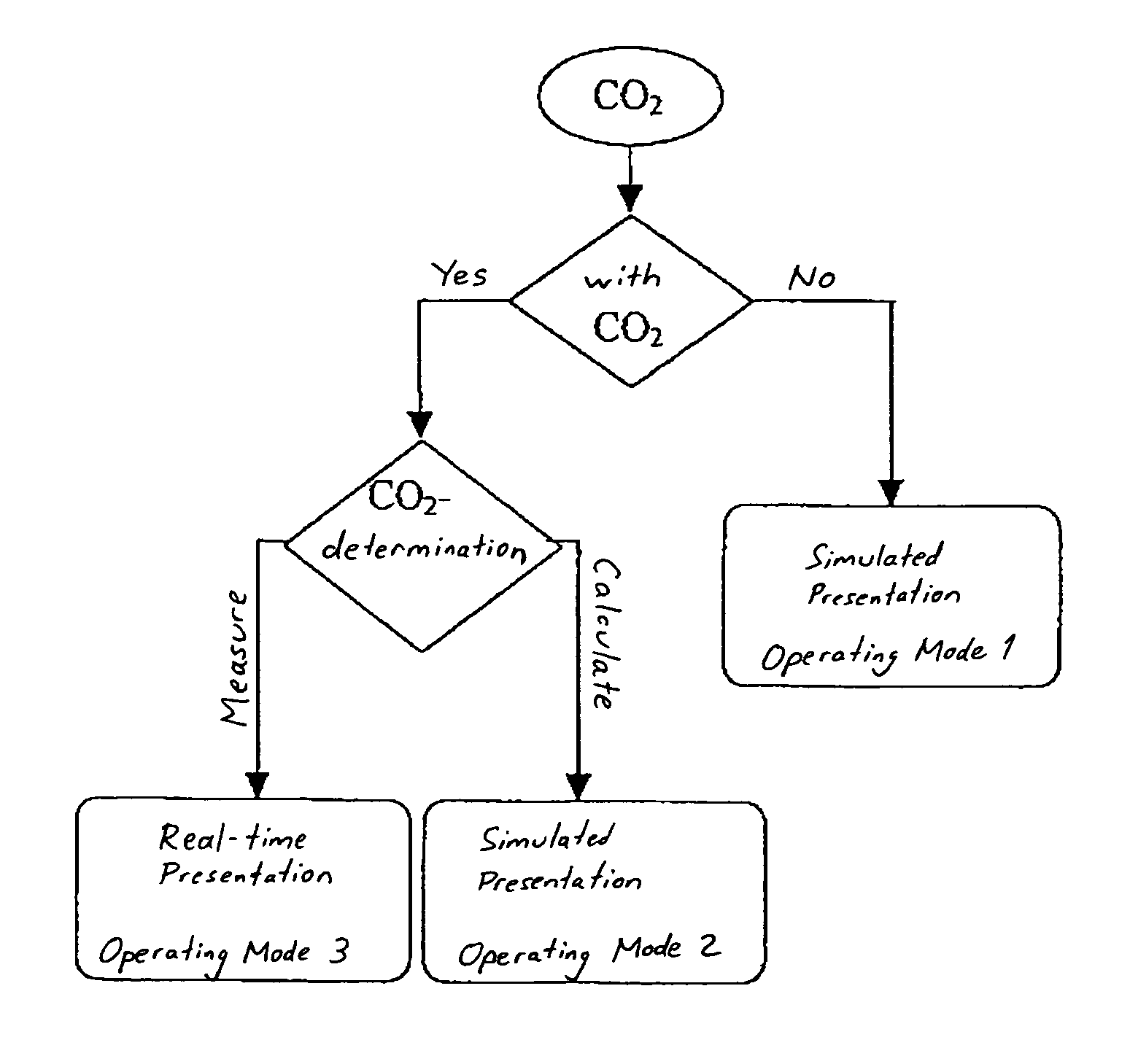
A system and method of delivering CO2 in a respiration closed-loop control system to a respiratory simulator includes (a) providing a CO2 supply to a respiratory simulator having a piston / cylinder arrangement; (b) providing flow control hardware between the CO2 supply and the piston / cylinder arrangement; (c) generating a first control signal representative of a predefined amount of CO2; and (d) providing the predefined amount of CO2 into the piston / cylinder arrangement. Thereafter, either (i) an end-tidal carbon dioxide partial pressure (EtCO2) value is determined based on an amount of CO2 emptied from the piston / cylinder arrangement during an exhalation phase of the respiratory simulator or (ii) an EtCO2 value is calculated via an equation. A second control signal is generated that is representative of a tidal volume and a breathing frequency representative of a physiological response to the EtCO2 value to effect corresponding movement of the piston in a next inhalation and exhalation phase.
Owner:INGMAR MEDICAL
Method of testing for atopic dermatitis by measuring expression of the NOR-1 gene
InactiveUS7115373B2Cell receptors/surface-antigens/surface-determinantsSugar derivativesStage iibDifferential display
Genes whose expression differ between that in eosinophils collected from atopic dermatitis patients of the exabartation stage and those of the remission stage were searched via a differential display method. As a result, NOR-1 (MINOR) gene was successfully identified whose expression significantly increased in eosinophils of patients in the remission stage, a stage associated with a decrease of eosinophils. The present inventors discovered that the gene can be successfully employed in testing for allergic diseases and screening for candidate compounds for therapeutic agents.
Owner:JAPAN REPRESENTED BY GENERAL DIRECTOR OF AGENCY OF NAT CENT FOR CHILD HEALTH & DEV
Rat embryonic stem cell
ActiveUS20120142092A1Microbiological testing/measurementHybrid cell preparationEmbryoNa k atpase activity
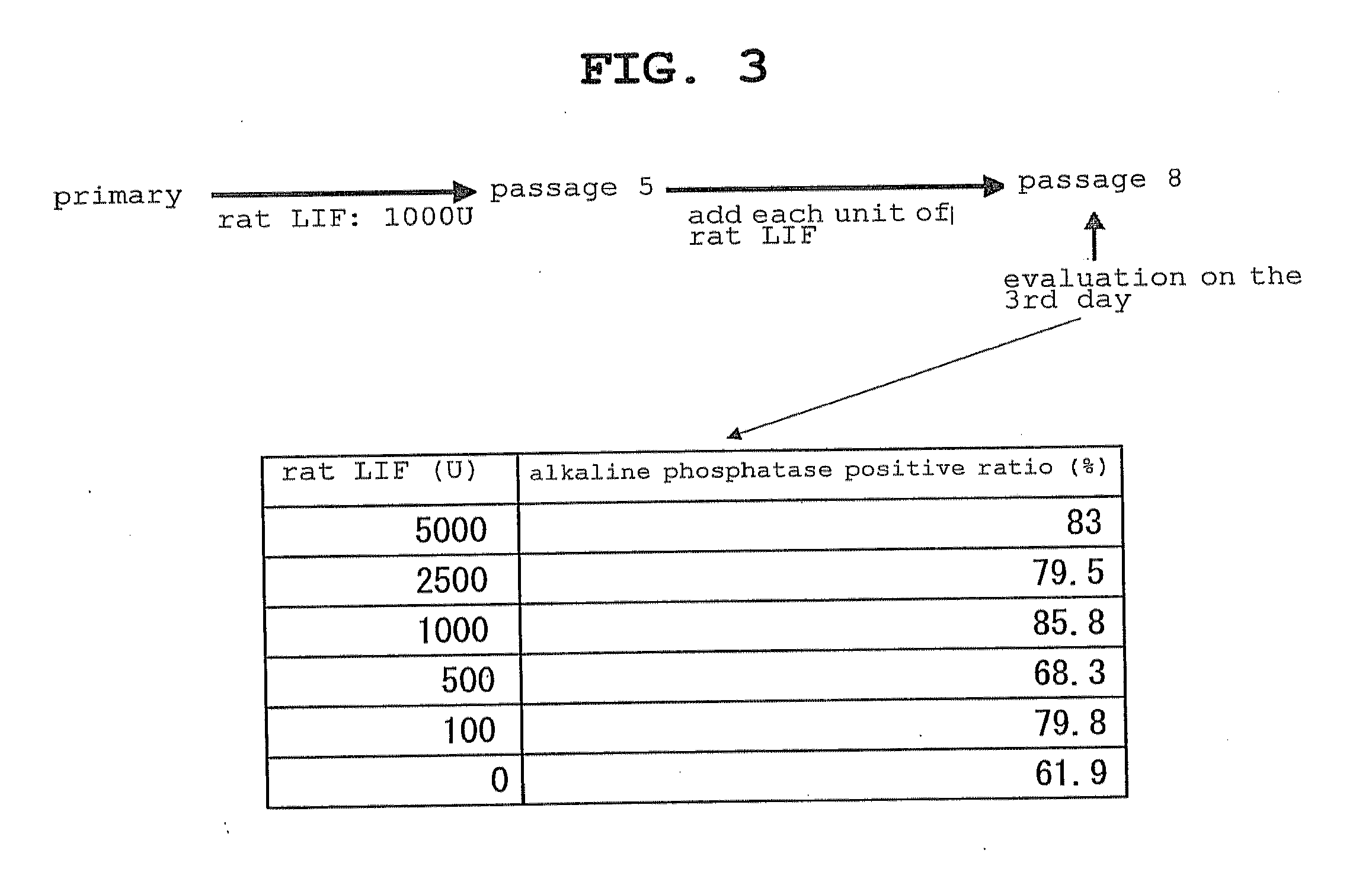
The present invention provides a rat embryonic stem cell characterized by having the following properties of (a) expressing Oct3 / 4 gene and Nanog gene, (b) positive for alkaline phosphatase activity, (c) having an embryoid body forming ability, (d) expressing SSEA (Stage-Specific Embryonic Antigen)-1 and SSEA-4, (e) having the same number of chromosomes as does a normal rat cell, (f) capable of being subcultured and holding the undifferentiated state, (g) having in vitro pluripotency, (h) having a potential to differentiate for cells of three embryonic germ lineages, (i) having teratoma formation ability, and (j) having an ability to produce a chimeric rat, a method of establishing the aforementioned rat embryonic stem cell and the like.
Owner:SUMITOMO CHEM CO LTD
Immunological analytical method and device for the determination of advanced glycosylation end products (AGEs)
The invention provides an immunological analytical method and devices for determination of the advanced glycosylation end products (AGEs), comprising a reagents for determining the AGEs, which comprises a displaying carrier suspension and the antigen or antibody immobilized on the surface of the said displaying carrier; and a test strip, comprising of: a base plate, and constitutive parts provided on the said base plate. After infusing the said reagent into the said test strip, the immunological reaction of the AGEs antigen or antibody can be determined based on the agglutination phenomenon or accompanied changes of absorbance or color and the presence or raised level of the AGEs in the diabetic patient can be known accordingly such that the practitioner can prevent the occurrence of the complications, or block further the progression of the complications at the earlier stage.
Owner:LIU YUNG HSIANG +1
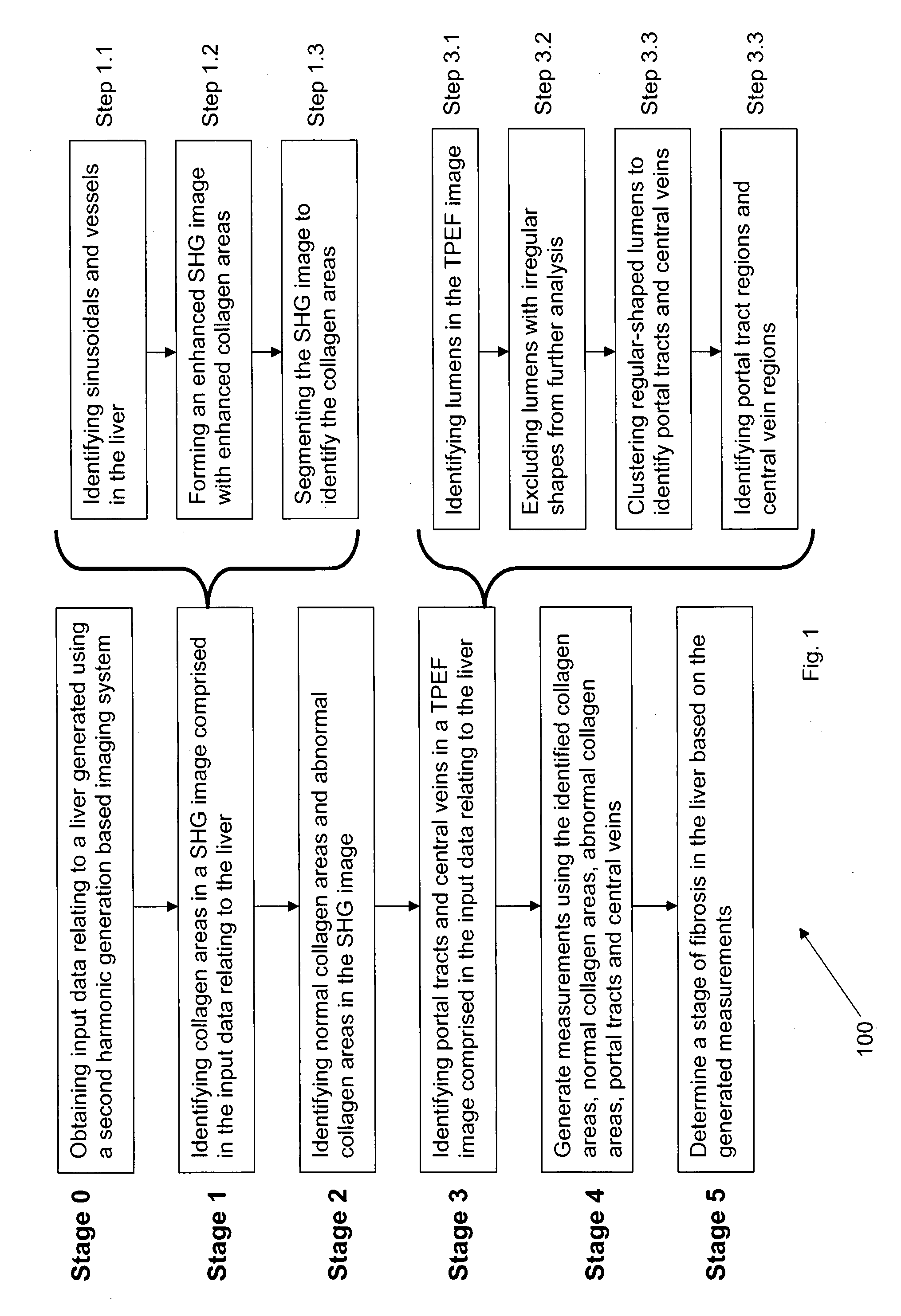
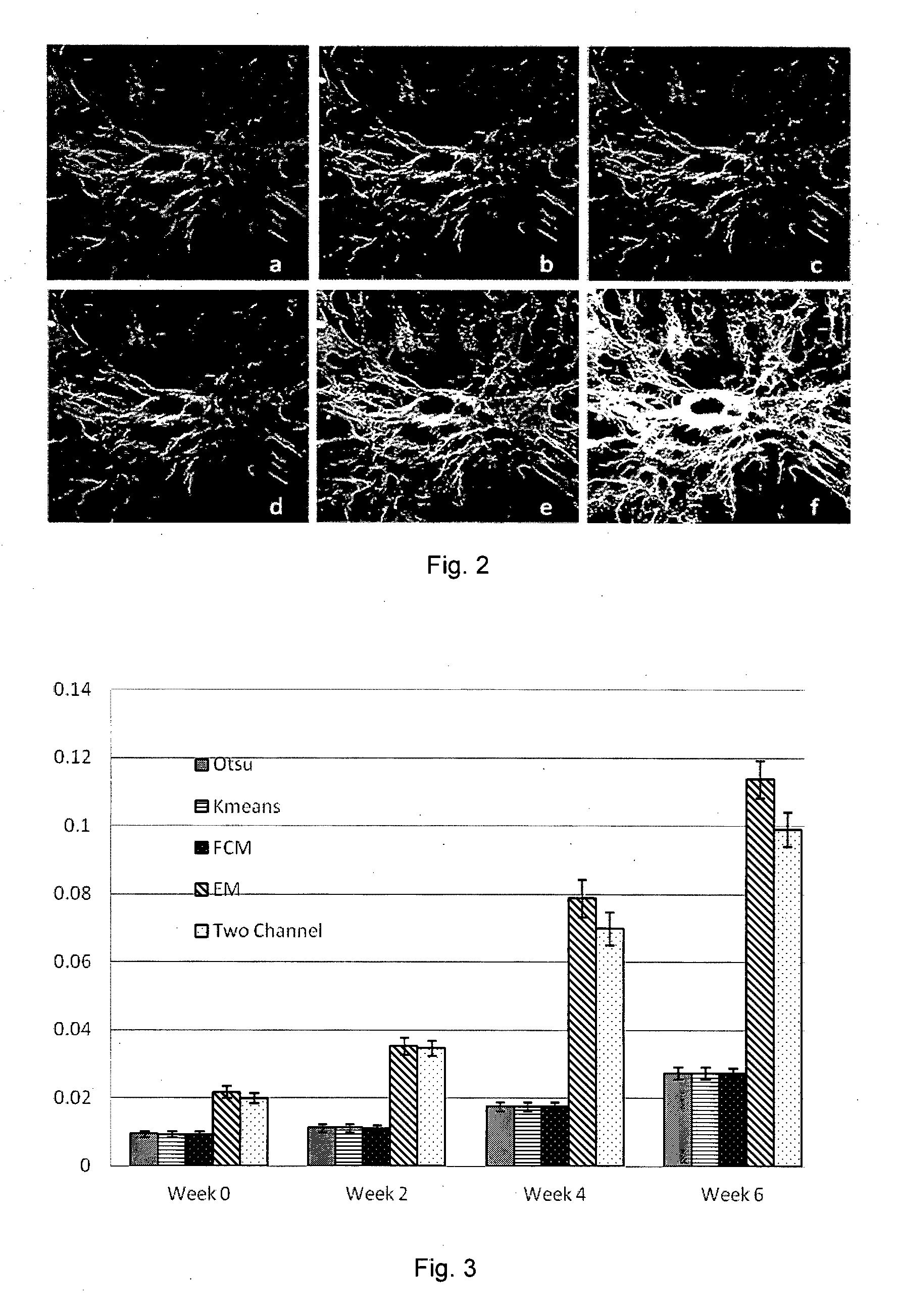
Method and system for determining a stage of fibrosis in a liver
A method for determining a stage of fibrosis in a liver is disclosed. The method comprises the steps of: (1a) obtaining input data relating to the liver, the input data being generated using a second harmonic generation based imaging system; (1b) identifying a plurality of morphological features of the liver from the input data relating to the liver; (1c) generating a plurality of measurements based on the identified plurality of morphological features; and (1d) determining the stage of fibrosis in the liver based, on the generated plurality of measurements.
Owner:AGENCY FOR SCI TECH & RES
Mimo Receiver Using Lattic Reduction and K-Best Detection
InactiveUS20140185716A1Powerful near-ML detectionAmplitude-modulated carrier systemsRadio transmissionStage iibCommunications system
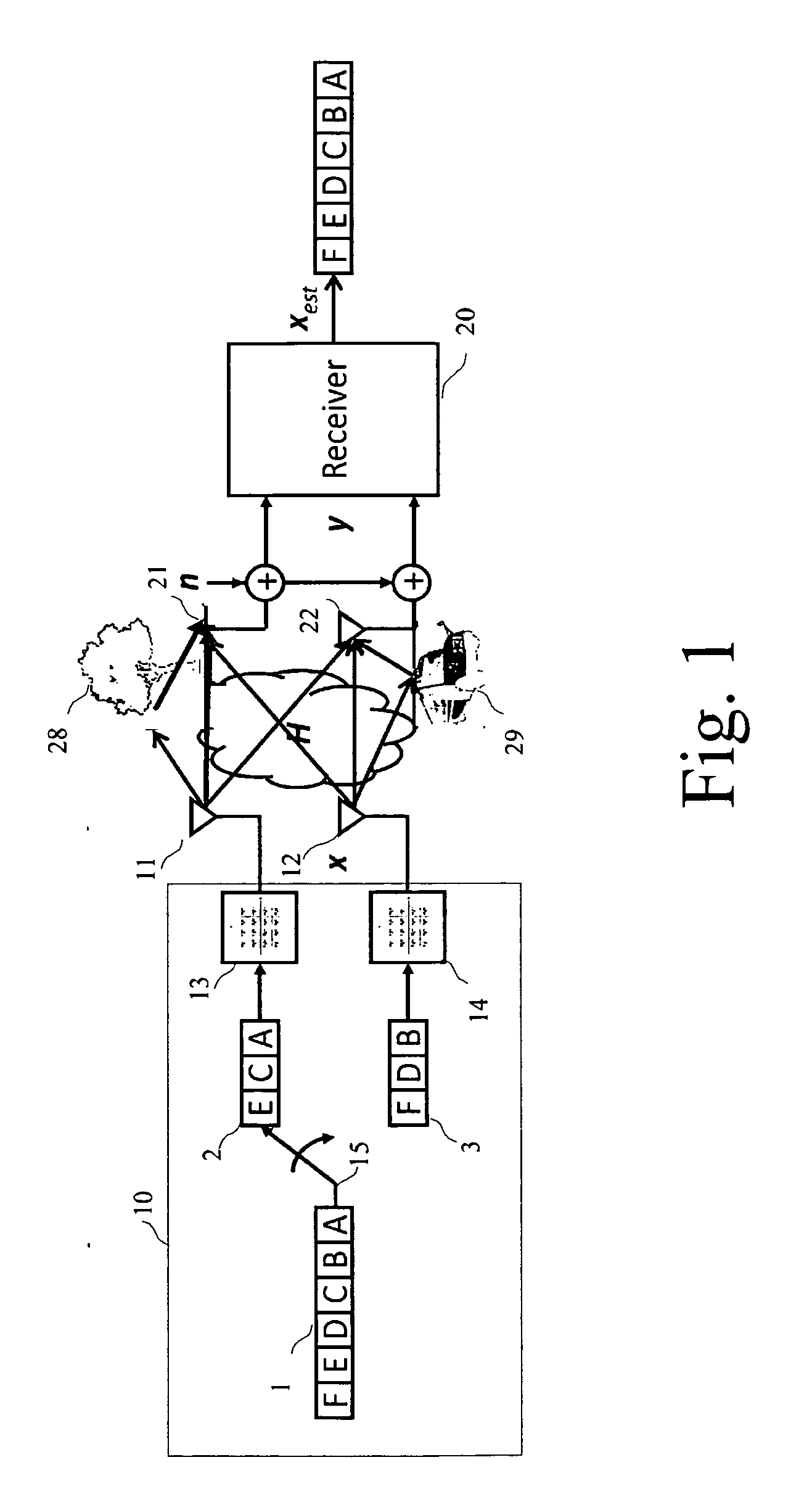
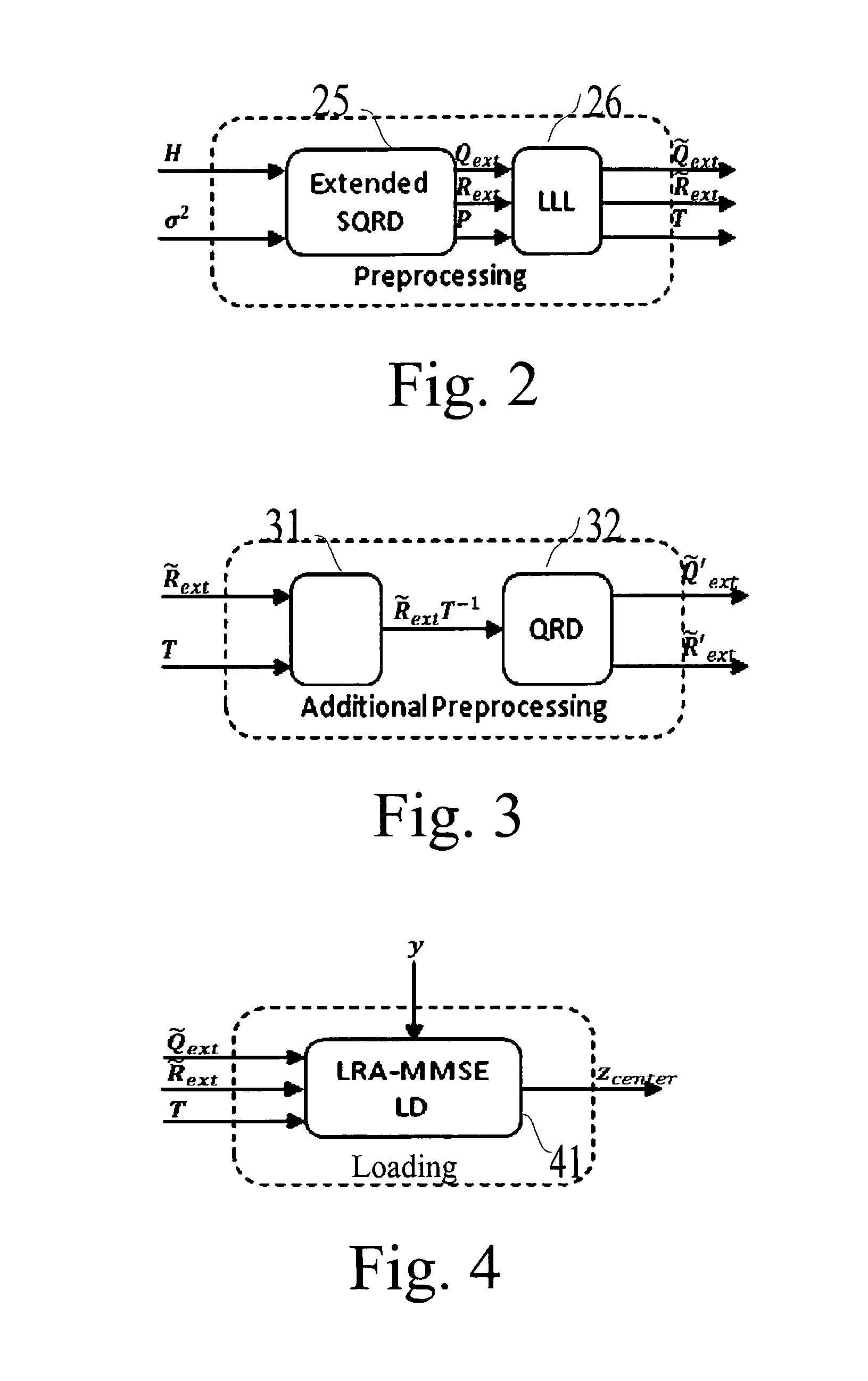
A detection process for a receiver of a wireless communication system based on Multiple-Input Multiple-Output antennas (nT, nR), said receiver processing observations symbols y derived from symbols x transmitted by an emitter through a channel H; characterized in that it involves: —a preprocessing which only depends on the channel H, said preprocessing involving: —a first QRD decomposition (61) for the purpose of decomposing said channel H into two Qext and Rext matrices, with QextHQext= / and Rext being upper triangular; —a lattice reduction (62) for the purpose of generating Qext, Rext and a transformation matrix T; —a second QRD decomposition (63) applied on the matrix Rext T−1 for the purpose of generating two matrixes Q′ext and R′ext, —a loading phase (64, 65, 66) comprising a linear detection process of the observations y for the purpose of generating a value xcenter; —a neighborhood search (67-70) performed in the Original Domain Neighborhood (ODN) with a search center being equal to the result xcenter of said loading phase, said neighborhood search determining a limited number of symbols (K-best).
Owner:ST ERICSSON SA
Anti-inflammatory peptide separated from haliotis discus hannai abalone visceral organ and use of anti-inflammatory peptide
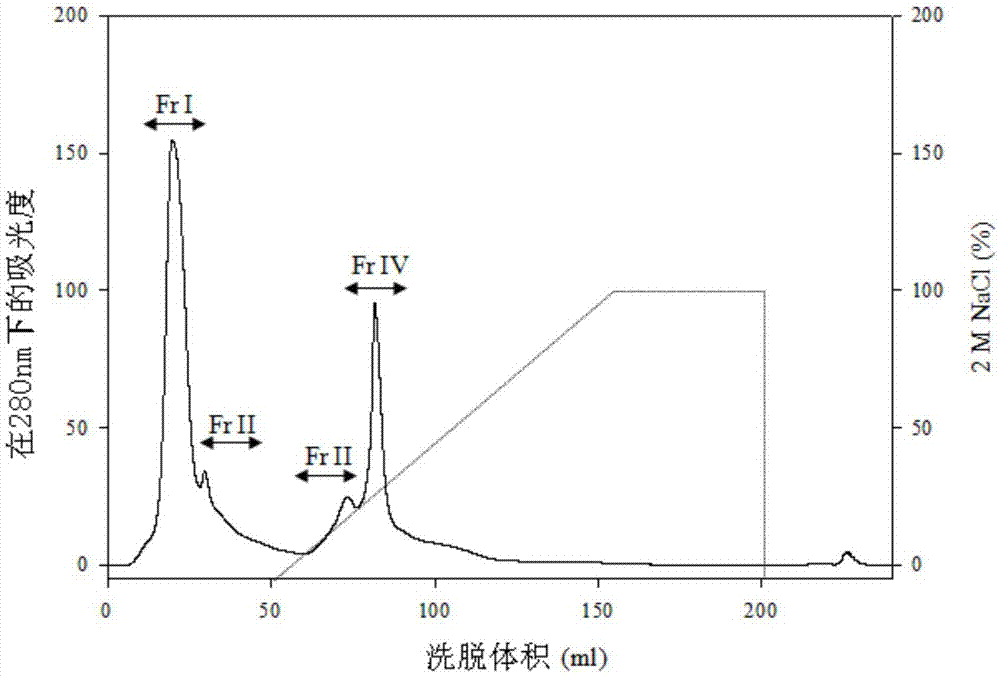
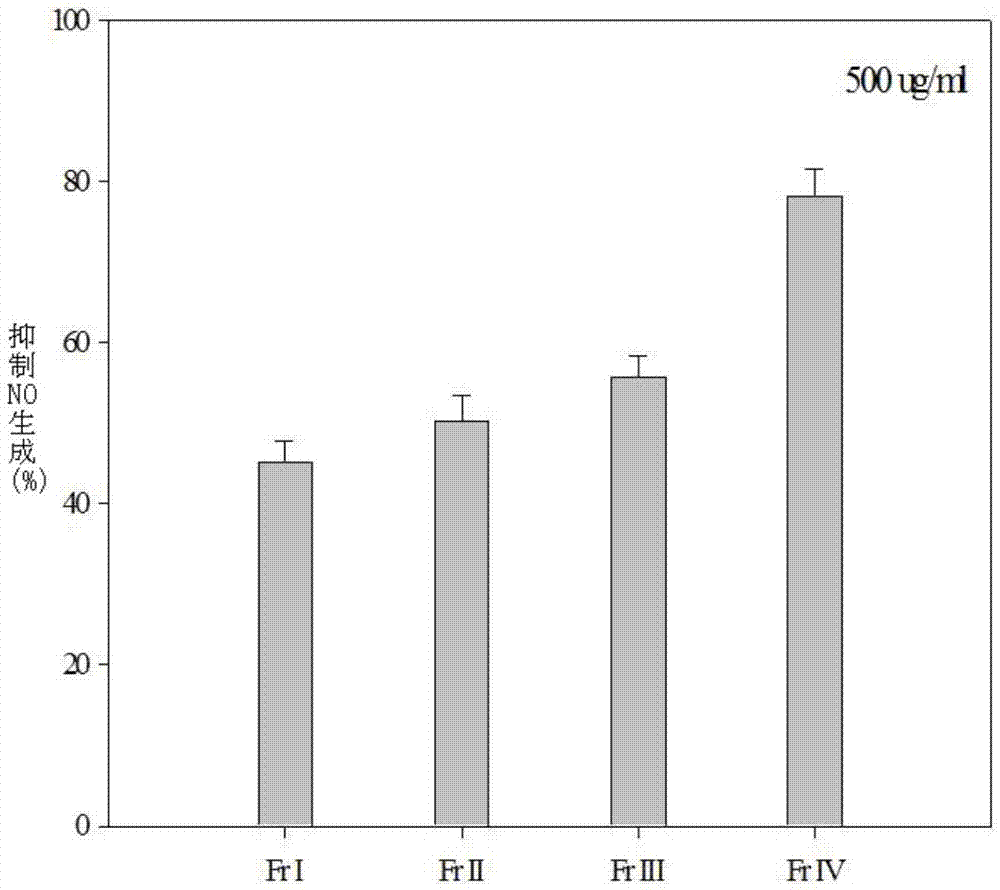
The present invention is used for evaluating the advantages of haliotis discus hannai. A multi-phase HPLC purification system is used to purify anti-inflammatory peptide from abalone (AAIP, abalone anti-inflammatory peptide). In tandem MS analysis, a fragmentation result shows that the amino acid sequence of the AAIP with nitrogen monoxide (NO) inhibitory activity (IC50=55.8[um]M) is Pro-Phe-Asn-Glu-Gly-Thr-Phe-Ala-Ser (1175.2Da). While the anti-inflammatory effect of RAW264.7 macrophages generated by the stimulus of the AAIP to lipopolysaccharides (LPS) is further studied, and the molecular mechanism is elaborated. The result shows that the AAIP peptide inhibits the nitrogen monoxide (NO) generation induced by the LPS through the expression of inducible nitric oxide synthase (iNOS) by a dose-dependent manner, and the gene transcription of pro-inflammatory cytokines is also obviously reduced, wherein the pro-inflammatory cytokines comprise such as interleukin (IL-1 beta), tumor necrosis factors (TNF-beta) and IL-6. In addition, the AAIP obviously inhibits phosphorylation of mitogen-activated protein kinases (MAPK), such as p-p38 and p-JNK. These results indicate that the AAIP inhibits inflammatory response induced by LPS by intercepting the MAPK pathway of the macrophages. Therefore, the AAIP can be applied to therapeutic drugs for inflammations treatment or healthcare food products.
Owner:千忠吉
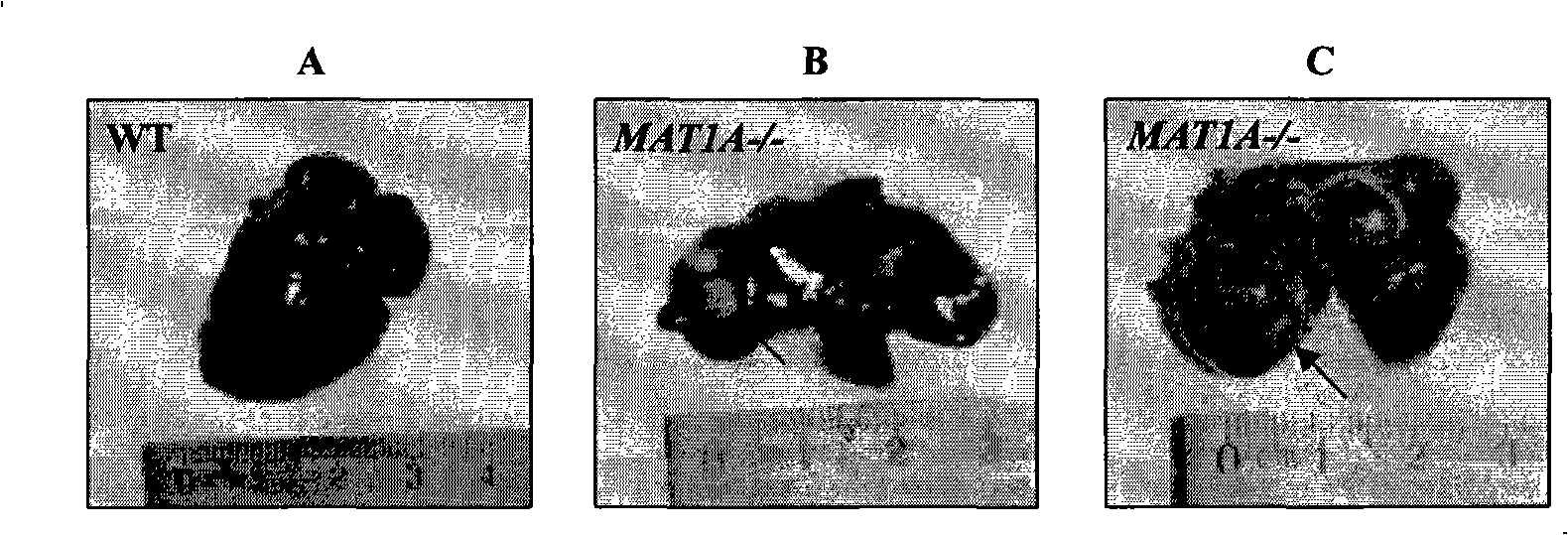
Molecular markers of hepatocellular carcinoma and their applications
InactiveUS20090181379A1Precise definitionFacilitate prognosisCompound screeningApoptosis detectionEtiologyProteomics methods
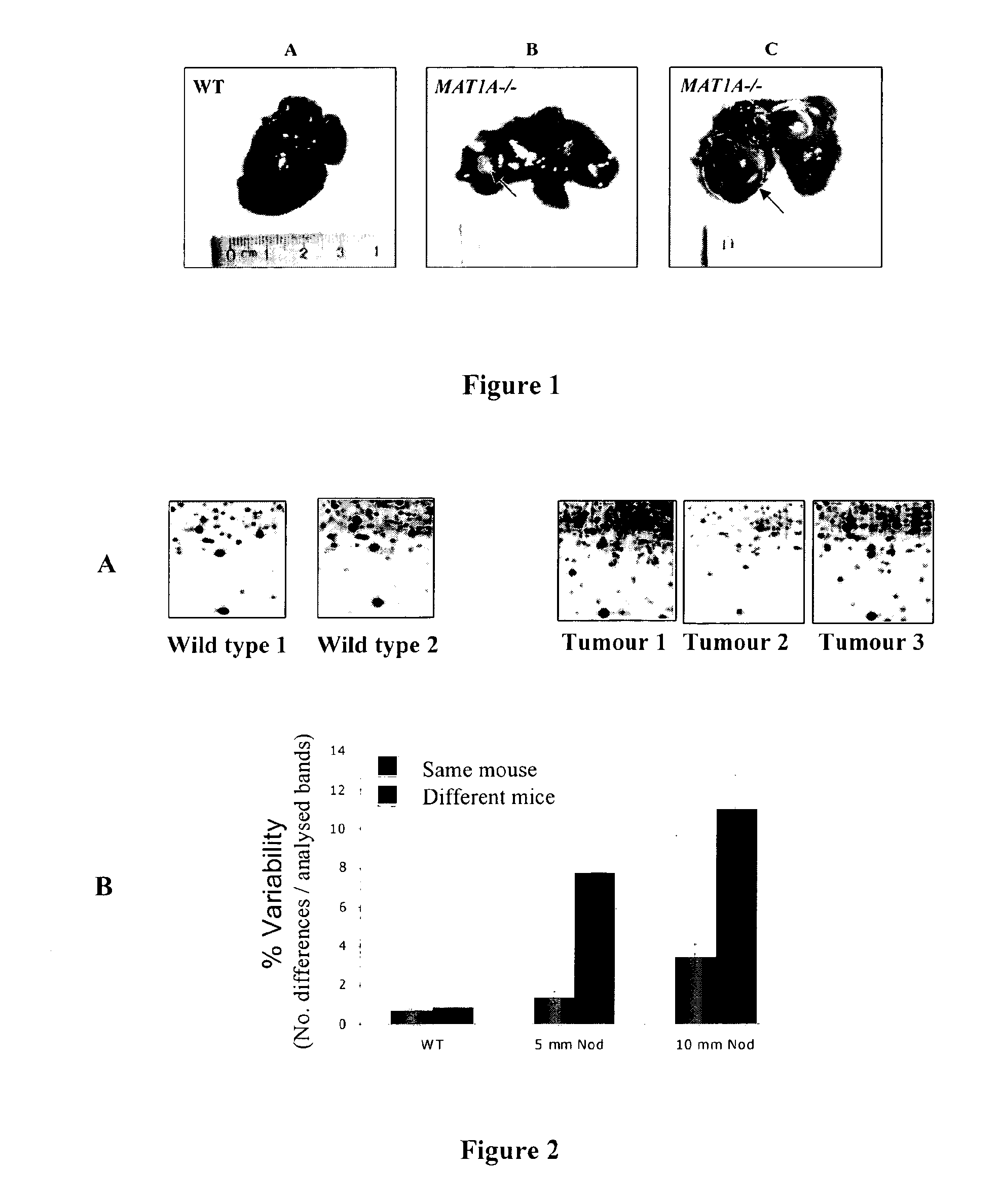
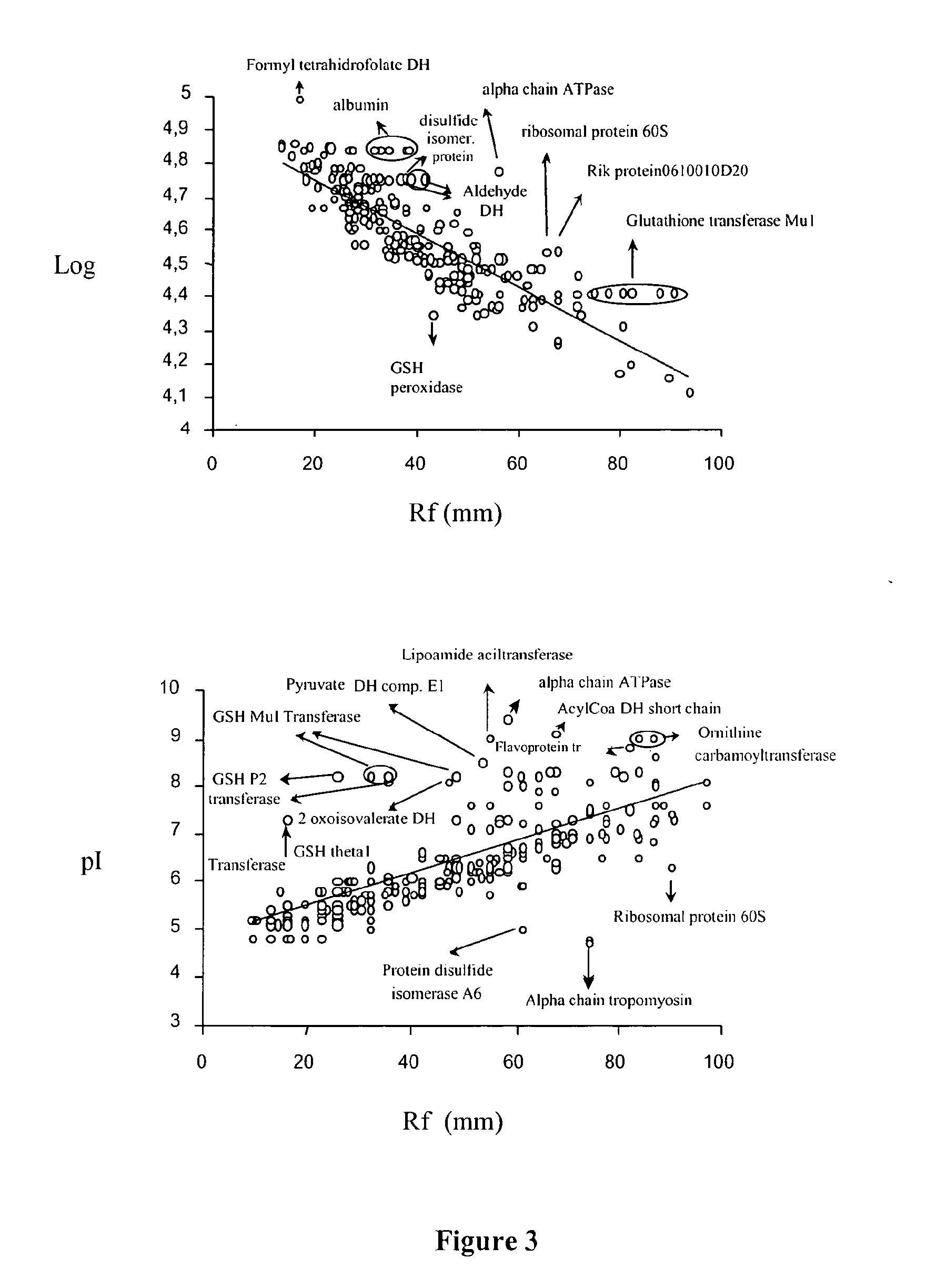
By means of a proteomic approach, the markers of hepatocellular carcinoma (HCC) in the liver of a knockout mouse (MAT1A− / −) have been identified for the MAT1A gene (deficient in the synthesis of S-adenosylmethionine). 27 proteins have been detected the expression thereof is altered in, at least, 50% of the analysed tumours. Amongst them, 13 proteins have been validated in biopsies of patients with HCC of different etiology, and 7 of them have been validated in biopsies of patients with liver cirrhosis, a stage prior to the development of HCC, which makes it possible to differentiate between prior stages of the disease and even between different etiologies (viral and alcoholic). Having a panel of markers available may contribute to more accurately defining the alterations associated with the development of HCC and thus facilitate prognosis and diagnosis of this disease.
Owner:PROYECTO DE BIOMEDICINA CIMA
Device And Active Component For Inhibiting Formation Of Thrombus-Inflammatory Cell Matrix
A combination drug treatment for inhibiting stenosis or restenosis is disclosed. The combination treatment is an active component containing both an anti-inflammatory substance and an anti-thrombotic substance which, together, contribute to an inhibiting effect on the initial stages of stenosis or restenosis. The active component can be delivered to a site of treatment by being carried on a device, such as a stent.
Owner:ABBOTT CARDIOVASCULAR
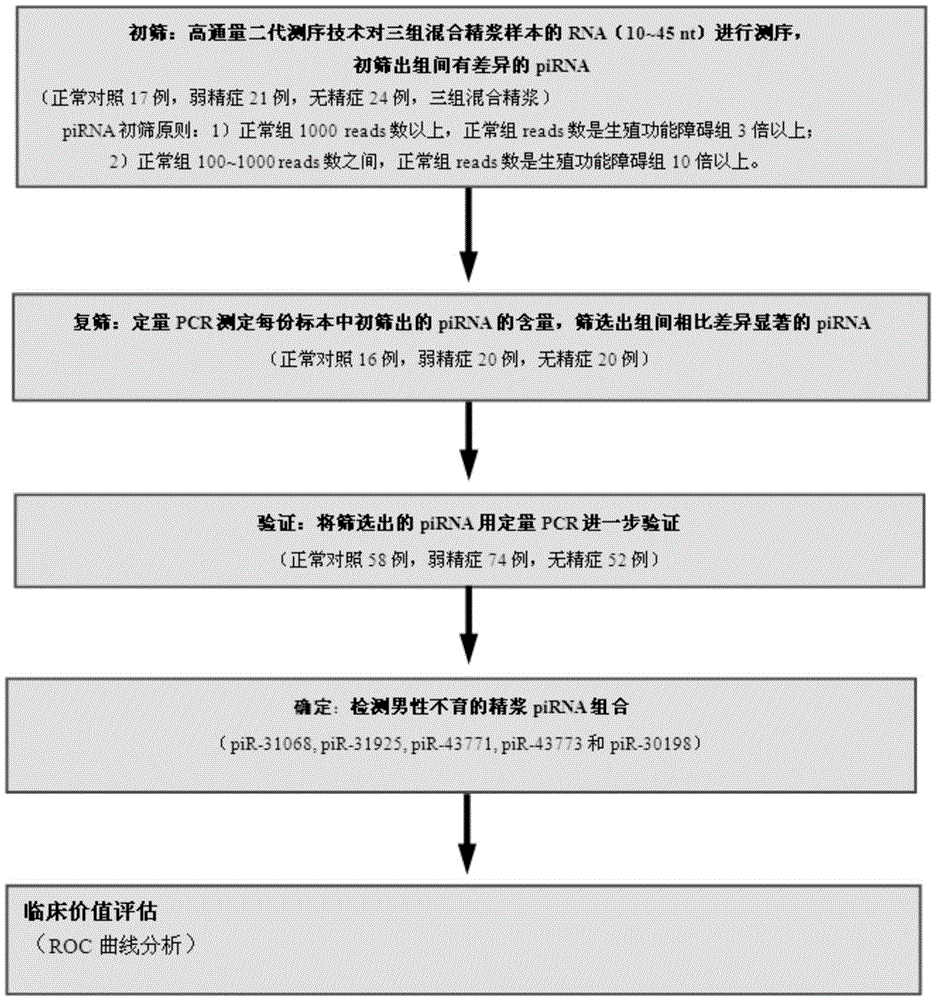
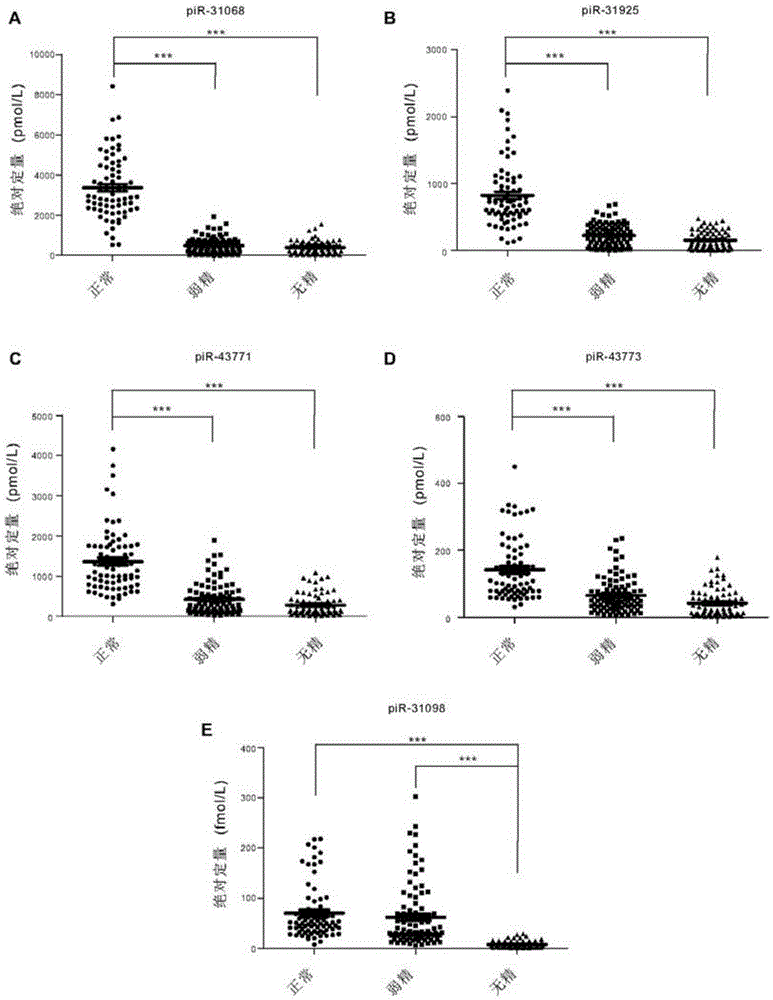
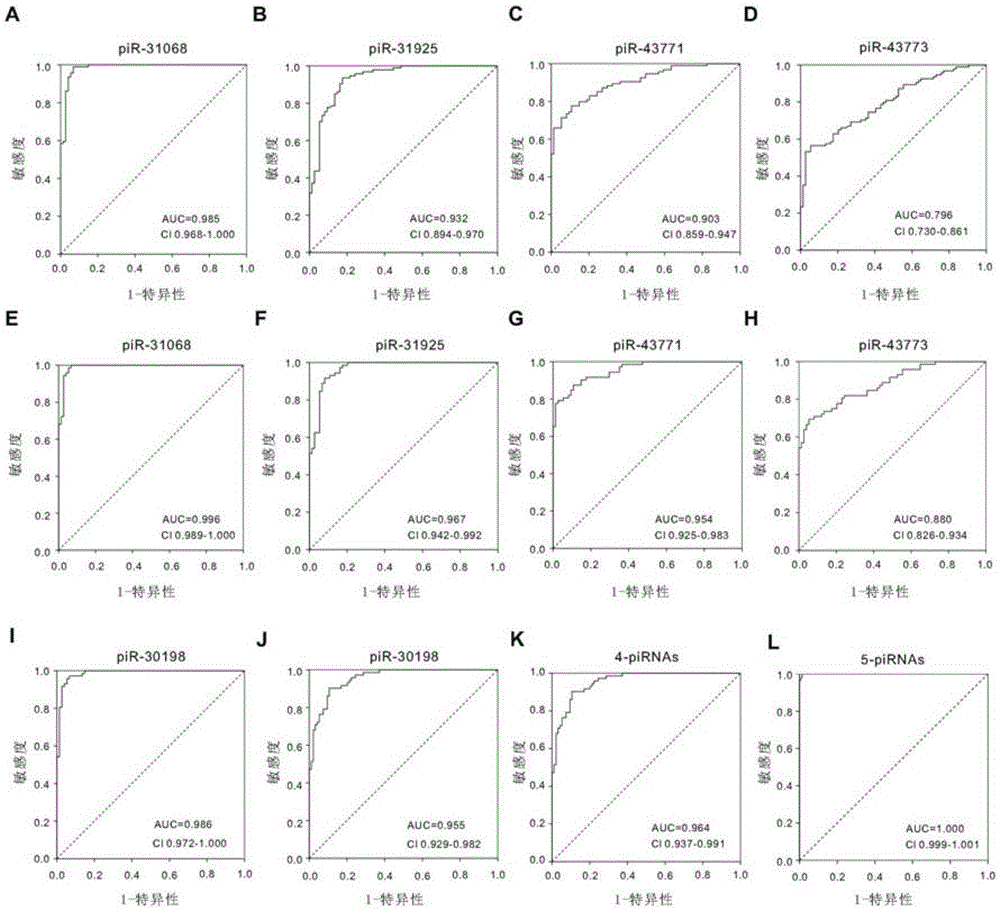
Seminal plasma piRNA markers or their combination for detecting and/or predicting male reproductive dysfunction and application thereof
ActiveCN105483218AEasy to useRelieve painMicrobiological testing/measurementDNA/RNA fragmentationStage iibBiological condition
The invention discloses seminal plasma piRNA markers or their combination for detecting and / or predicting male reproductive dysfunction and application thereof. The piRNA markers or their combination are 61 in quantity and mainly include piR-31068, piR-31925, piR-43771, piR-43773 and piR-30198; this combination can be used to detect and / or predict asthenospermia and azoospermia. In the invention, seminal plasma is easy to obtain, no other tissues are required, and examinations are noninvasive; seminal plasma piRNA can indicate pathological and biological conditions of a whole spermatogenic stage, accuracy level of detection can be increased using the seminal plasma piRNA as auxiliary detection indexes, the pathological and biological conditions the whole spermatogenic process are indicated on molecular level, and a latent target is provided for the diagnosis and treatment of male reproductive dysfunction.
Owner:NANJING YOUZHIYUAN PHARMACEUTICAL TECHNOLOGY CO LTD +1
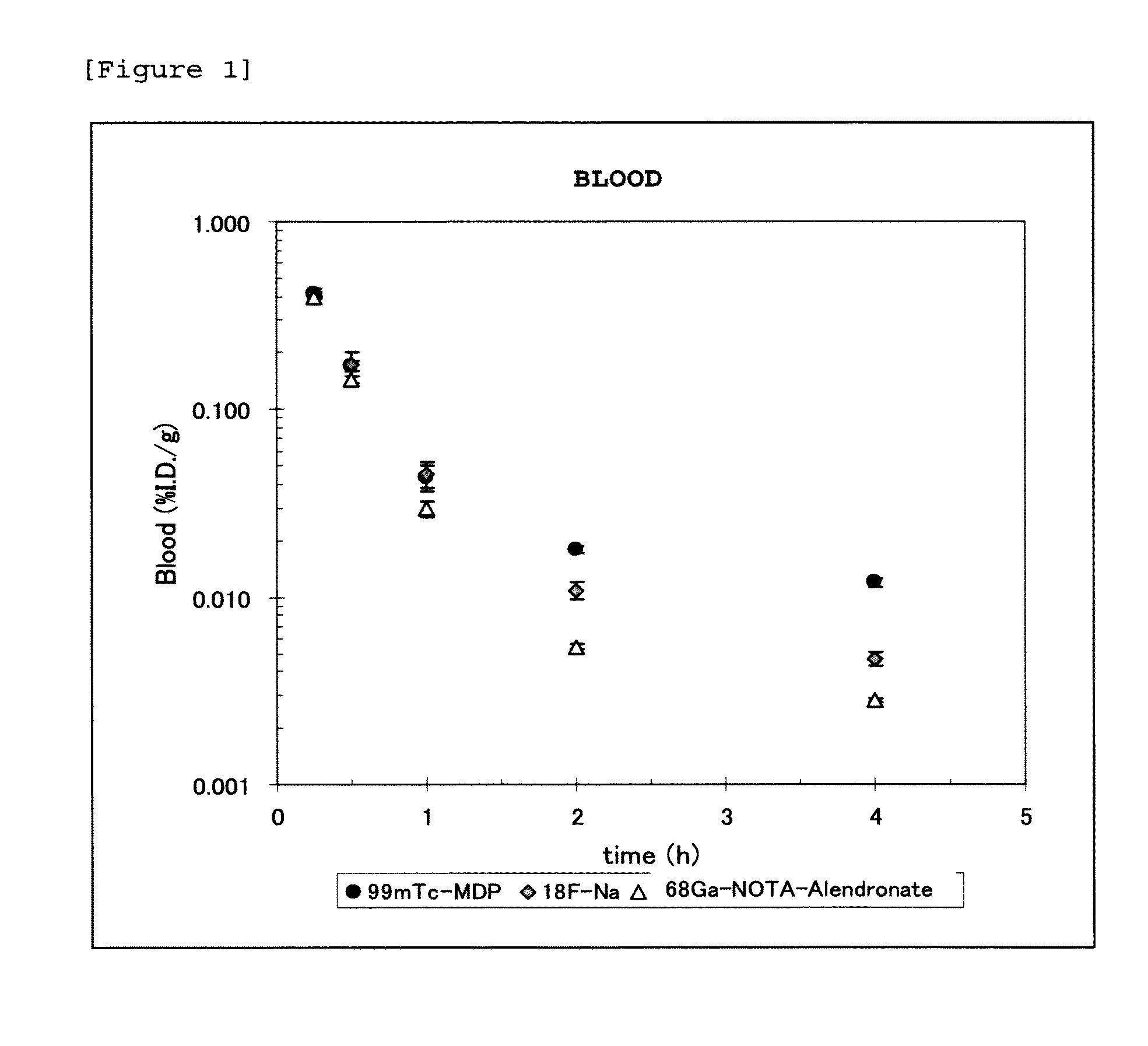
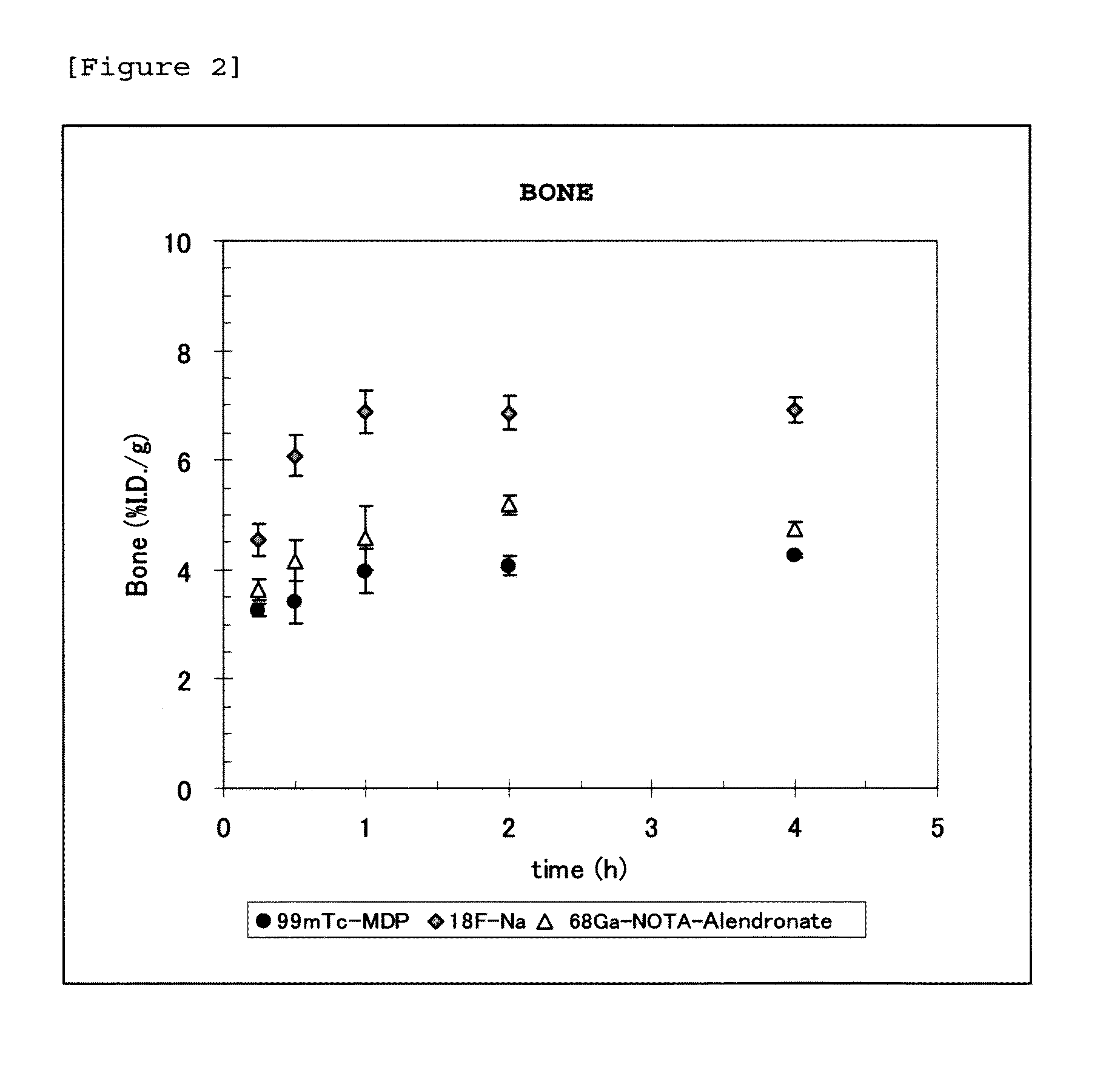
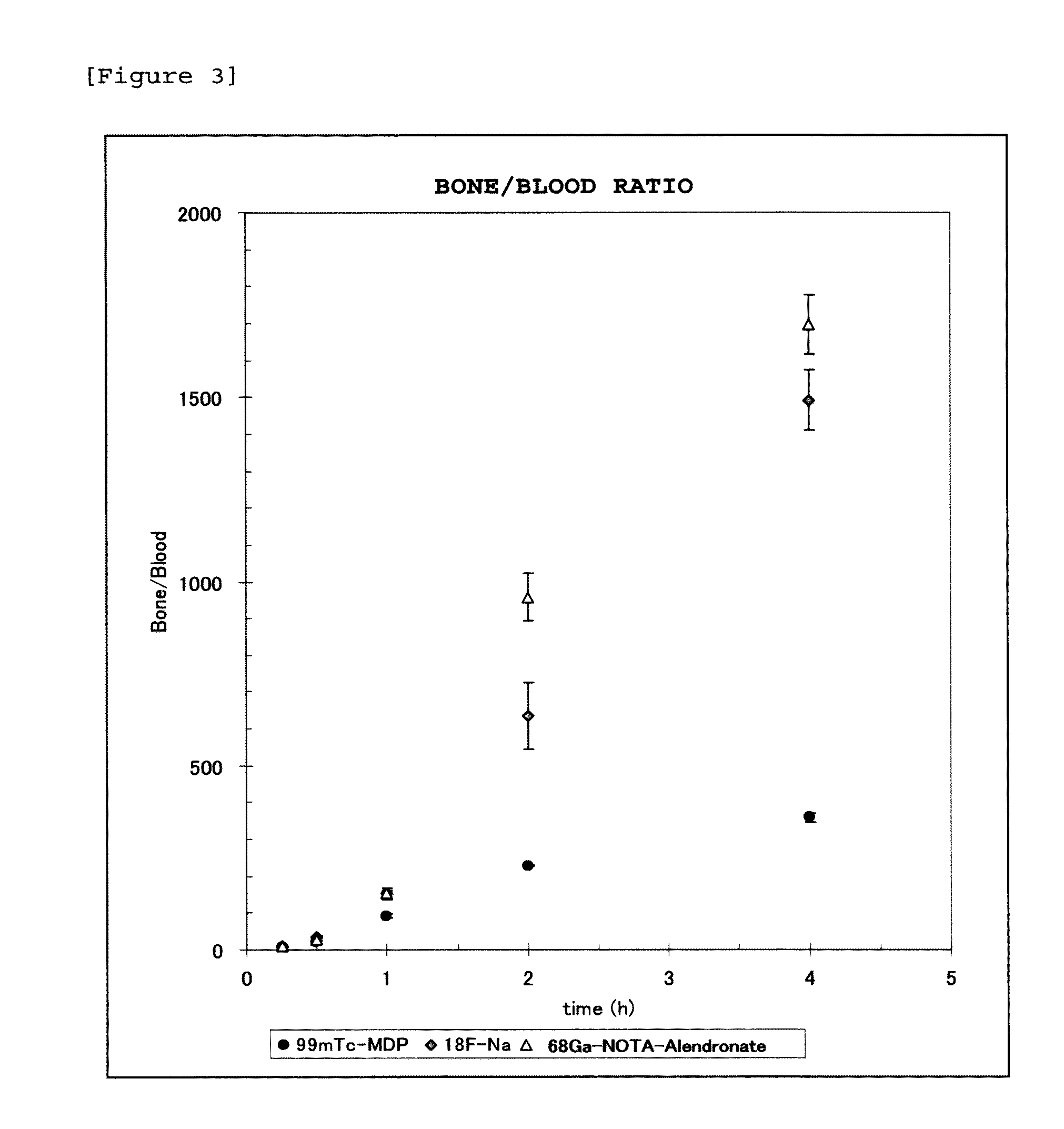
Bisphosphonic acid derivative and compound thereof labeled with radioactive metal nuclide
InactiveUS20120148492A1Rapid clearanceRaise the ratioIsotope introduction to heterocyclic compoundsGroup 5/15 element organic compoundsStage iibHalogen
Disclosed is a radioactive bone diagnostic agent which gives a high ratio of radioactivity accumulation in bone to that in blood from an early stage after administration of the agent and allows capturing an image in a short time after administration. Also disclosed is a bisphosphonic acid derivative represented by the following chemical formula (II) or a salt thereof, wherein X represents —(CH2)mCO—, Y represents —(CH2)n—, R represents H, OH, or a halogen atom, m and n are independent of each other and m represents an integer of 1 to 3, and n represents an integer of 0 to 4.
Owner:FUJIFILM RI PHARMA
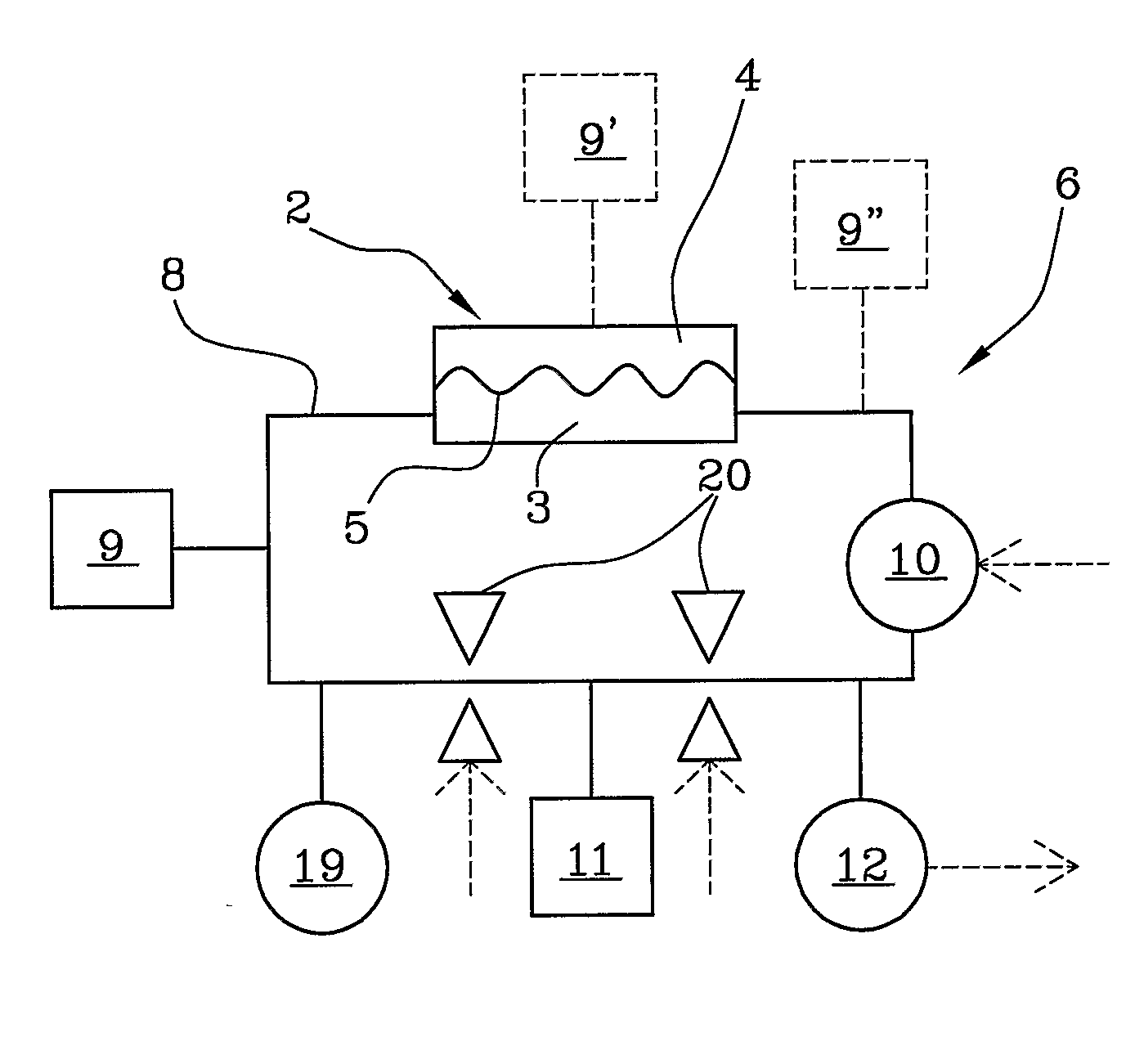
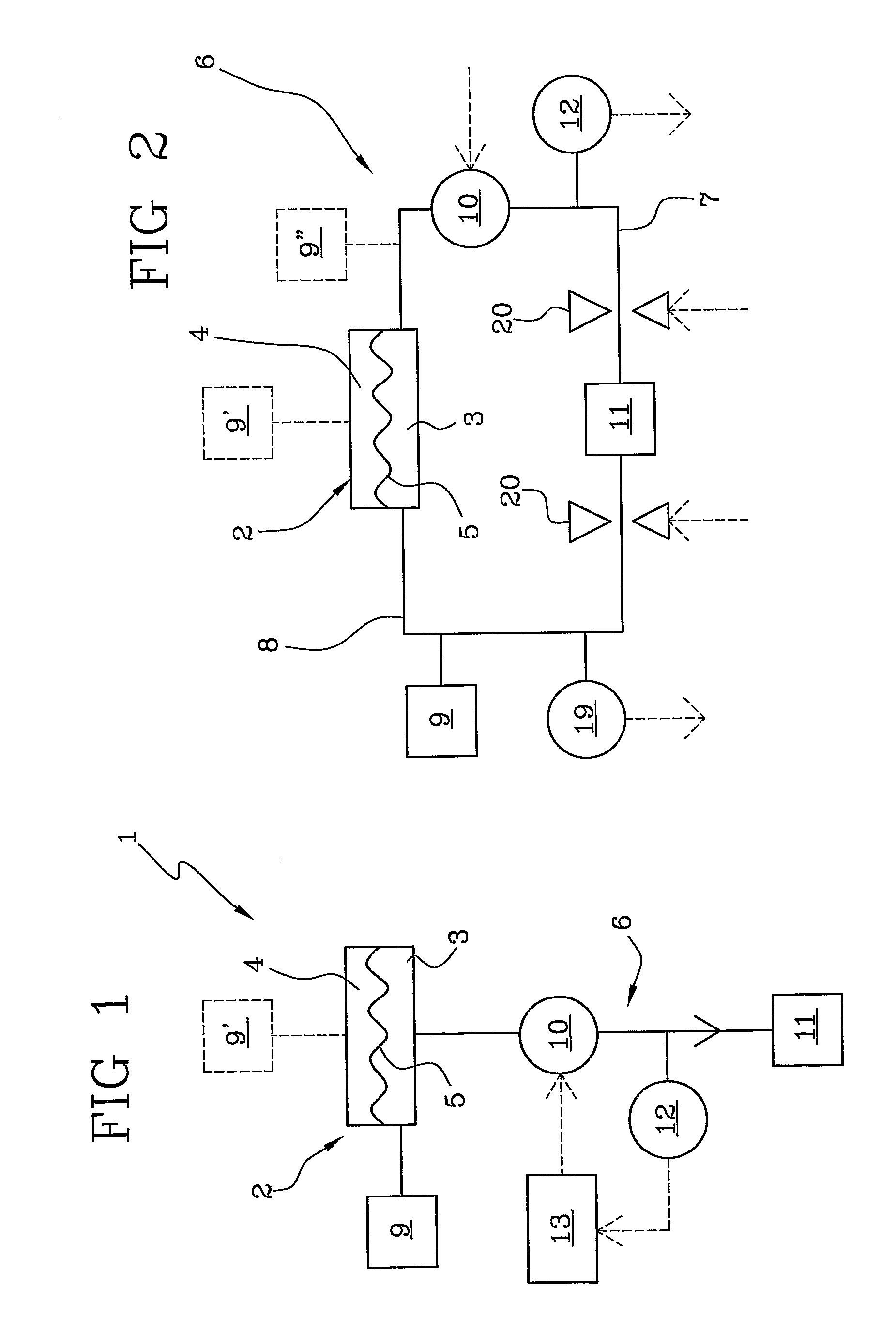
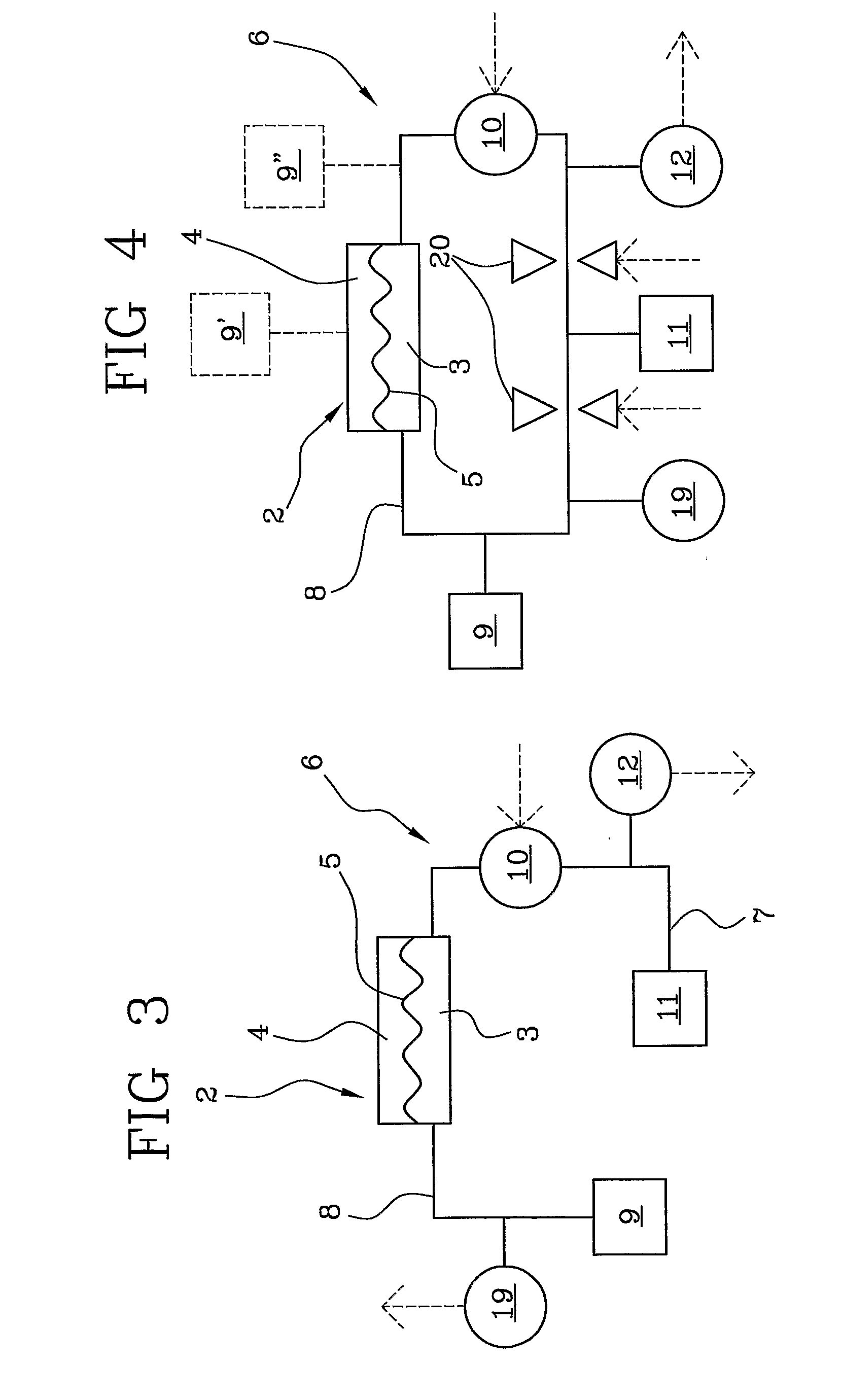
Apparatus for extracorporeal blood treatment
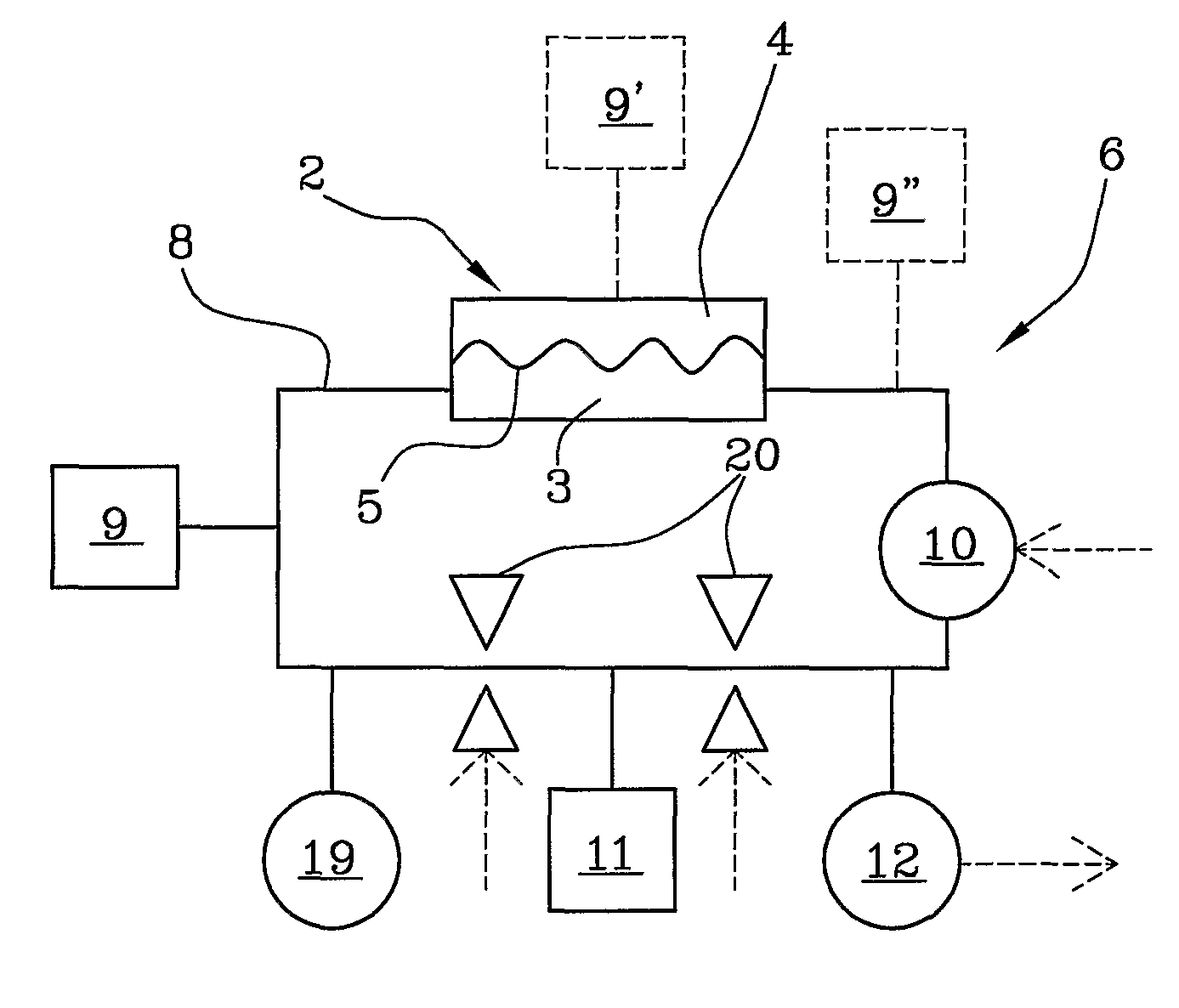
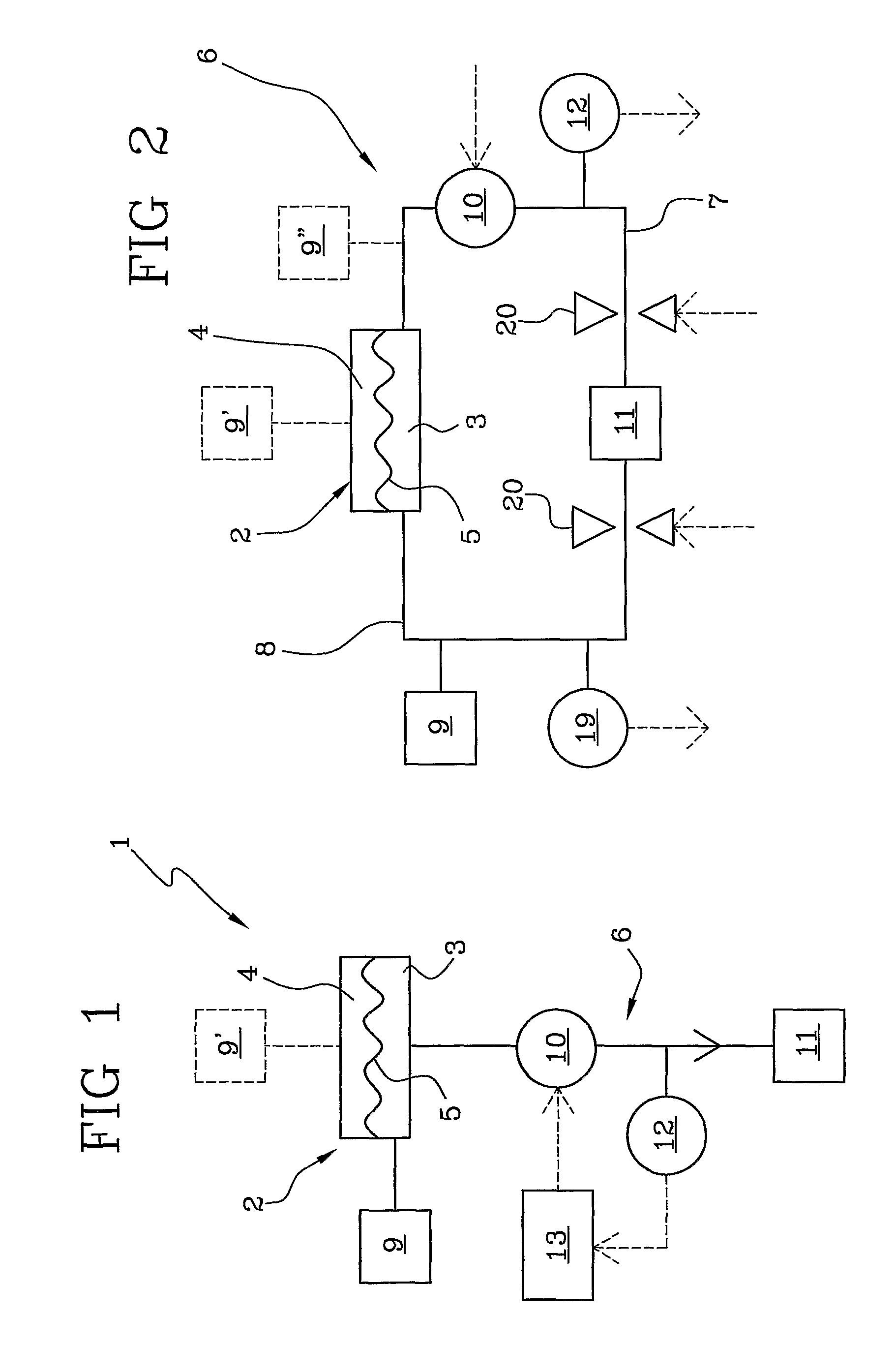
ActiveUS20100114005A1Economical and simpleDistinguish clearlyHaemofiltrationDialysis systemsStage iibBlood treatments
In an apparatus for extracorporeal blood treatment, an extracorporeal circuit (6) is connected to a blood chamber (3) of a membrane device (2). A pump (10) displaces a priming fluid from a source of a priming fluid (9) to a drainage (11) for discharging the priming fluid. A control unit (13) is provided with a processor which controls the pump at a preset first flow rate value, and receives from a pressure sensor (12) a first pressure value, compares the first pressure value with a reference pressure value and, on the basis of this comparison, determines whether or not the extracorporeal circuit is of a pediatric type or of an adult type. The invention is particularly useful during a stage of readying a dialysis apparatus.
Owner:GAMBRO LUNDIA AB
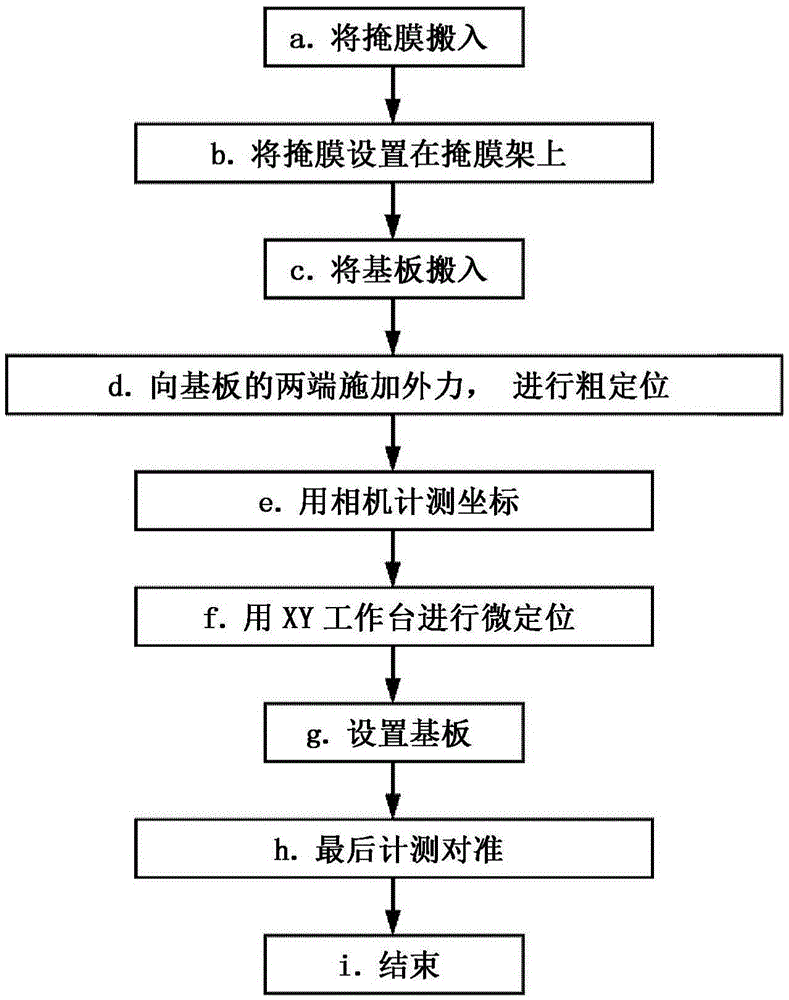
Alignment method and alignment device
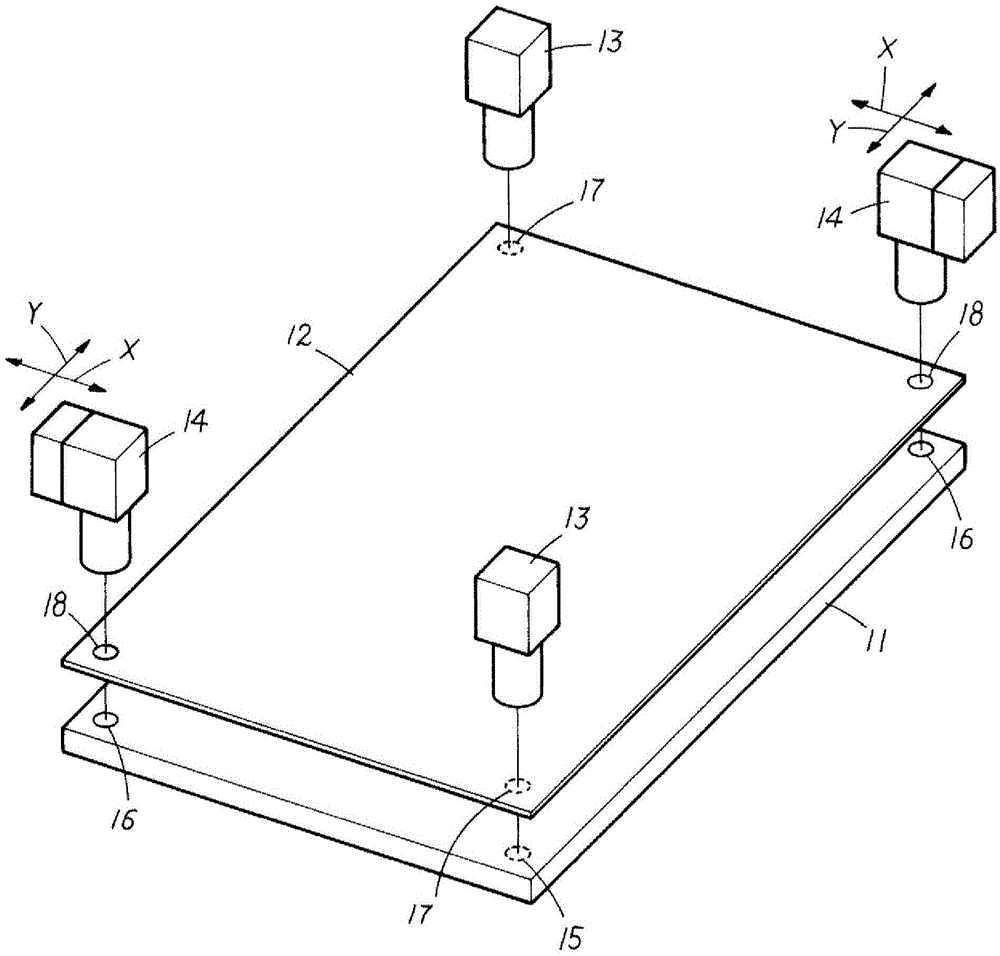
ActiveCN105593396AHigh precisionPracticalVacuum evaporation coatingSemiconductor/solid-state device manufacturingStage iibMagnification
Provided is an alignment method which is practically superior in capabilities for carrying out high precision alignment. When alignment of a substrate (12) and the mask (11) is carried out, a second imaging unit (14) with a high imaging magnification is positioned using imaging data imaged by a first imaging unit (13) with a low imaging magnification. Two stages of steps are carried out for positional correction for the substrate (12) and the mask (11) with the imaging range of the second imaging unit (14) as a reference: a first alignment step that is carried out using imaging data imaged by the first imaging unit (13), and a second alignment step that is carried out using imaging data imaged by the second imaging unit (14).
Owner:CANON TOKKI CORP
Apparatus for extracorporeal blood treatment
ActiveUS8388567B2Economical and simpleDistinguish clearlyHaemofiltrationDialysis systemsStage iibBlood treatments
In an apparatus for extracorporeal blood treatment, an extracorporeal circuit (6) is connected to a blood chamber (3) of a membrane device (2). A pump (10) displaces a priming fluid from a source of a priming fluid (9) to a drainage (11) for discharging the priming fluid. A control unit (13) is provided with a processor which controls the pump at a preset first flow rate value, and receives from a pressure sensor (12) a first pressure value, compares the first pressure value with a reference pressure value and, on the basis of this comparison, determines whether or not the extracorporeal circuit is of a pediatric type or of an adult type. The invention is particularly useful during a stage of readying a dialysis apparatus.
Owner:GAMBRO LUNDIA AB
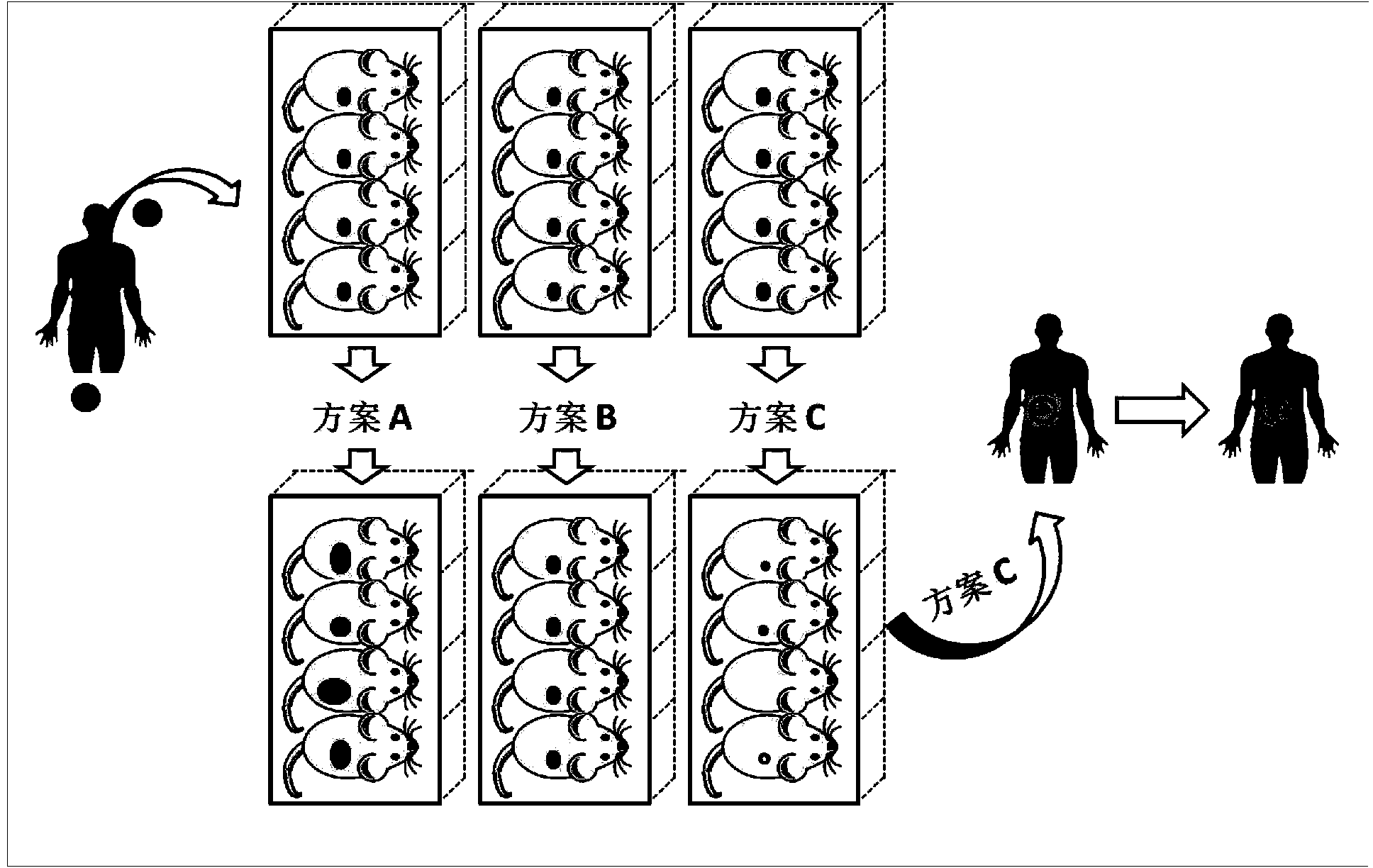
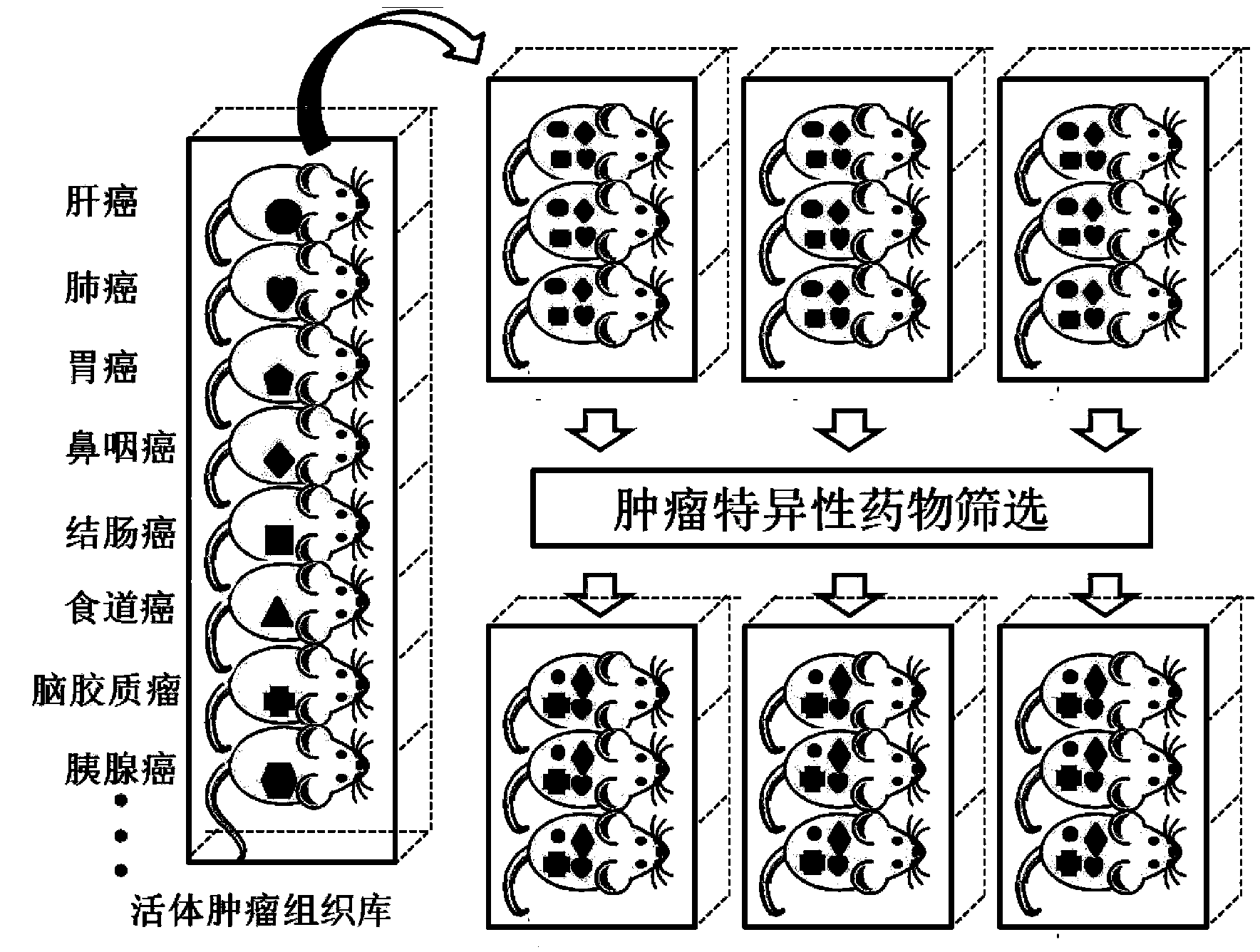
Building method and applications of living tumor tissue bank
The invention provides a building method and applications of a living tumor tissue bank. Tumor tissues of a cancerous patient subjected to an operation are transplanted to a part under the renal capsules of SCID (severe combined immunodeficient mice) and survive in the bodies of the mice for passage conservation, so that the living tumor tissue bank is obtained; the living tumor tissue bank means that based on single tumor tissue heterograft, numerous human body tumor tissues are conserved in animal bodies in a living tissue bank form for standby application; the living tumor tissue bank comprises transverse multiple tumors and various subtypes or various development phase of a certain longitudinal tumor. The living tumor tissue bank has multiple applications in the aspects of determining the personalized therapeutic schedule of the cancerous patient subjected to the operation, screening tumor specificities of anti-cancer drugs, and building animal models for human body cancer research.
Owner:AFFILIATED TAIHE HOSPITAL OF HUBEI MEDICAL COLLEGE
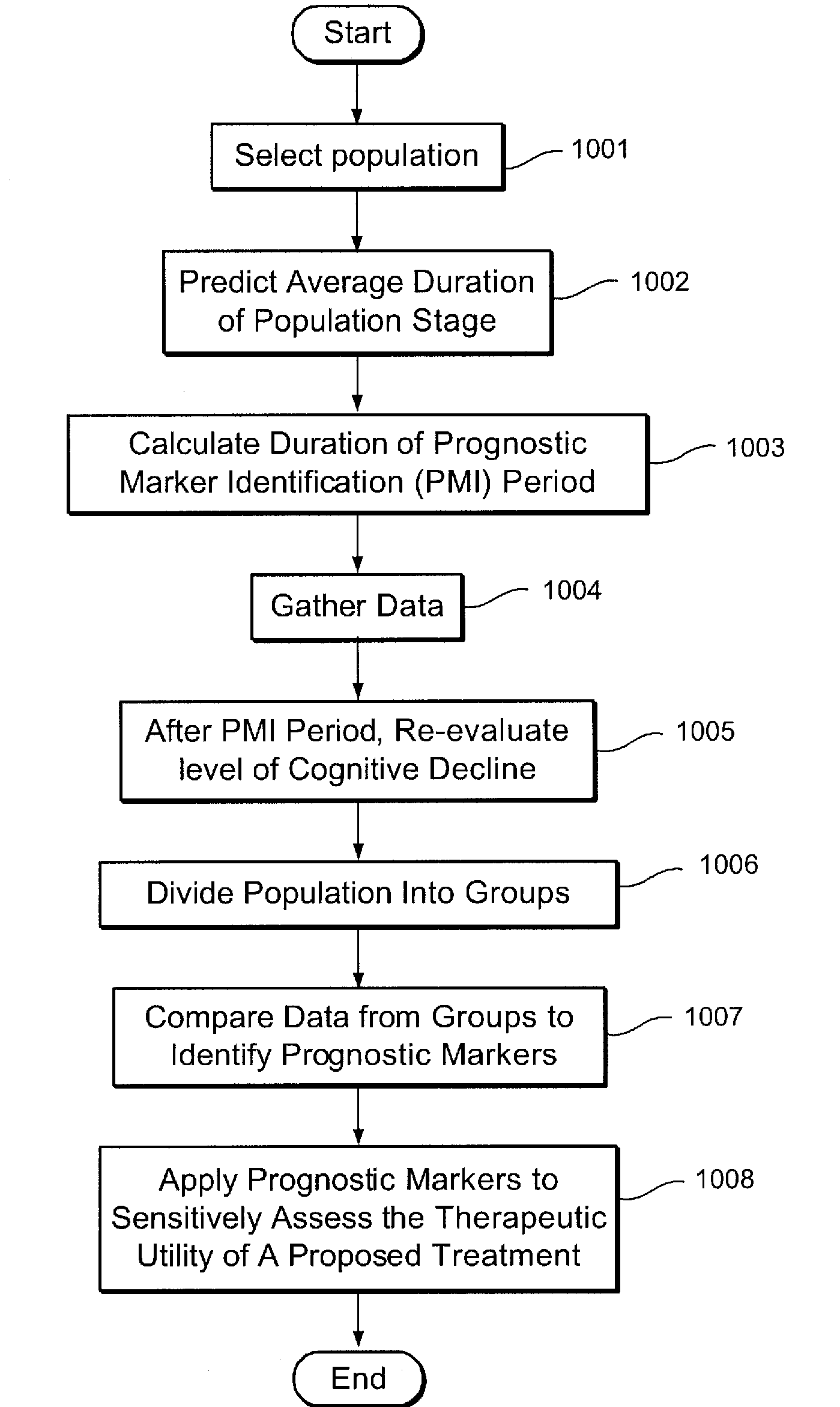
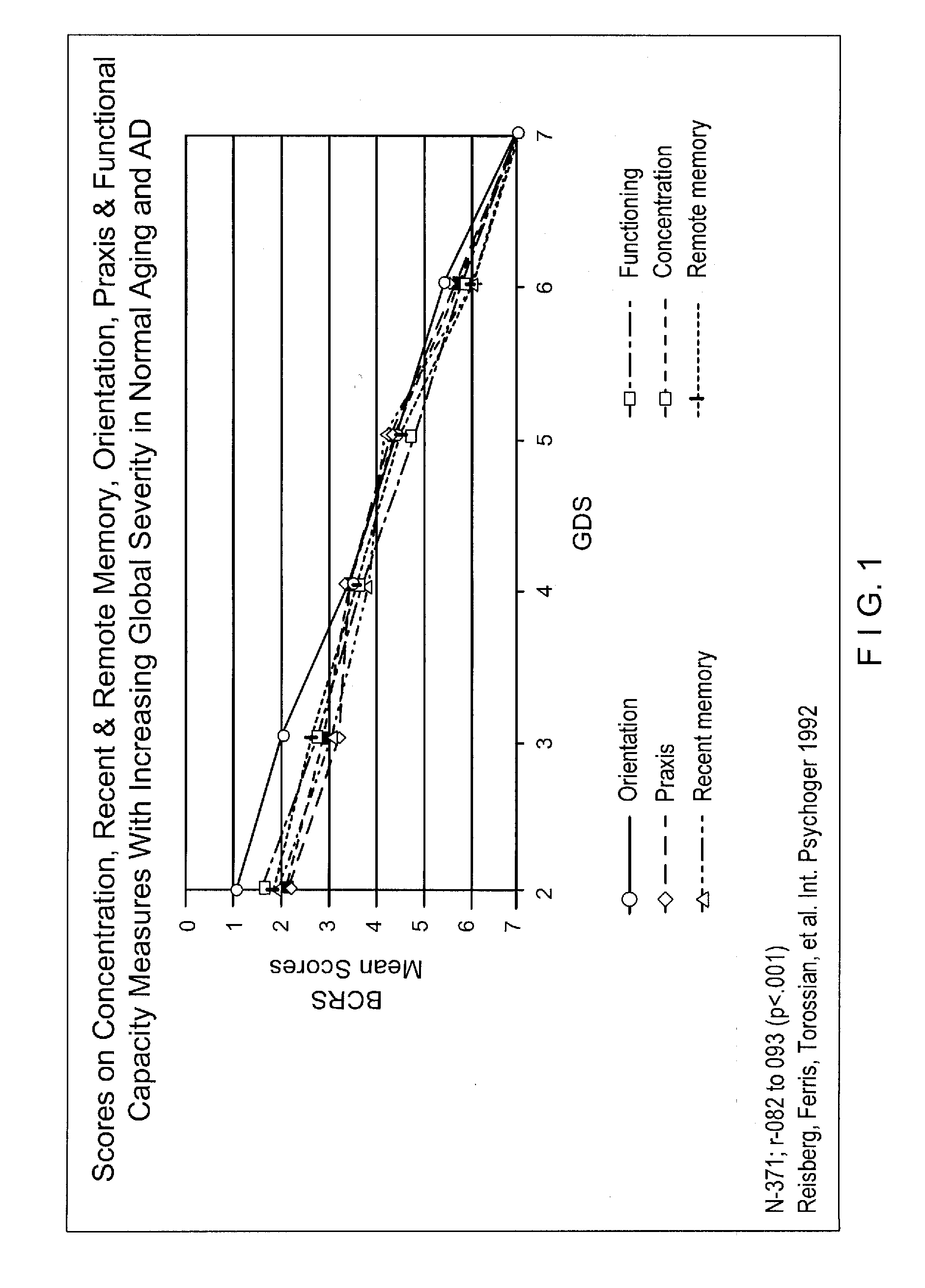
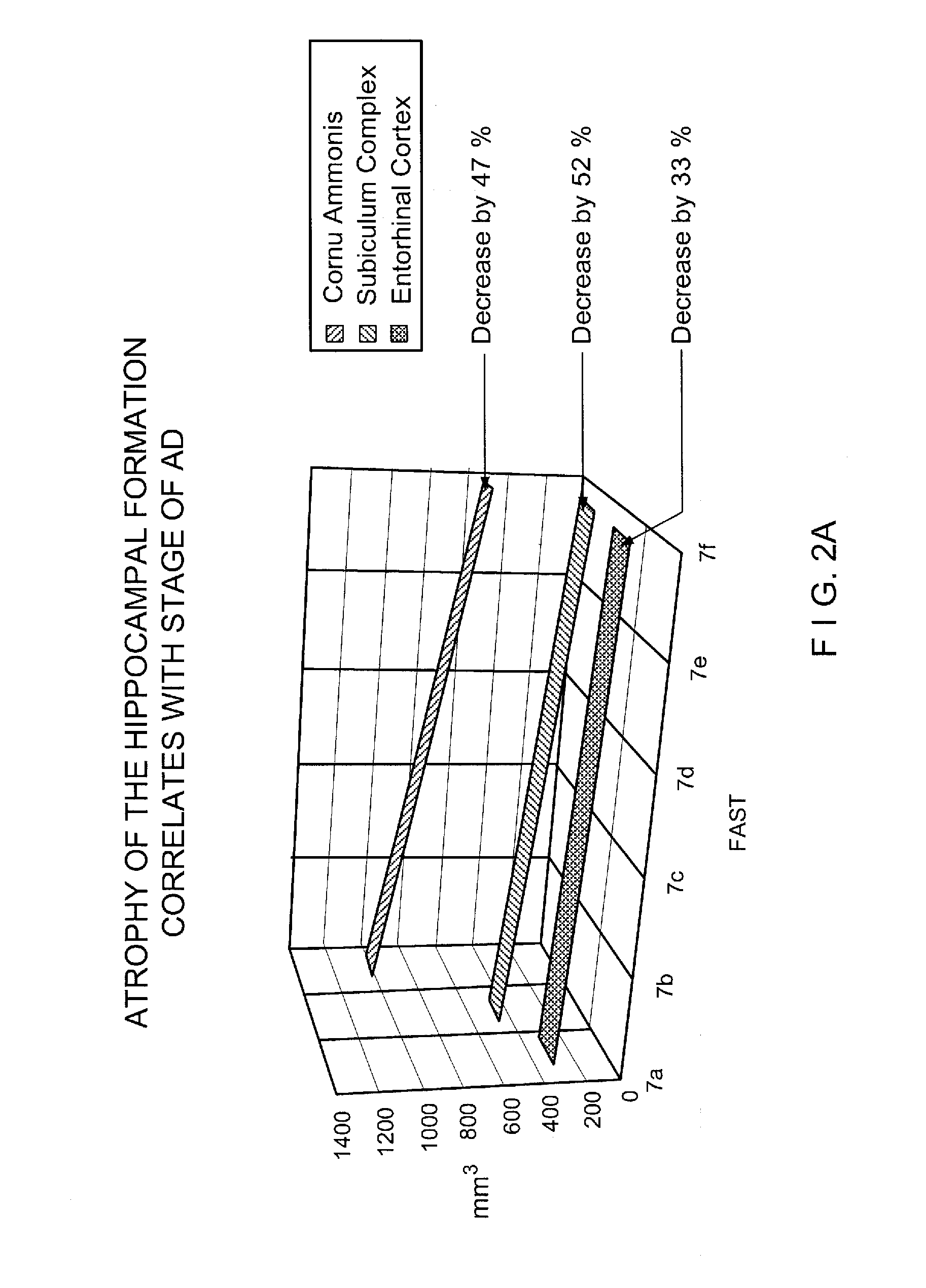
Stage Specific Prognostic In Vivo Markers of Brain Aging and Dementia
InactiveUS20090099783A1SurgeryEpidemiological alert systemsStage specificMild cognitive impairment (MCI)
A method for the identification of treatments and preventative agents for brain aging, subjective cognitive impairment (SCI), mild cognitive impairment (MCI), Alzheimer's disease (AD) and other degenerative dementias, the method including (a) the identification of the diagnosis and stage of the subject, (b) the identification of the duration of the stage of the condition and / or disorder, (c) the identification of prognostic markers based upon a formula incorporating the duration of the condition and / or stage, (d) the prospective separation of prognostic subgroups based upon outcome wherein the outcome is defined in these conditions as progression to a subsequent stage or stages, (e) the employment of a putative prognostic marker for an appropriate period of time, based upon the formula incorporating the duration of the condition and / or stage, (f) the application of in vivo, methodology specific techniques, in conjunction with stage specific prognostic subgroups, for the appropriate time period, for the identification, prospectively, of useful markers, (g) the employment of these markers to identify useful therapeutic agents for prevention and treatment.
Owner:NEW YORK UNIV
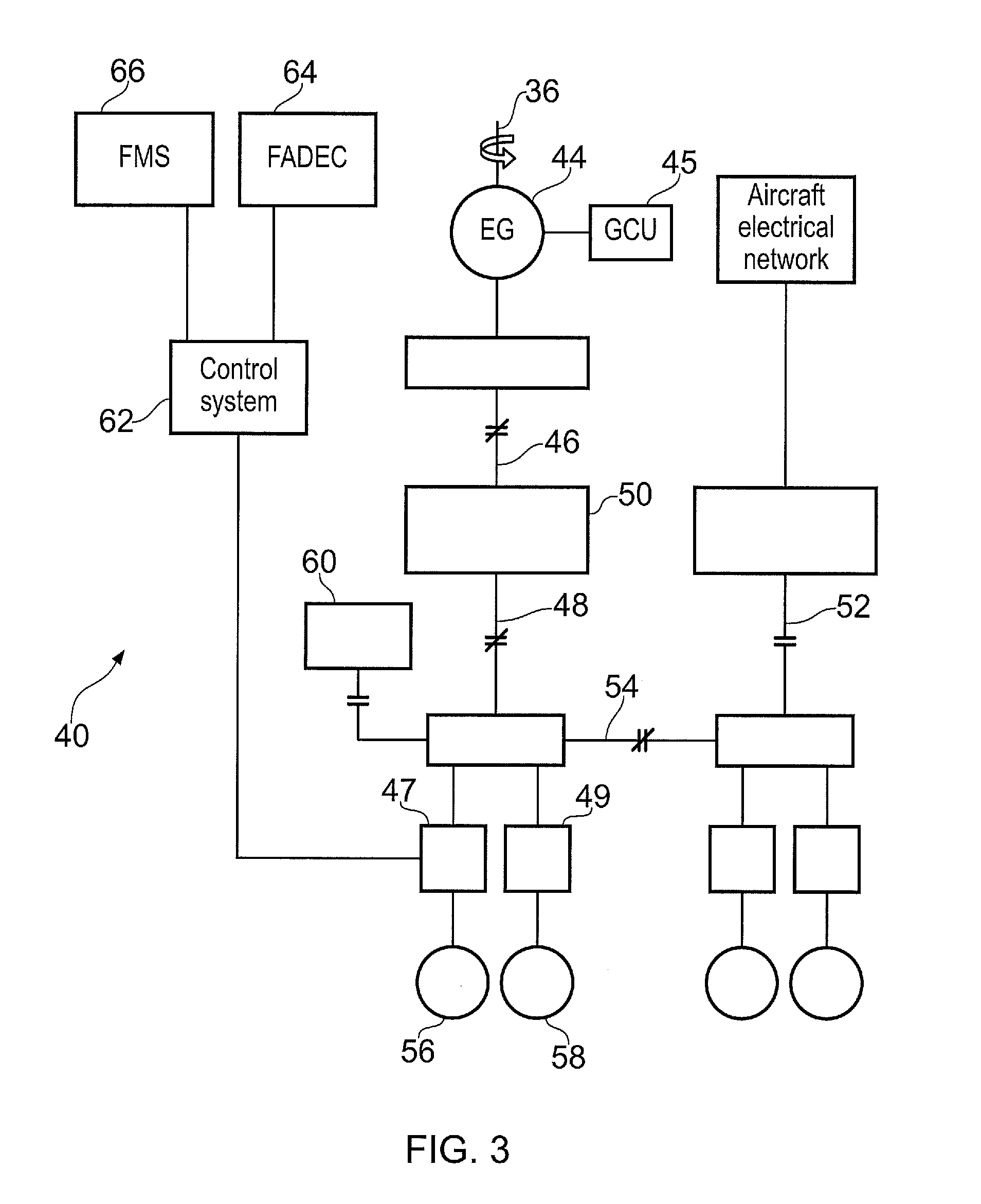
Aircraft electrical system operating method
ActiveUS20160185462A1Continuous operationPrevent leakageVehicle testingElectric power distributionStage iibFlight computer
A method of controlling an aircraft electrical system (40). The electrical system (40) comprises an alternating current electrical machine (56, 58) comprising a plurality of phases, each phase having a power rating, the electrical machine (56, 58) being configured to operate on failure of one or more phases. The method comprises: determining a power requirement; sensing a fault of one or more phases of the electrical machine (56, 58), and controlling the electrical machine (56, 58) to operate in a fault condition, in which the remaining phases of the electrical machine (56, 58) provide the electrical power requirement; and providing an overrating signal to a flight computer (66) of the aircraft (2) where the power provided by each phase exceeds the power rating of the respective phase.
Owner:ROLLS ROYCE PLC
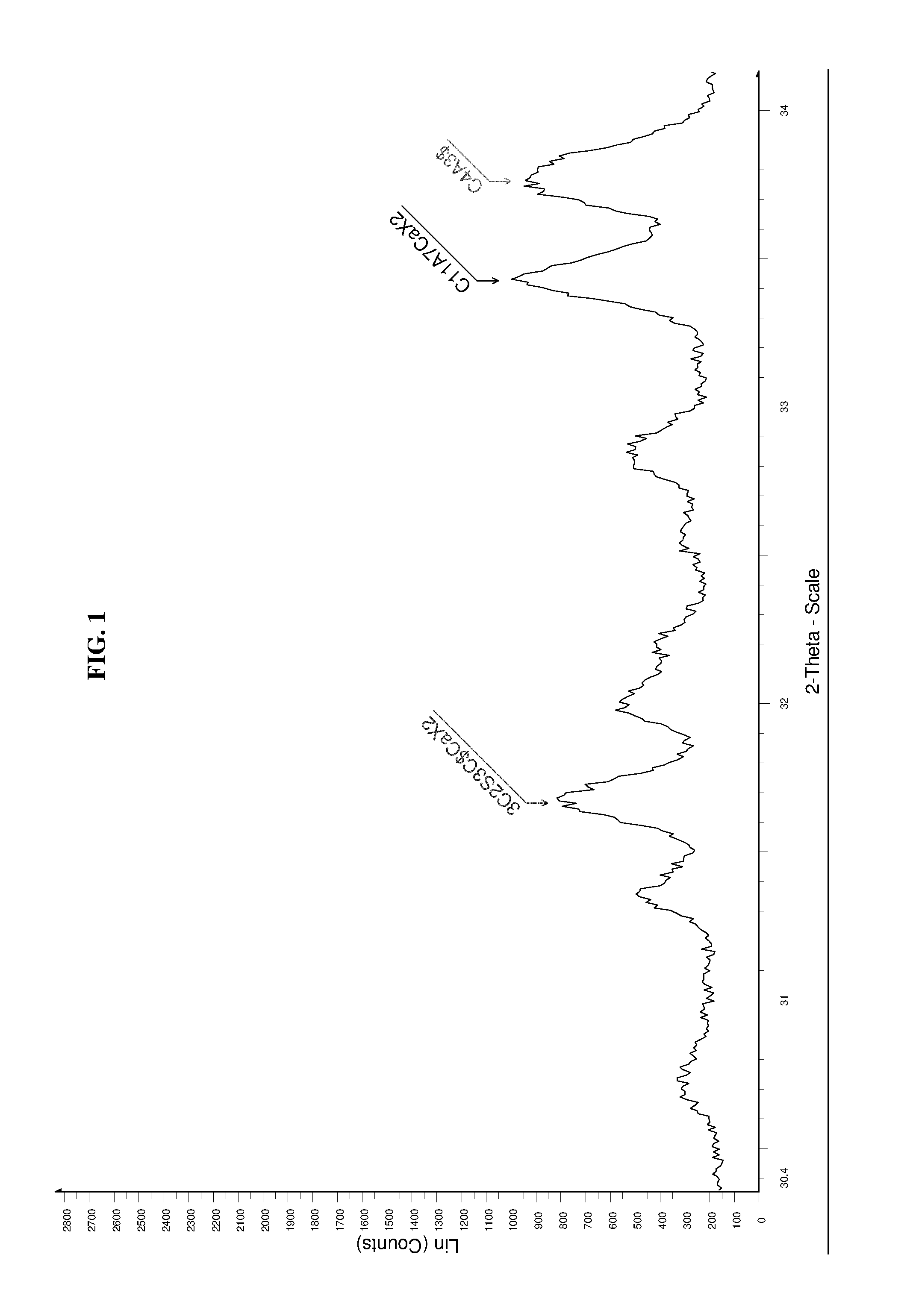
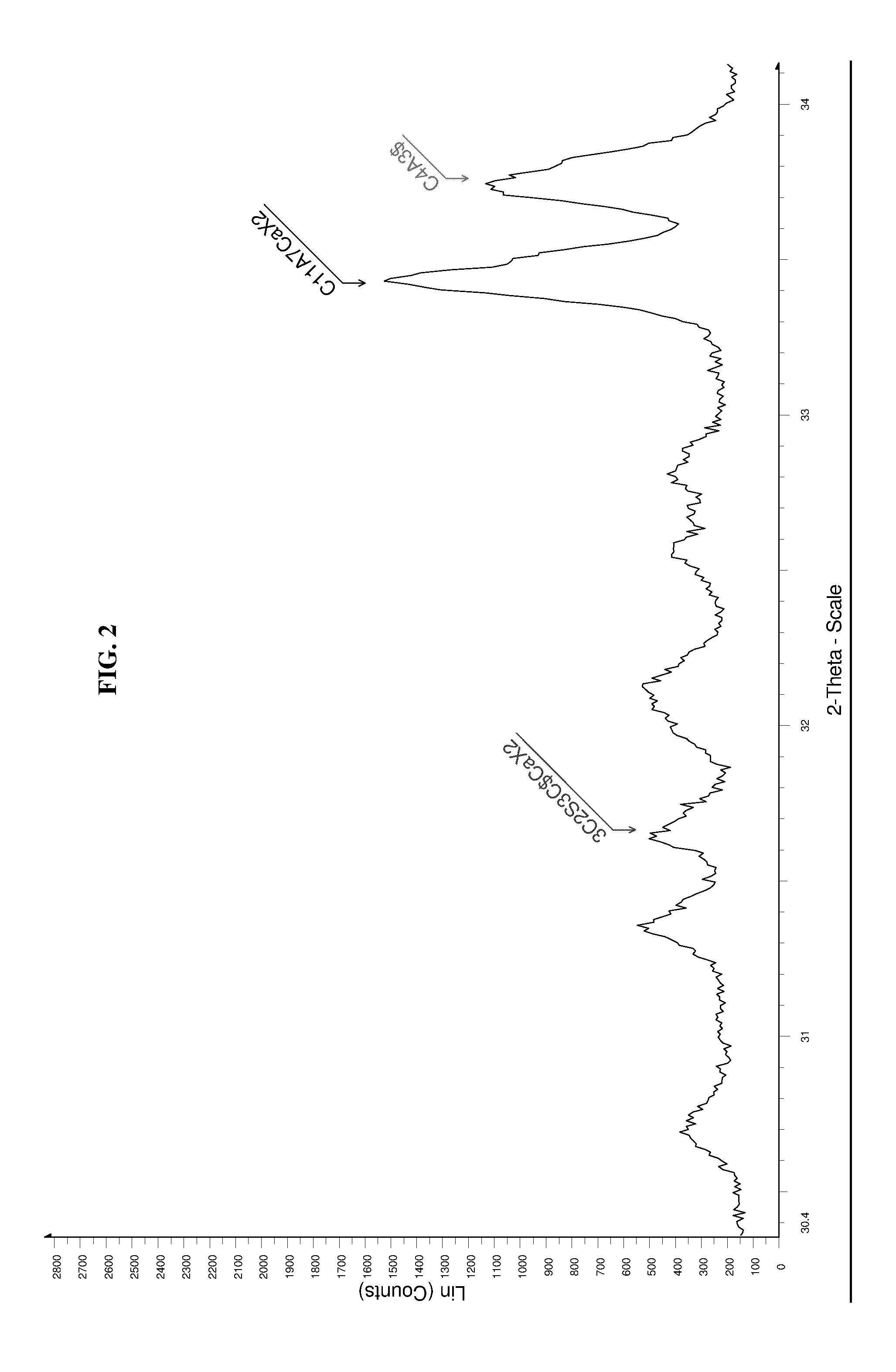
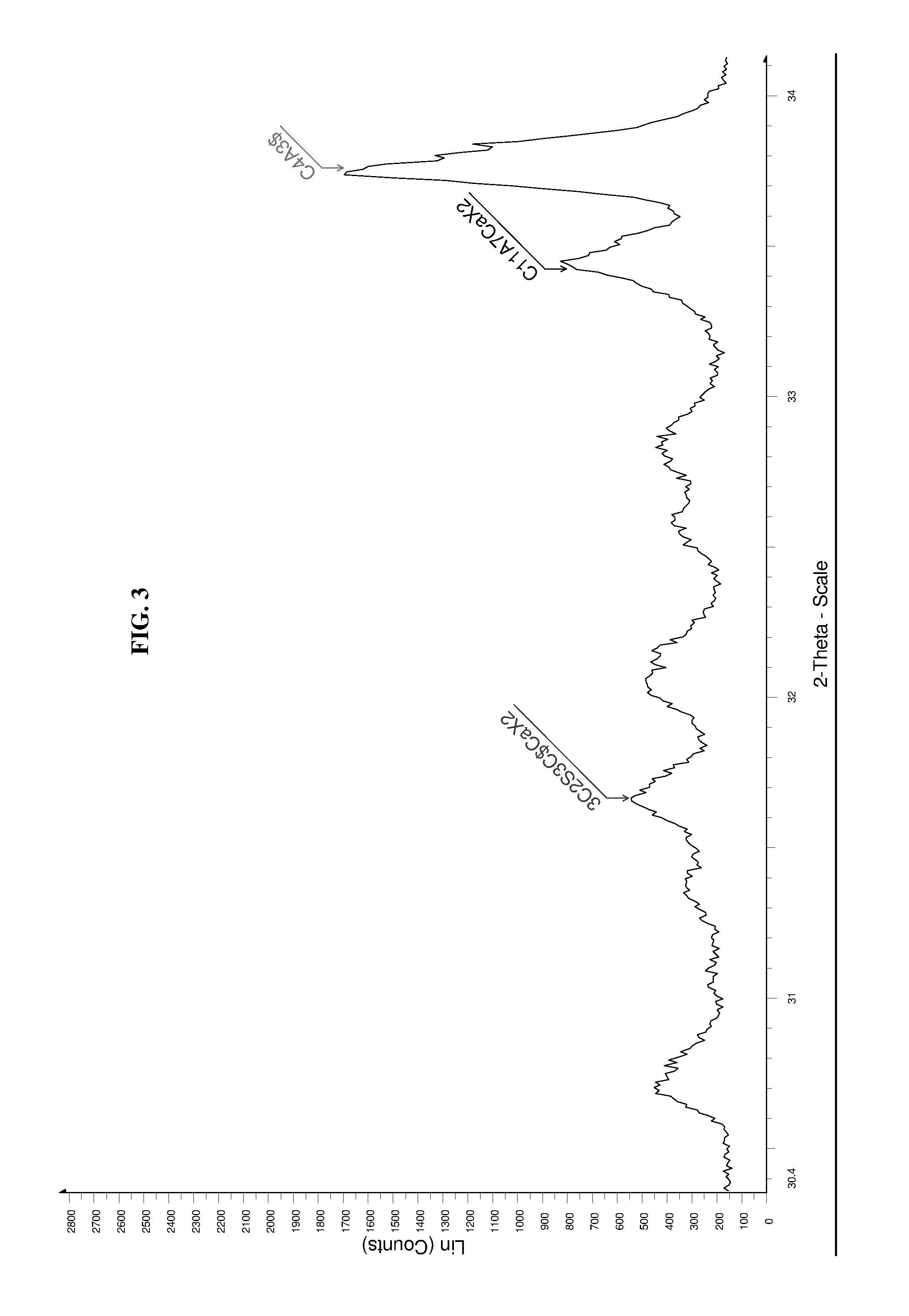
High performance sulfo-aluminous clinker
The invention relates to a sulfo-aluminous clinker with optimal setting time and short term compressive strengths, comprising a mixture of the following phases: —calcium sulfoaluminate, or C4A3$, in amounts higher than 50% by weight of the mixture, —belite, or C25, in amounts between 2 and 23%, —3C25 3C$ CaX2, X being fluorine or chlorine, between 3 and 15% —C11A7CaX2, X being fluorine or chlorine, between 2 and 12%, both fluorine and chlorine being altogether present in the mixture, and phase C5S2$ being absent. The invention also relates to a method for preparing this sulfo-aluminous clinker, and hydraulic binders comprising this clinker.
Owner:ITALCEMENTI
Use of annellated pyrrole compounds in the treatment of articular cartilage or subchondral bone degeneration
InactiveUS7425573B2Deterioration progressAvoid damageBiocideOrganic chemistrySubchondral boneStage iib
Treating or preventing degeneration or destruction of articular cartilage and / or subchondral bone in the affected joint of a mammal is accomplished by administering a compound of formula (I), wherein the variables have the meanings given in the present description. A preferred compound of formula (I) is formula (II). This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration
Owner:MERCKLE GMBH DE +1
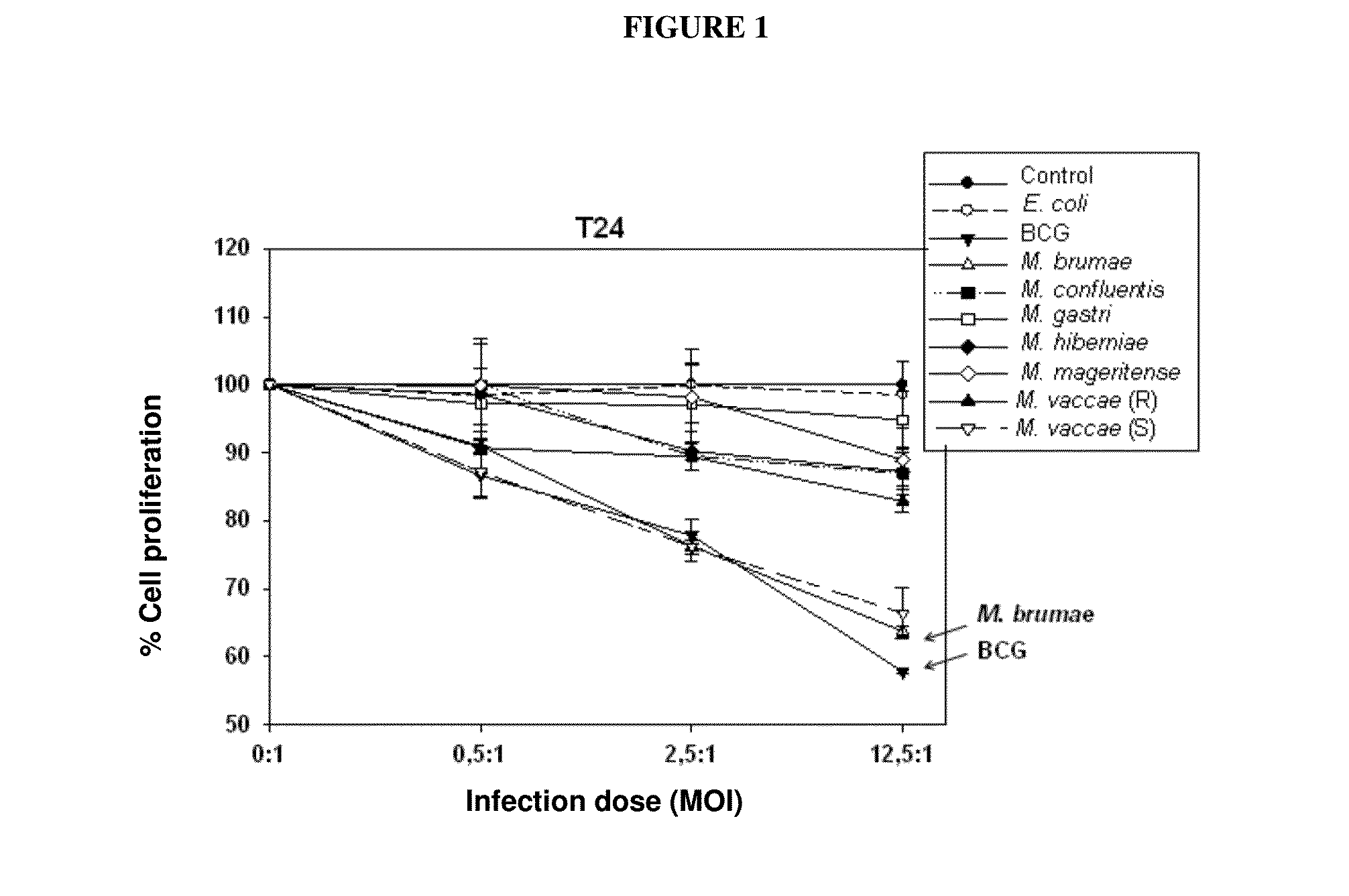
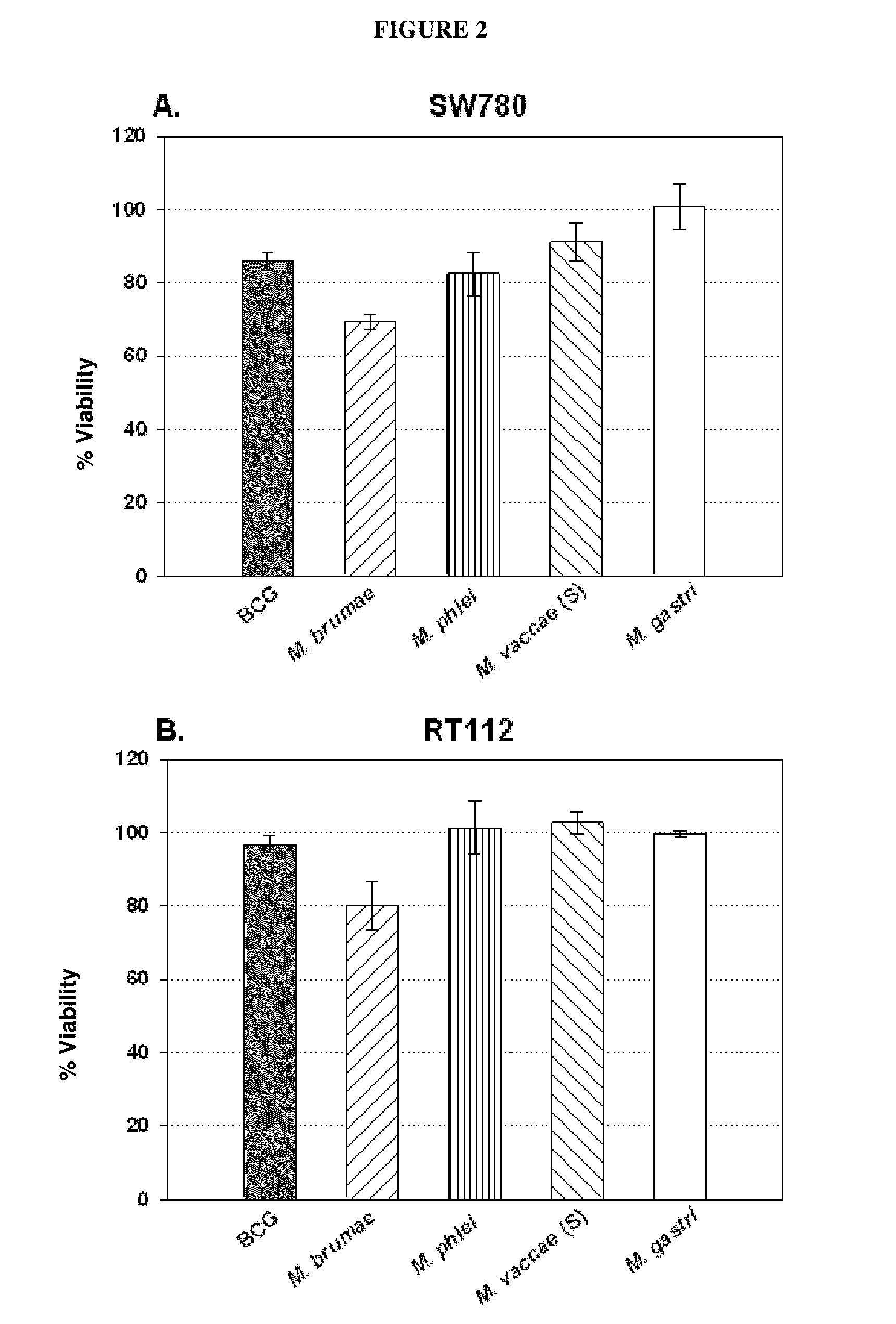
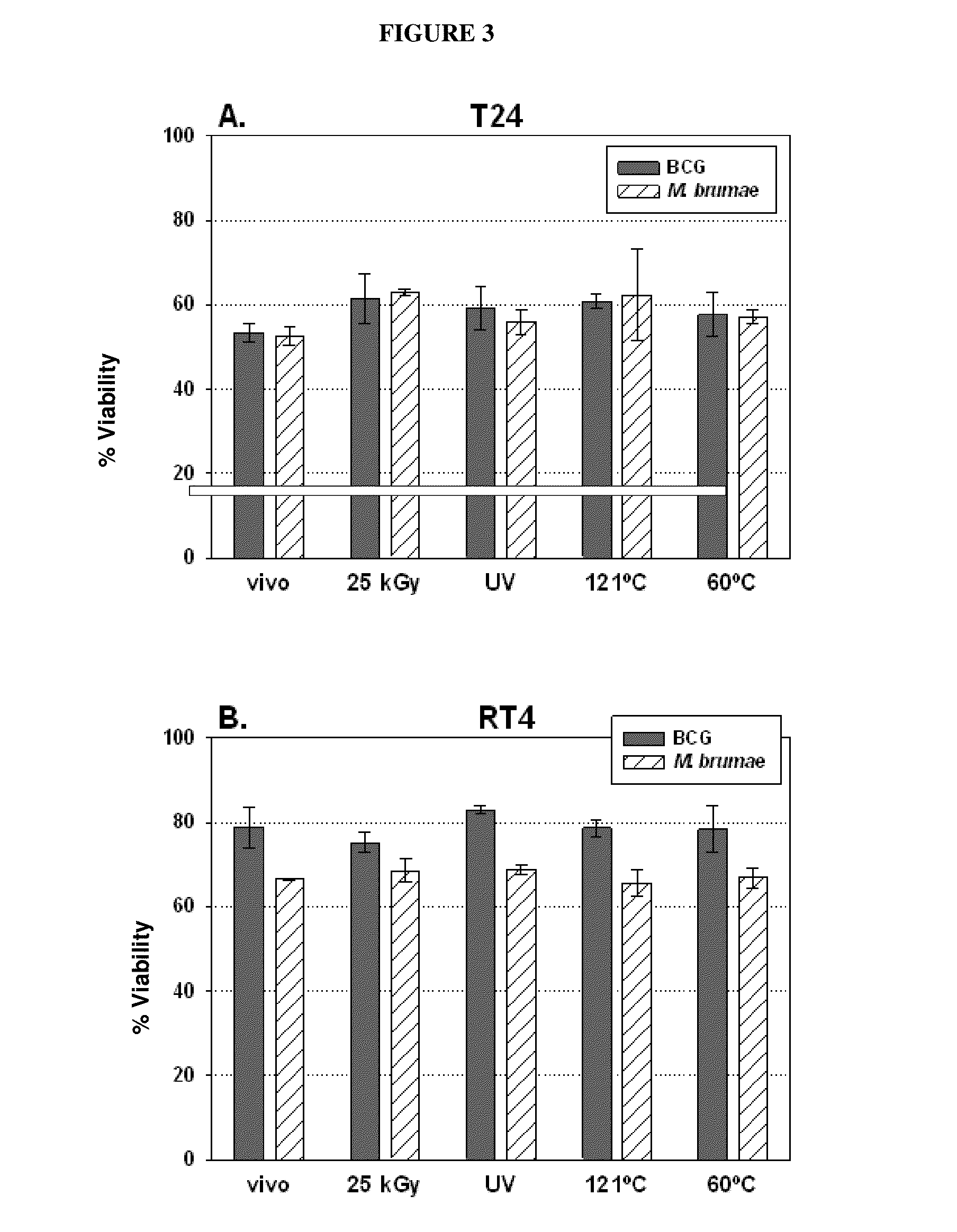
Use of mycobacterium brumae to treat bladder cancer
InactiveUS20150224151A1Reduce riskOrganic active ingredientsBiocideMycobacterium brumaeLymphatic Spread
Use of Mycobacterium brumae for the treatment of bladder cancer. The present invention relates to the treatment of bladder cancer, preferably superficial, non-invasive bladder cancer by using mycobacteria Mycobacterium brumae. Therapy described in this invention is particularly effective in grade 1 (well differentiated) and grade 2 (moderately differentiated) tumor cells. This makes it possible to treat the disease, which is still in an early stage, by reducing the risk of disease progression to a more harmful stage and / or preventing the risk of metastasis.
Owner:AUTONOMOUS UNIVERSITY OF BARCELONA
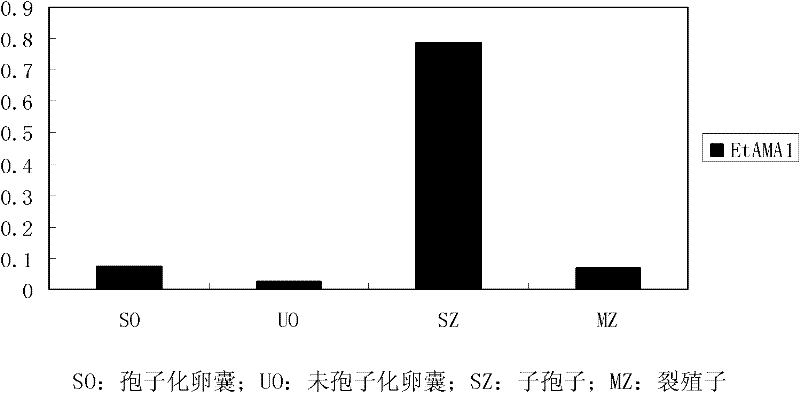
Eimeria tenella apical membrane antigen 1 (AMA 1) gene and application thereof
The invention discloses an Eimeria tenella AMA 1 gene, the nucleotide sequence of which includes a DNA sequence that codes the amino acid sequence represented by SEQ ID No.1 or a DNA sequence that codes the amino acid sequence which has AMA 1 protein activity and is obtained by omitting, adding, inserting or substituting one or more amino acid in the amino acid sequence represented by SEQ ID No.1. The invention also discloses proteins coded by the above-mentioned genes. The Eimeria tenella AMA 1 gene and proteins provided in the invention are closely related to invasion of daughter spores, escape and invasion of merozoites and escape and invasion of microgametes in the stage of sexual reproduction, and are suitable for being developed into novel vaccines or medicines for controlling coccidiosis.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Molecular markers of hepatocellular carcinoma and their applications
InactiveCN101313220AAccurate Prognosis SystemCompound screeningApoptosis detectionEtiologyProteomics methods
Owner:PROYECTO DE BIOMEDICINA CIMA
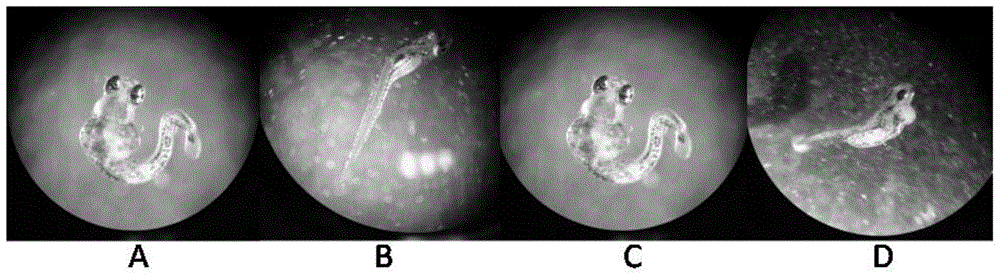
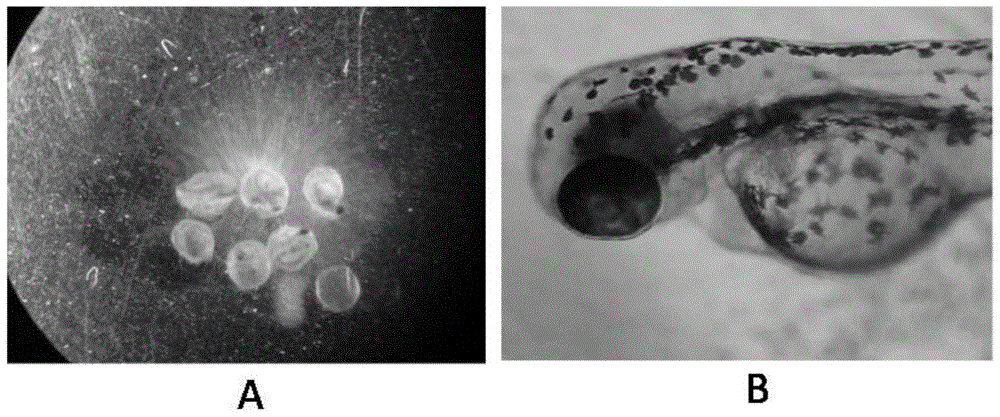
Method for predicating embryotoxicity of non-steroidal anti-inflammatory drug type novel pollutants on early-phase life stage of zebra fish
ActiveCN105044317AAddressing non-reflective typical NSAID sewage biotoxicityBiological testingLife stageFish embryo
The invention discloses a method for predicating the embryotoxicity of non-steroidal anti-inflammatory drug type novel pollutants on an early-phase life stage of zebra fish. The method comprises the following steps: exposing zebra fish embryos in the non-steroidal anti-inflammatory drug type novel pollutants which have equal logarithm space concentrations; recording the death rate and the aberration rate of the zebra fish embryos 7 days after exposing; and calculating by using SPSS software to obtain corresponding LC50 and teratogenetic EC50, which are used for evaluating the toxicity of the non-steroidal anti-inflammatory drug type novel pollutants. With the adoption of the method, the toxicity feature and the toxicity level of the non-steroidal anti-inflammatory drug type novel pollutants are subjected to an analytical test and quantitative description; and meanwhile, the method also can be used as an index for monitoring and evaluating the wastewater biological toxicity of non-steroidal anti-inflammatory drugs, and reference is provided for risk predication and evaluation of the potential biological toxicity of the pollutants in a water body.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY
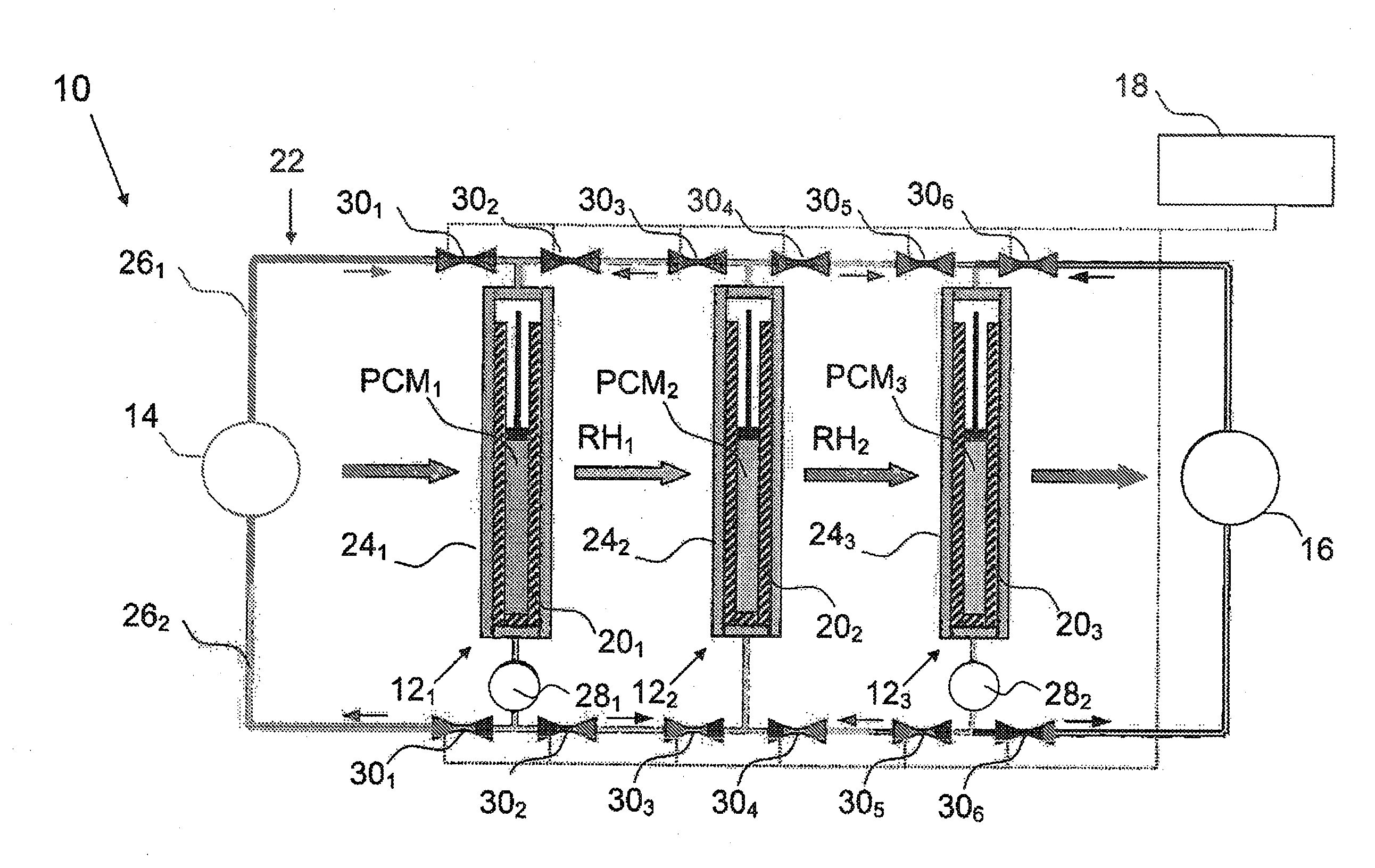
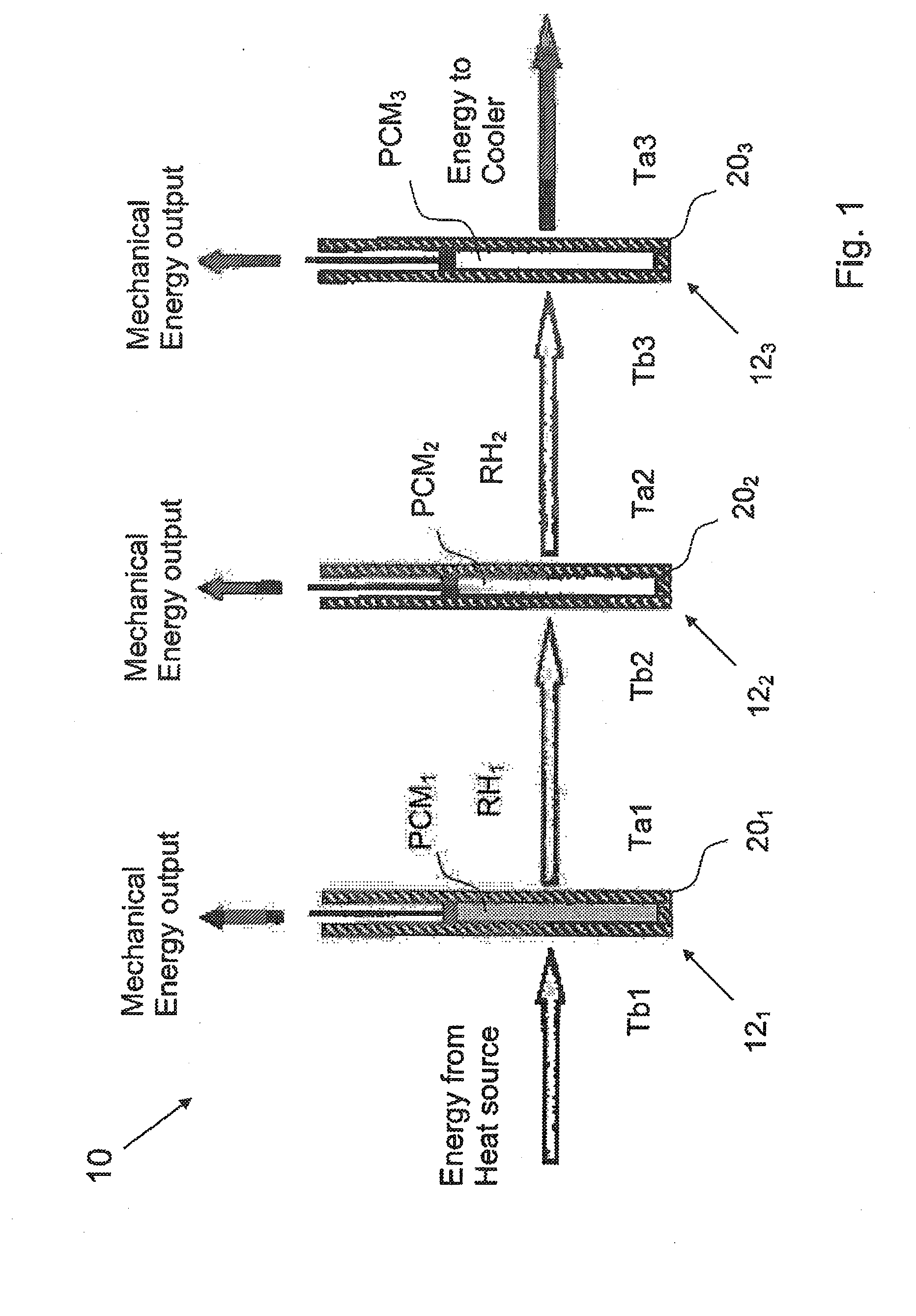
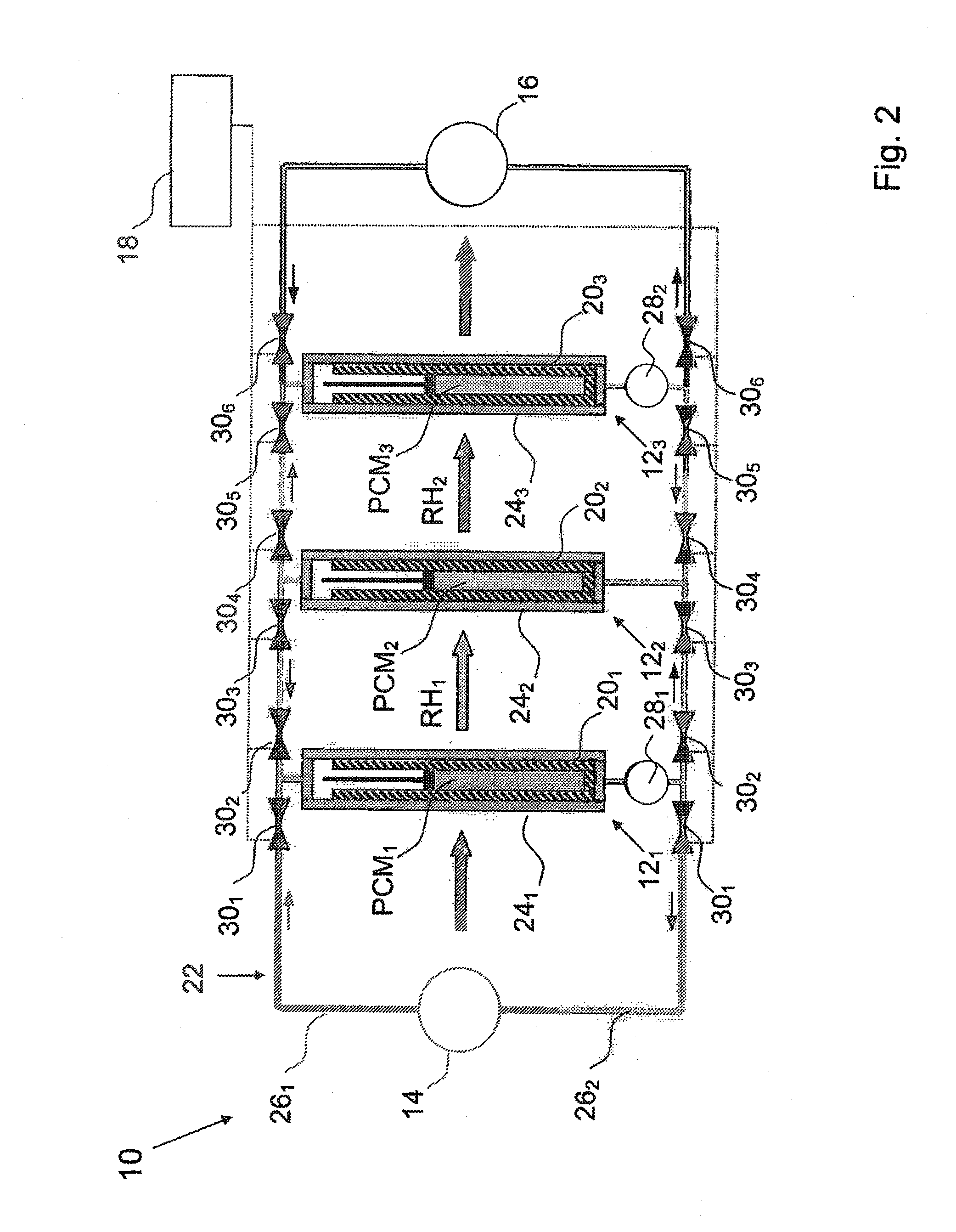
System and method for regenerating heat energy
InactiveUS20110024075A1Simple methodEfficient methodMachines/enginesMechanical power devicesStage iibMechanical energy
The present invention relates to a method for regenerating heat energy with the aid of an energy system comprising n number of energy cells (121, . . . ,12n), wherein n is an integer, and n>2. The energy cells (121, . . . ,12n) are connected in sequence. Each energy cell (121, . . . ,12n) comprises a phase change material (PCM1, . . . ,PCMn), wherein PCMT1>PCMT2>, . . . , >PCMTn. The energy cells (121, . . . ,12n) performs the steps:to produce mechanical energy, which also causes rest heat energy (RH1, . . . ,RHn) stored in said energy cell (121, . . . ,12n) when the phase change material (PCM1, . . . ,PCMn) changes from solid phase to liquid phase; orto cool down when the phase change material (PCM1, . . . ,PCMn) changes from liquid phase to solid phase;to transfer said rest heat energy (RHx) from one energy cell (12x) which is cooling down as input energy to the next energy cell (12x+1), said method comprises the step:with the aid of a control means connected to said energy cells (121, . . . ,12n), to control said system alternately between a first phase, and a second phase, wherein, during said first phase, every two energy cells (121, 123, 125, . . . ) produces mechanical energy, and every two energy cells (122, 124, 126, . . . ) are cooling down, and vice versa during said second phase.
Owner:EXENCOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com