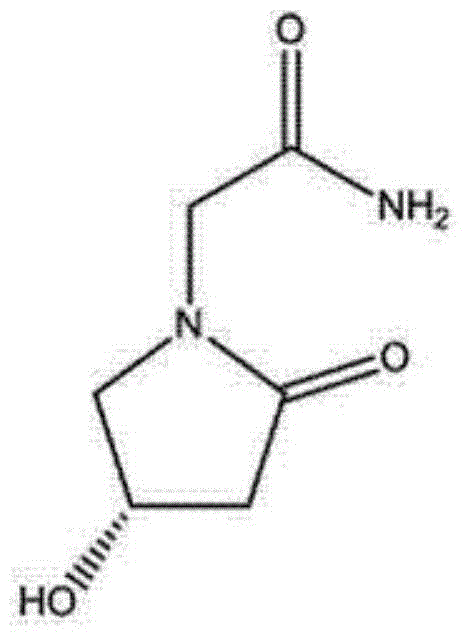
(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide injection and preparation method of (S)-4-hydroxyl-2oxo-1-pyrrolidineacetamide injection
A technology for pyrrolidine acetamide and injections, which can be used in pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as poor product stability, increased impurities, and easy crystallization , to achieve the effect of simple and feasible preparation process, not easy to be oxidized, and less impurities in the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide injection prepared according to the following steps:
[0022] Element Dosage (S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide 100g Propylene Glycol 19g Lecithin 18g Vitamin C 3g Ethylenediaminetetraacetic acid 5g Sterile water for injection Add to 2000ml
[0023] Make 1000 pieces
[0024] Preparation process:
[0025] 1. Concentrated preparation: Add 1 / 3 of the prescription amount of sterile water for injection into the batching tank, add the prescription amount of raw and auxiliary materials, stir, dissolve, and obtain a concentrated liquid preparation;
[0026] 2. Dilute preparation: take the concentrated preparation, add sodium bicarbonate or hydrochloric acid to adjust the pH to 6.0-7.0, add activated carbon with a mass fraction of 0.1%-0.3%, absorb and decolorize, filter through a 0.45 μm filter membrane, and collect the filtrate. Add sterilized water for injection to the ...
Embodiment 2
[0060] A (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide injection prepared according to the following steps:
[0061] Element Dosage (S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide 100g Propylene Glycol 15g Lecithin 17g Vitamin C 2g Ethylenediaminetetraacetic acid 3g Sterile water for injection Add to 2000ml
[0062] Make 1000 pieces
[0063] Preparation process: prepared according to the preparation process of Example 1.
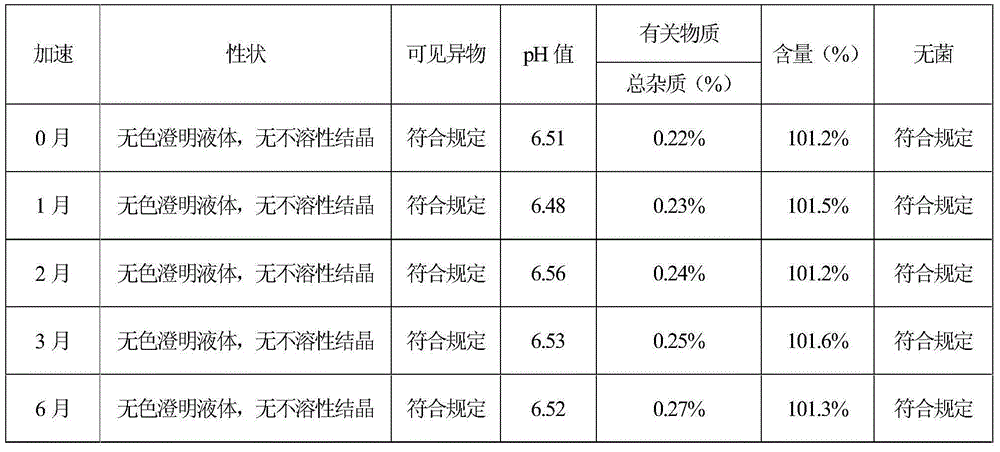
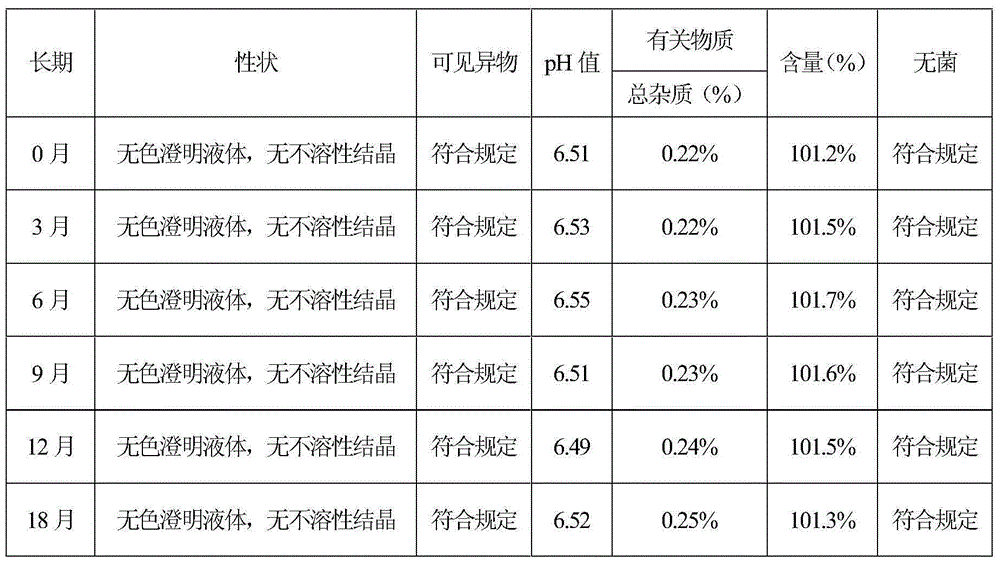
[0064] According to the test method of Example 1, the stability test investigation and the impact test of the sterilization process on the increase of impurities were carried out respectively. The stability test results showed that the sample quality was stable in 6 months, and the quality was stable in 18 months for a long time. Therefore, the validity period of this product is at least 18 months. months. The test results of the effect of sterilization process on the increase of impurities show that the prescri...
Embodiment 3
[0066] A (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide injection prepared according to the following steps:
[0067] Element Dosage (S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide 100g Propylene Glycol 18g Lecithin 20g Vitamin C 2g Ethylenediaminetetraacetic acid 3g Sterile water for injection Add to 2000ml
[0068] Make 1000 pieces
[0069] Preparation process: prepared according to the preparation process of Example 1.
[0070] According to the test method of Example 1, the stability test investigation and the impact test of the sterilization process on the increase of impurities were carried out respectively. The stability test results showed that the sample quality was stable in 6 months, and the quality was stable in 18 months for a long time. Therefore, the validity period of this product is at least 18 months. months. The test results of the effect of sterilization process on the increase of impurities show that the prescri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com