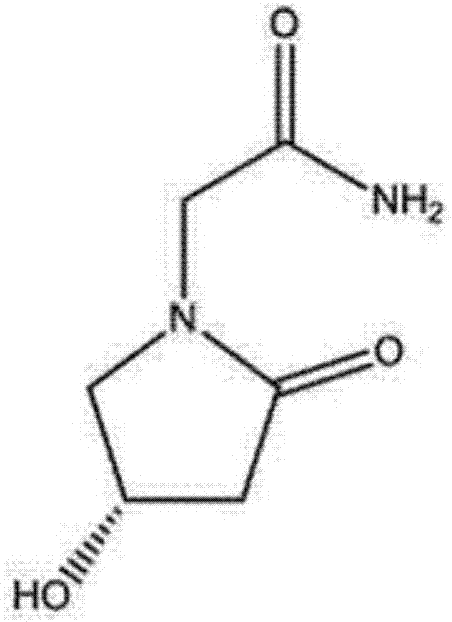
(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide sterile powder for injection
A technology of pyrrolidine acetamide and sterile powder, which is applied in powder delivery, inorganic non-active ingredients, medical preparations of non-active ingredients, etc., and can solve the problems of prolonging the production cycle of preparations, powder scattering of preparations, and low sublimation temperature. Achieve the effects of reducing adverse drug reactions, improving safety, and increasing sublimation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
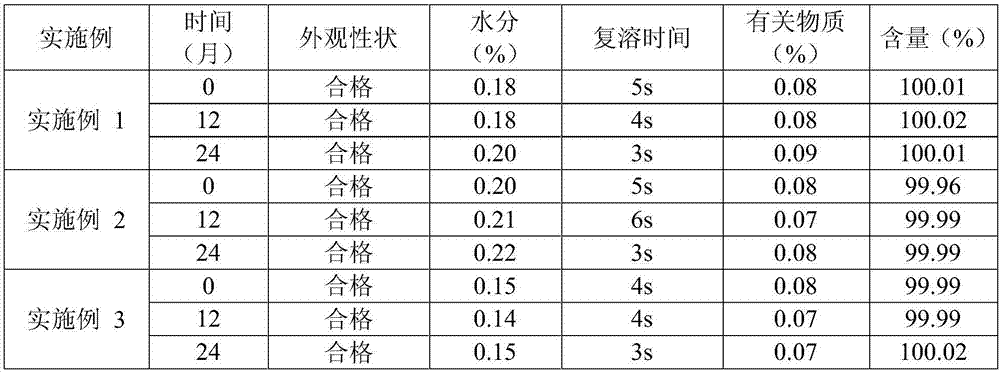
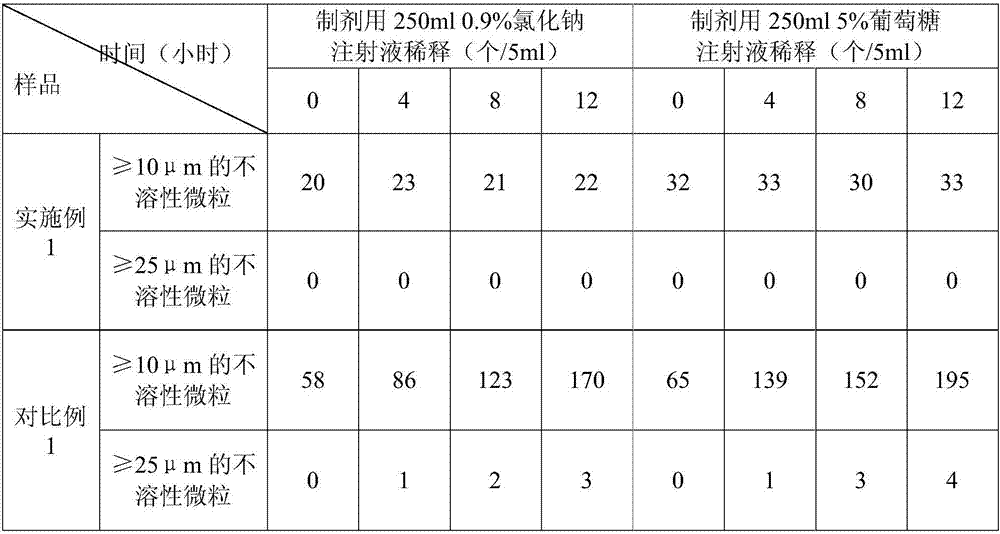
[0017] The prescription of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide sterile powder for injection of embodiment 1 is shown in the table below:
[0018] prescription
weight percentage
(S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide
50g
25g
35g
15g
7.5g
Water for Injection
Add to 250mL
[0019] The preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide sterile powder for injection of embodiment 1 comprises the following steps:
[0020] (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, methionine, lactose, triethanolamine and sodium bisulfite were dissolved in water for injection, and 0.1mol / L hydrochloric acid The solution was adjusted to pH 3.5, then freeze-dried as follows:
[0021] (1) Pre-freezing stage: freeze the temperature to -15°C within 10 minutes; then raise the temperature to -10°C, and the heating time is 40 minutes; freeze the temper...
Embodiment 2
[0025] The prescription of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide sterile powder for injection of embodiment 2 is shown in the table below:
[0026] prescription
weight percentage
(S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide
50g
20g
lactose
25g
10g
5g
Water for Injection
Add to 250mL
[0027] The preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide sterile powder for injection of embodiment 2 comprises the following steps:
[0028] (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, methionine, lactose, triethanolamine and sodium bisulfite were dissolved in water for injection, and 0.1mol / L hydrochloric acid The solution was adjusted to pH 3.3 and then freeze-dried as follows:
[0029] (1) Pre-freezing stage: Freeze the temperature to -15°C within 5 minutes; then raise the temperature to -9°C for 30 minutes; freeze the temperature to -32°C within ...
Embodiment 3
[0033] The prescription of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide sterile powder for injection of embodiment 3 is shown in the table below:
[0034] prescription
weight percentage
(S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide
50g
30g
lactose
45g
20g
sodium bisulfite
10g
Water for Injection
Add to 250mL
[0035] The preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide sterile powder for injection of embodiment 3 comprises the following steps:
[0036] (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, methionine, lactose, triethanolamine and sodium bisulfite were dissolved in water for injection, and 0.1mol / L hydrochloric acid The solution was adjusted to pH 3.5, then freeze-dried as follows:
[0037] (1) Pre-freezing stage: freeze the temperature to -18°C within 15 minutes; then raise the temperature to -8°C, and the heating time is 60 minutes; freeze the temperat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com