(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide granule having good stability, and preparation method thereof
A pyrrolidine acetamide and stability technology, which is applied in the field of -4-hydroxy-2 oxo-1-pyrrolidine acetamide particles and their preparation, can solve the problem that the particle size is not easy to control, the particles have strong hygroscopicity and easy adhesion. In order to achieve the effect of simple and feasible preparation process, uniform particle size, and not easy to absorb moisture and agglomerate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
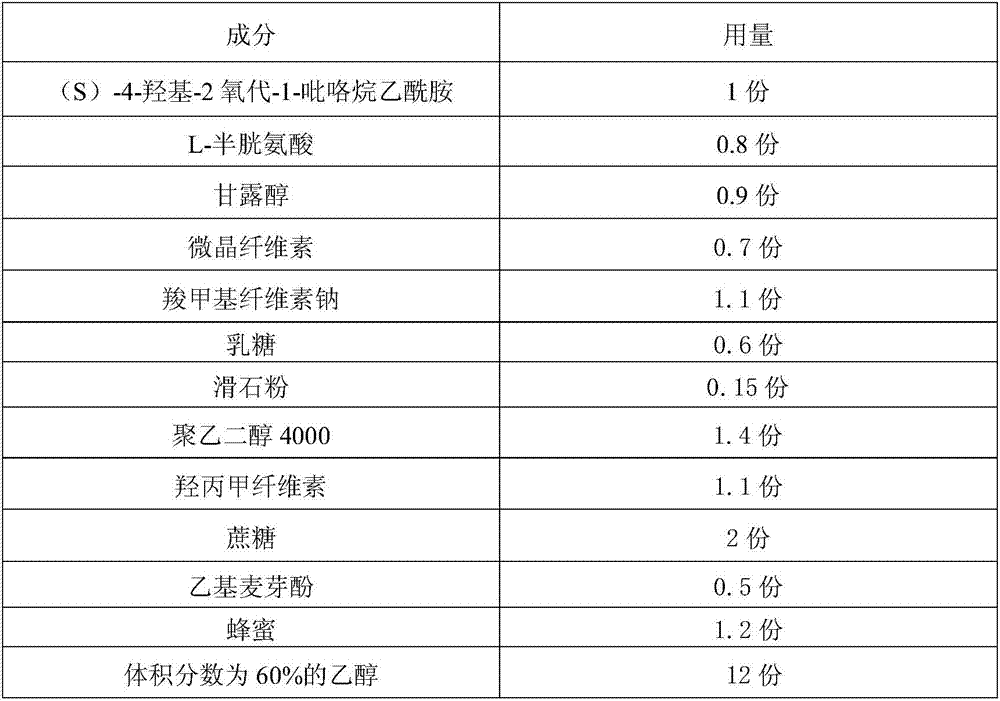
[0025] A stable (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide granule is prepared according to the following steps:
[0026]
[0027]
[0028] Preparation process:
[0029] 1. Preparation of adhesive: Take the prescribed amount of honey, put it in an iron pot, add purified water with 2 times the weight of honey, stir evenly, heat to 100-105°C, keep warm for 20-25 minutes, take it out, and use 80 Filter through a mesh sieve, take the filtrate, let it cool, add ethanol in the prescribed amount, stir to dissolve, and set aside;
[0030] 2. Pretreatment of raw and auxiliary materials: take the prescribed amount of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, L-cysteine, mannitol, microcrystalline cellulose, carboxymethyl cellulose Sodium, lactose, sucrose, and ethyl maltol are placed in a universal grinder, crushed through a 100-mesh sieve, and set aside;
[0031] 3. Granulation: Take the mixed powder obtained after pretreatment, put it in a wet granulator, add the previously...
Embodiment 2
[0087] A stable (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide granule is prepared according to the following steps:
[0088]
[0089]
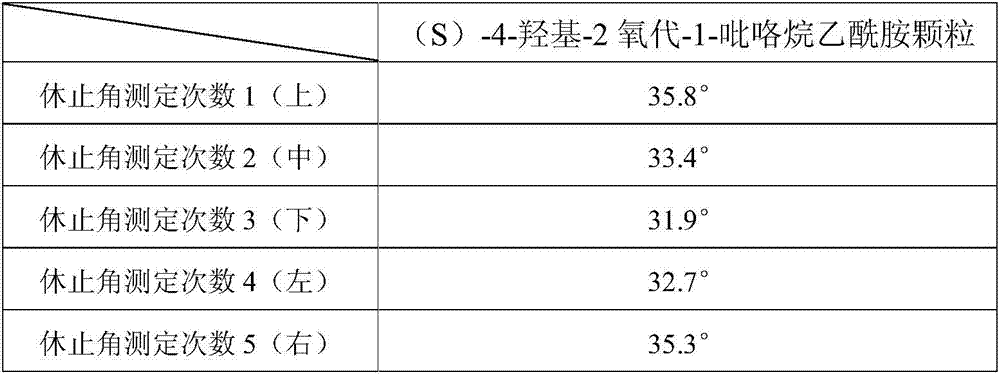
[0090]Preparation process: prepared according to the preparation process of Example 1. Observing the granulation process of the product did not find the phenomenon of sticking to the screen, and the product was easy to granulate. Carry out the test by the test method of embodiment 1, the Hughes angle test measurement result shows that this product granule fluidity is good, and the Hughes angle is lower than 37 °, and the loading difference test shows that the loading difference of this product is less than ± 5%. The amount is stable and controllable, and the test results of the influence of product prescription on the increase of impurities in the preparation process show that the increase of impurities in the preparation process of this product is small, and the related substances in the preparation process only increase by 0.03%. The qu...
Embodiment 3
[0092] A kind of (S)-4-hydroxyl-2 oxo-1-pyrrolidineacetamide granules, prepared according to the following steps:
[0093]
[0094] Preparation process: prepared according to the preparation process of Example 1. Observing the granulation process of the product did not find the phenomenon of sticking to the screen, and the product was easy to granulate. Carry out the test by the test method of embodiment 1, the Hughes angle test measurement result shows that this product granule fluidity is good, and the Hughes angle is lower than 35 °, and the loading difference test shows that the loading difference of this product is less than ± 4%. The amount is stable and controllable, and the test results of the influence of product prescription on the increase of impurities in the preparation process show that the increase of impurities in the preparation process of this product is small, and the related substances in the preparation process only increase by 0.03%. The quality is st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com