Preparation method of (R,S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide
A technology of pyrrolidine acetamide and oxo substitution, which is applied in the direction of organic chemistry, can solve the problems of high cost, large amount of solvent, and difficult recovery, etc., and achieve the effects of improved service life, short reaction cycle, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A kind of preparation method of (R, S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, its concrete steps are:
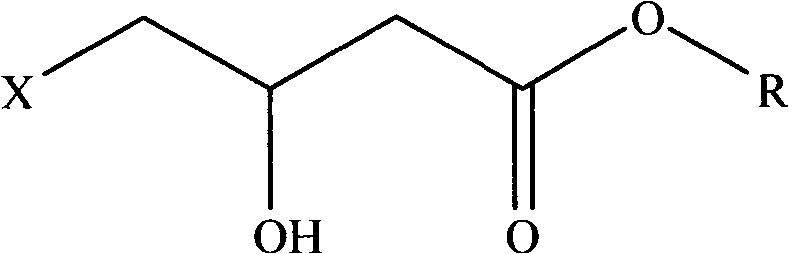
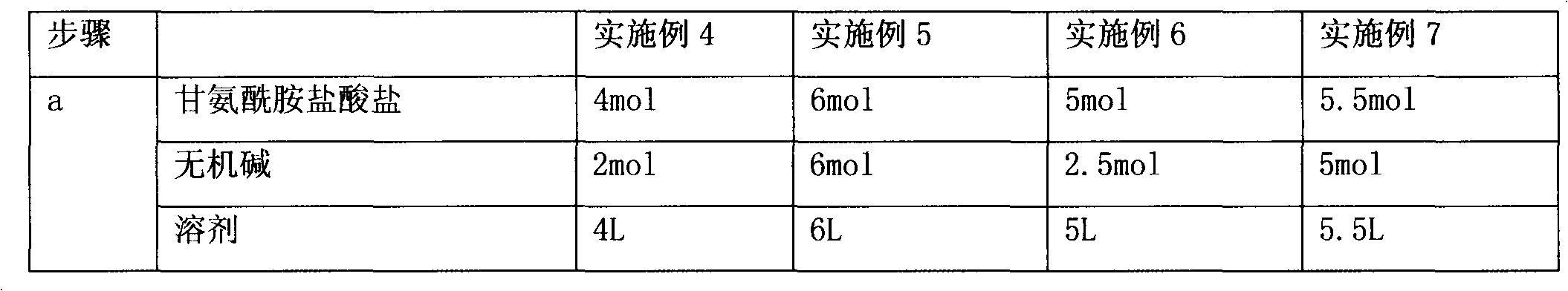
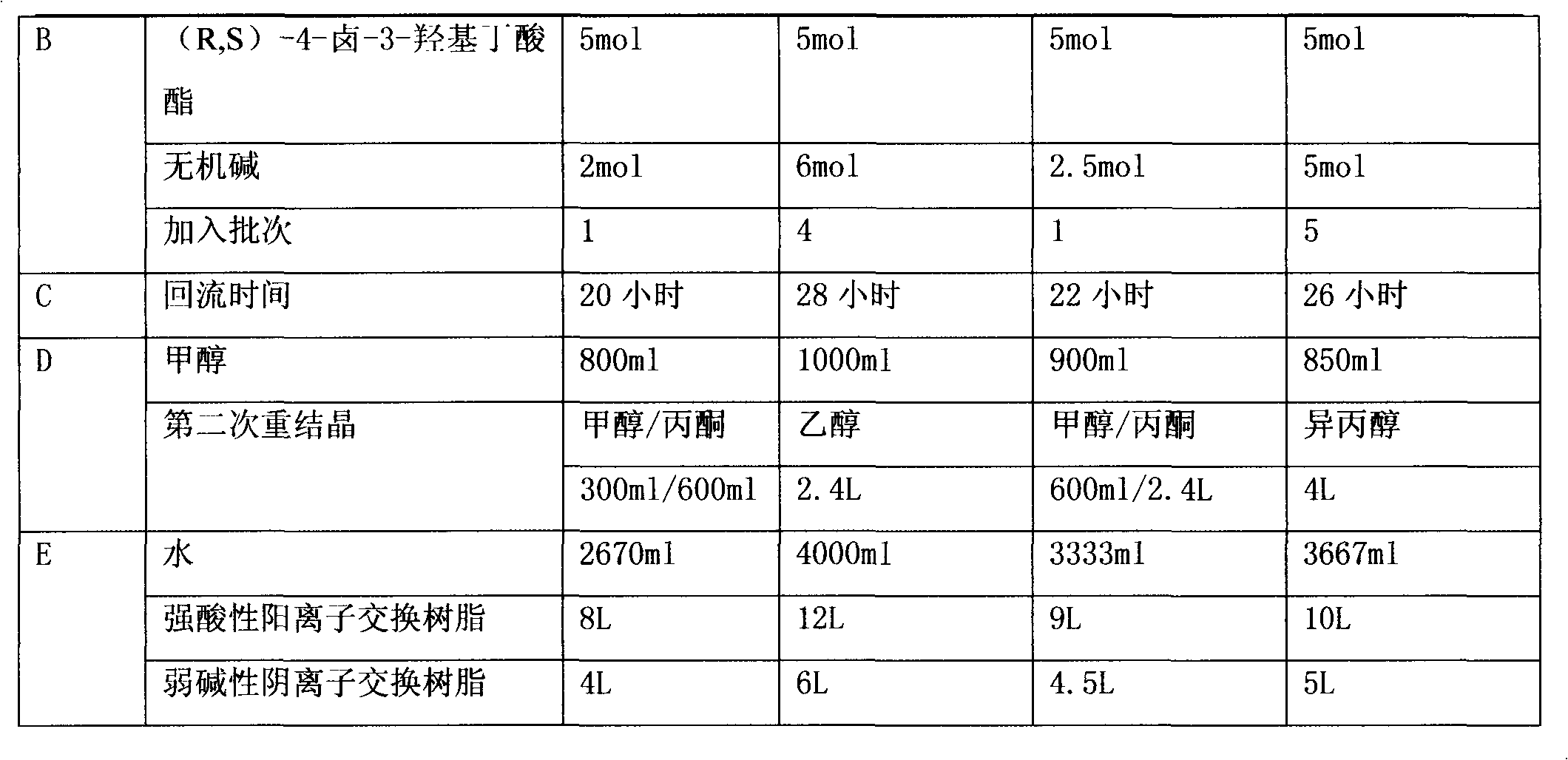
[0056] (a) 55.0g of glycinamide hydrochloride, 29.2g of sodium carbonate, 99.6g of (R, S)-4-chloro-3-hydroxybutyric acid ethyl ester, and 500ml of absolute ethanol are dropped into the reaction flask During the process, the reaction was heated to reflux for 23 hours, and then the reaction vessel was sealed to raise the internal temperature to 90° C. and kept for 1 hour to terminate the reaction. After cooling to 60°C, filter with suction, concentrate the mother liquor to nearly dryness, add 40 ml of methanol for beating and filter, then use 90 ml of ethanol for beating at 60°C for 1 hour, cool to room temperature, and filter to obtain (R,S)-4-hydroxy-2 - crude product 37g of oxo-1-pyrrolidineacetamide;
[0057] (d) The above crude product was dissolved in 370ml of water, treated with 120g of Pleinlight 732 strong acidic styrene-based cation exchange resin, and the ...
Embodiment 2
[0060] A kind of preparation method of (R, S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, its concrete steps are:
[0061] (a) 55.0g of glycinamide hydrochloride, 29.2g of sodium carbonate, 99.6g of (R, S)-4-chloro-3-hydroxybutyric acid ethyl ester, and 500ml of absolute ethanol are dropped into the reaction flask During the process, heat to reflux for 24 hours, then cool the inner temperature of the reaction vessel to 60°C and filter with suction, concentrate the mother liquor to nearly dryness, add 40 ml of methanol for beating and filtering, and then use 90 ml of ethanol to beat the crude product at 60°C 1h, after cooling to room temperature, filtered to obtain 32g of crude product of (R,S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide;
[0062] (d) The above crude product was dissolved in 370ml of water, treated with 120g of Pleinlight 732 strong acidic styrene-based cation exchange resin, and the product part was collected. Neutralize and collect the aqueous solution obtained with...
Embodiment 3
[0065] A kind of preparation method of (R, S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, its concrete steps are:
[0066] (a) 55.0g of glycinamide hydrochloride, 29.2g of sodium carbonate, 99.6g of (R, S)-4-chloro-3-hydroxybutyric acid ethyl ester, and 500ml of absolute ethanol are dropped into the reaction flask During the process, the reaction was heated to reflux for 28 hours, and then the reaction vessel was sealed to raise the internal temperature to 90° C. and kept for 1 hour to terminate the reaction. After cooling to 60°C, filter with suction, concentrate the mother liquor to nearly dryness, add 40 ml of methanol for beating and filter, then use 90 ml of ethanol for beating at 60°C for 1 hour, cool to room temperature, and filter to obtain (R,S)-4-hydroxy-2 - crude product 32g of oxo-1-pyrrolidineacetamide;
[0067] (d) The above crude product was dissolved in 370ml of water, treated with 120g of Pleinlight 732 strong acidic styrene-based cation exchange resin, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com