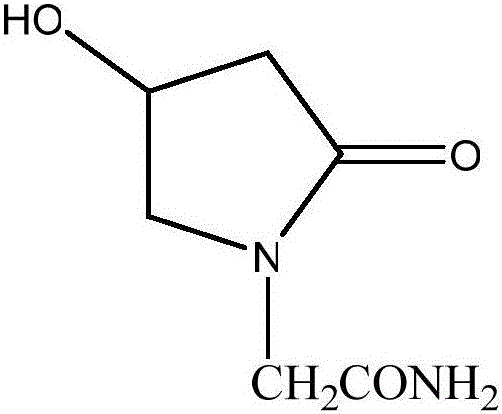
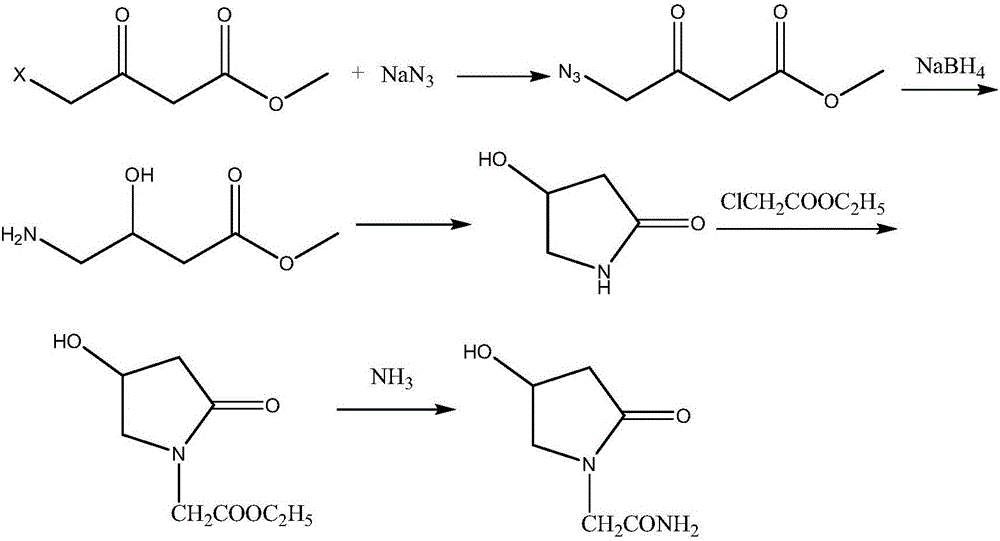
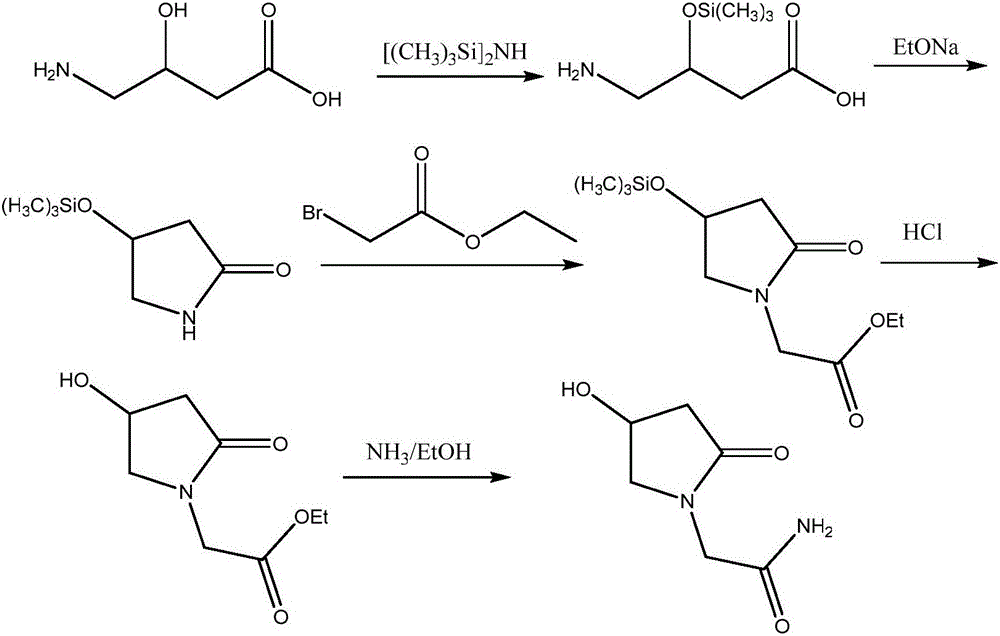
Oxiracetam synthesis technology
A synthesis process and technology of the process route, applied in the field of medicine and chemical industry, can solve the problems of adding protection and deprotection steps, expensive amino protecting agents, difficult starting materials, etc., and achieves low cost, good industrial value, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of preparation of 4-hydroxyl-2-oxo-1-pyrrolidine acetamide
[0031] 1) Preparation of ethyl 4-bromoacetoacetate: Control the temperature at -5~5°C, mix 26.03g of ethyl acetoacetate and 15mL of dichloromethane evenly, add 35.16g of bromine drop by drop, keep the reaction for 2h, pass into Dry nitrogen for 1 h, concentrate under reduced pressure to constant weight, and collect fractions at 85°C by oil pump vacuum distillation to obtain 38.69 g of transparent oily liquid ethyl 4-bromoacetoacetate, with a yield of 92.54%;
[0032] 2) Preparation of glycine methyl ester hydrochloride: Control the temperature at -5-5°C, add 23.56g of acetyl chloride dropwise to 60ml of anhydrous methanol, keep it warm for 1 hour after the drop, add 7.51g of glycine in 2 batches, After the addition, the system was heated to 70°C for 5 hours, concentrated under reduced pressure to remove excess solvent, and the residue was added with 30 ml of acetone and stirred for 5 hours, filtered, an...
Embodiment 2
[0036] A kind of preparation of 4-hydroxyl-2-oxo-1-pyrrolidine acetamide
[0037] 1) Preparation of ethyl 4-bromoacetoacetate: Control the temperature at -5~5°C, mix 30.03g of ethyl acetoacetate and 15mL of dichloromethane evenly, add 44.24g of bromine drop by drop, keep the reaction for 2h, pass into Dry nitrogen for 1 h, concentrate under reduced pressure to constant weight, and collect fractions at 85°C by oil pump vacuum distillation to obtain 38.69 g of transparent oily liquid ethyl 4-bromoacetoacetate, with a yield of 92.66%.
[0038] 2) Preparation of glycine ethyl ester: Control the temperature at -5~5°C, add 23.56g of acetyl chloride dropwise to 70ml of absolute ethanol, keep the reaction for 1h after dropping, add 7.51g of glycine in 2 batches, and complete the system Raise the temperature to 70°C for 5 hours, concentrate under reduced pressure to remove excess solvent, add 40ml of acetone to the residue and stir for 5h, filter, and vacuum-dry the filter cake to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com