Patents
Literature
82 results about "Glycine methyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
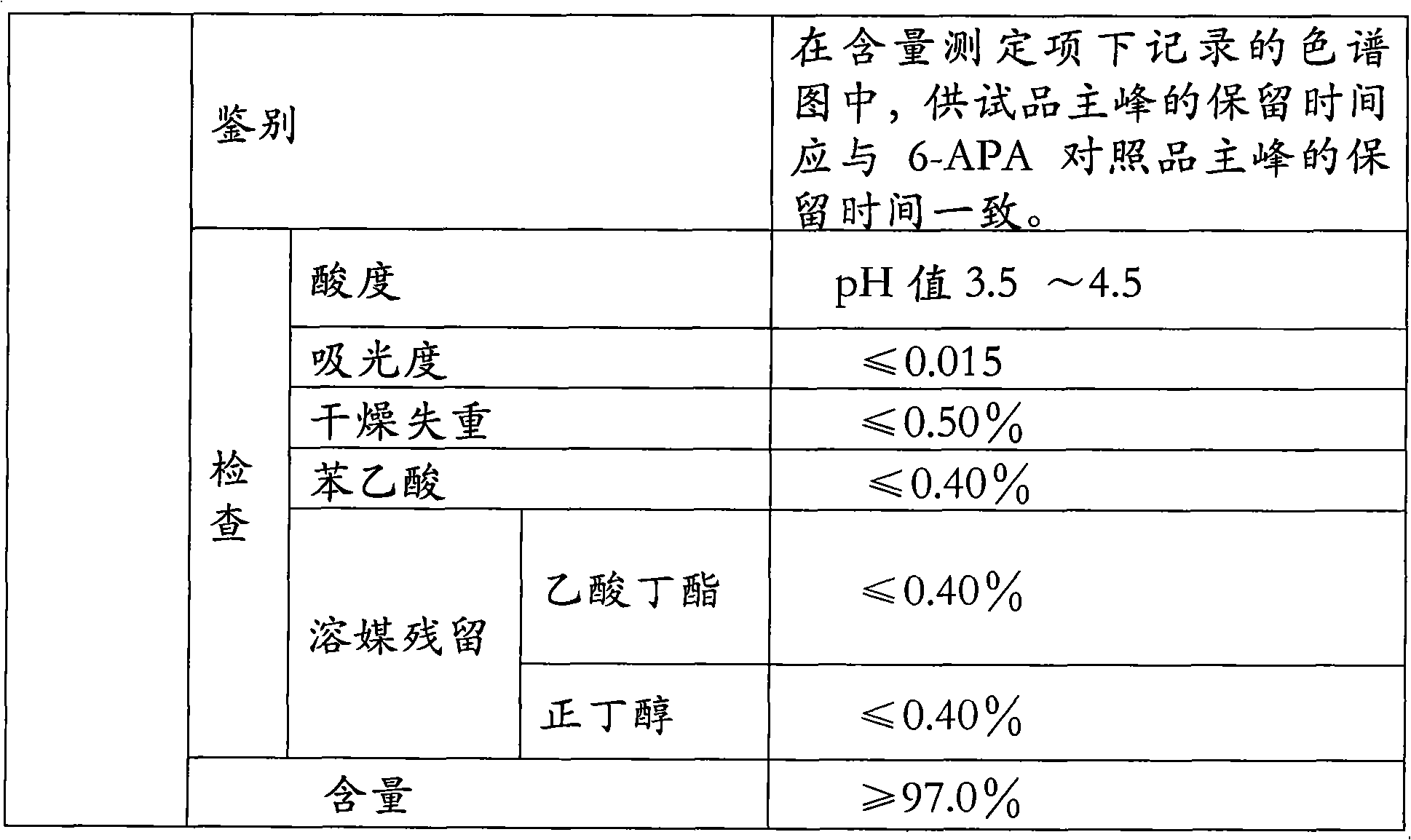
Improved method for preparing amoxicillin by enzymic method
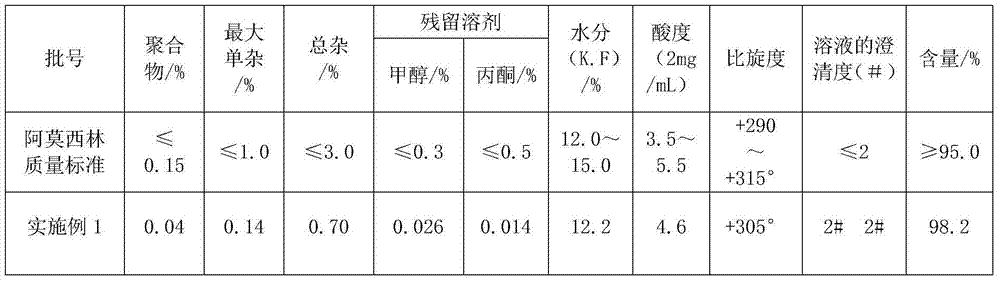
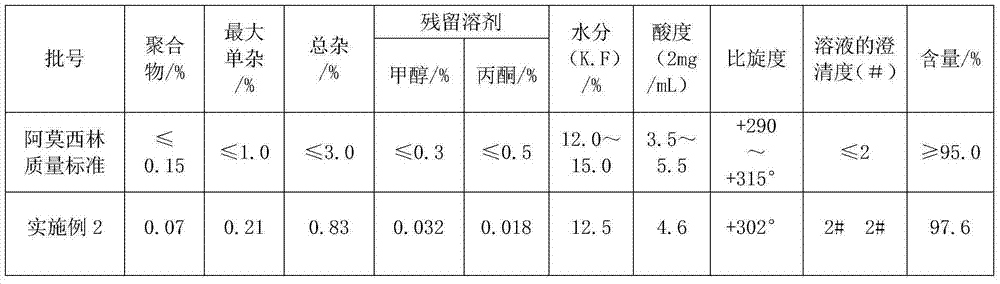
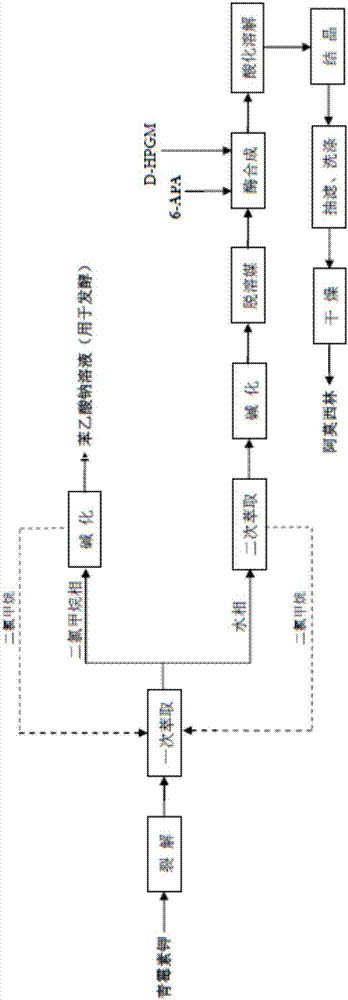
The invention relates to the field of pharmacy, and provides an improved method for preparing amoxicillin by an enzymic method, and a product obtained by the improved method for preparing amoxicillin by the enzymic method. The method comprises the following steps of: 1) dissolving 6-aminopenicillanic acid (6-APA) at the temperature of between 10 and 20 DEG C by using water or / and aqueous solution of ammonia which has the pH value of 7.0 to 8.0, and adding D-p-Hydroxyphenylglycine methyl ester hydrochlorid and penicillin G acyltransferase; 2) adjusting the pH value of a solution obtained in the step 1) to be 6.0 to 6.5, and reacting at the temperature of between 21 and 30 DEG C until the content of 6-APA is less than 5mg / ml to obtain a solution of an amoxicillin product; and 3) separating the penicillin G acyltransferase from the solution of the amoxicillin product, adjusting by using hydrochloric acid until the solution of the amoxicillin product is clarified, adding the aqueous solution of ammonia, adjusting the pH value to be 5.5 to 6.5, and crystallizing at the temperature of between 0 and 5 DEG C to obtain amoxicillin. By the improved method for preparing amoxicillin by the enzymic method, the quality of the amoxicillin product is greatly improved, and the medication safety of the amoxicillin product is further improved.
Owner:UNITED LAB INNER MONGOLIA CO LTD
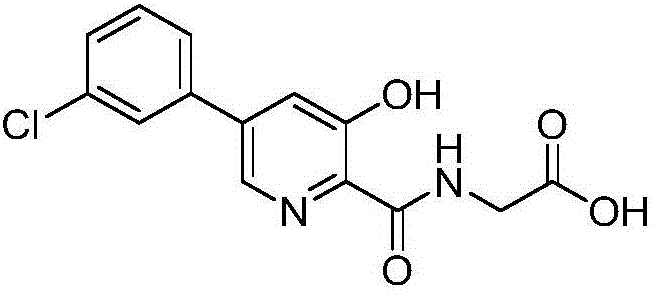
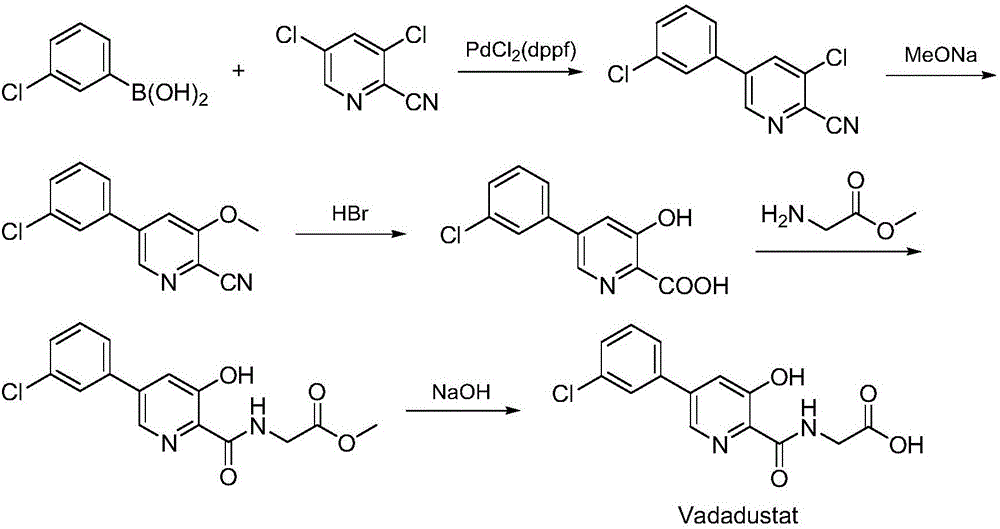
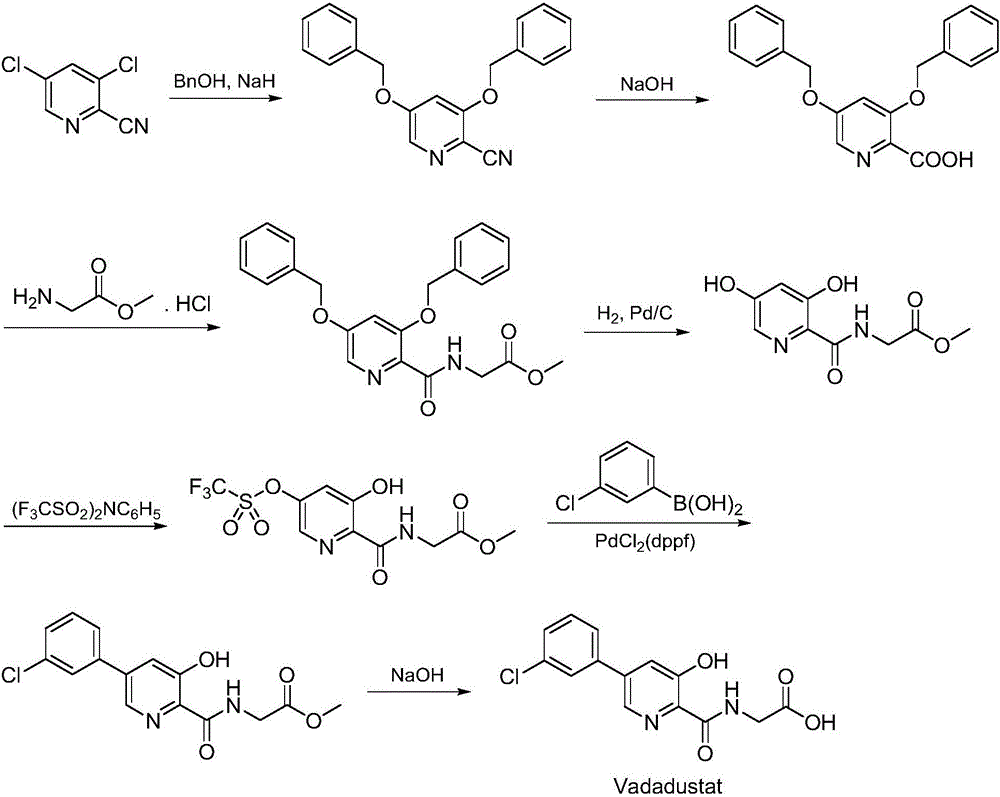
Synthesis method of Vadadustat
InactiveCN105837502AMeet the needs of useLess impuritiesOrganic chemistrySodium methoxideGlycine methyl ester hydrochloride
The invention discloses a synthesis method of Vadadustat. The method 3,5-dichloro-2-picolinic acid and glycine methyl ester hydrochloride carry out condensation reaction; the obtained N-(3,5-dichloropyridine-2-carbonyl) glycine methyl ester and 3-chlorobenzene Boronic acid is carried out catalytic coupling reaction; The N-[5-(3-chlorophenyl)-3-chloropyridine-2-carbonyl] glycine methyl ester obtained and sodium methylate are carried out methoxy substitution reaction; The obtained N- [5-(3-chlorophenyl)-3-methoxypyridine-2-carbonyl]glycine is hydrolyzed to obtain finished product Vadadustat. The synthesis method has short route steps, simplified operation and low cost, is a green and environment-friendly method, and is suitable for industrial production.
Owner:湖南欧亚药业有限公司
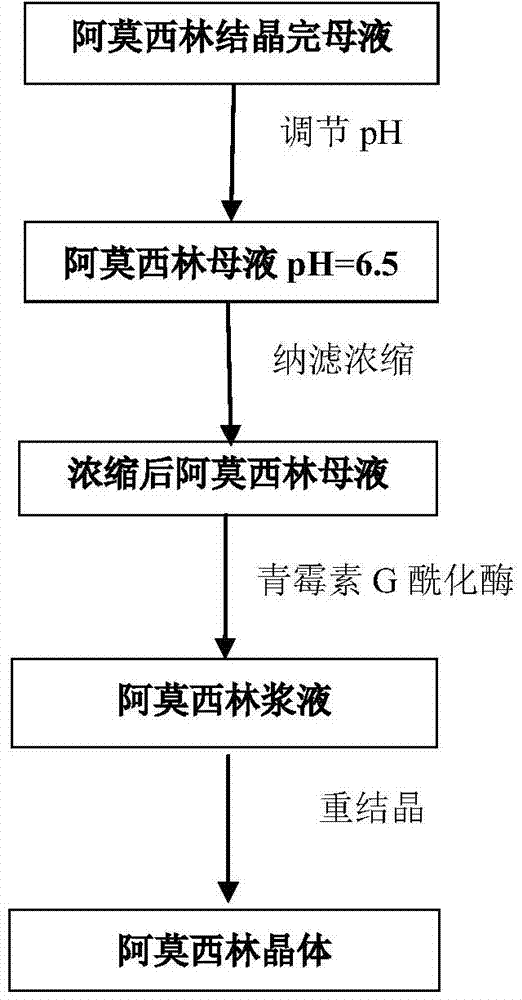
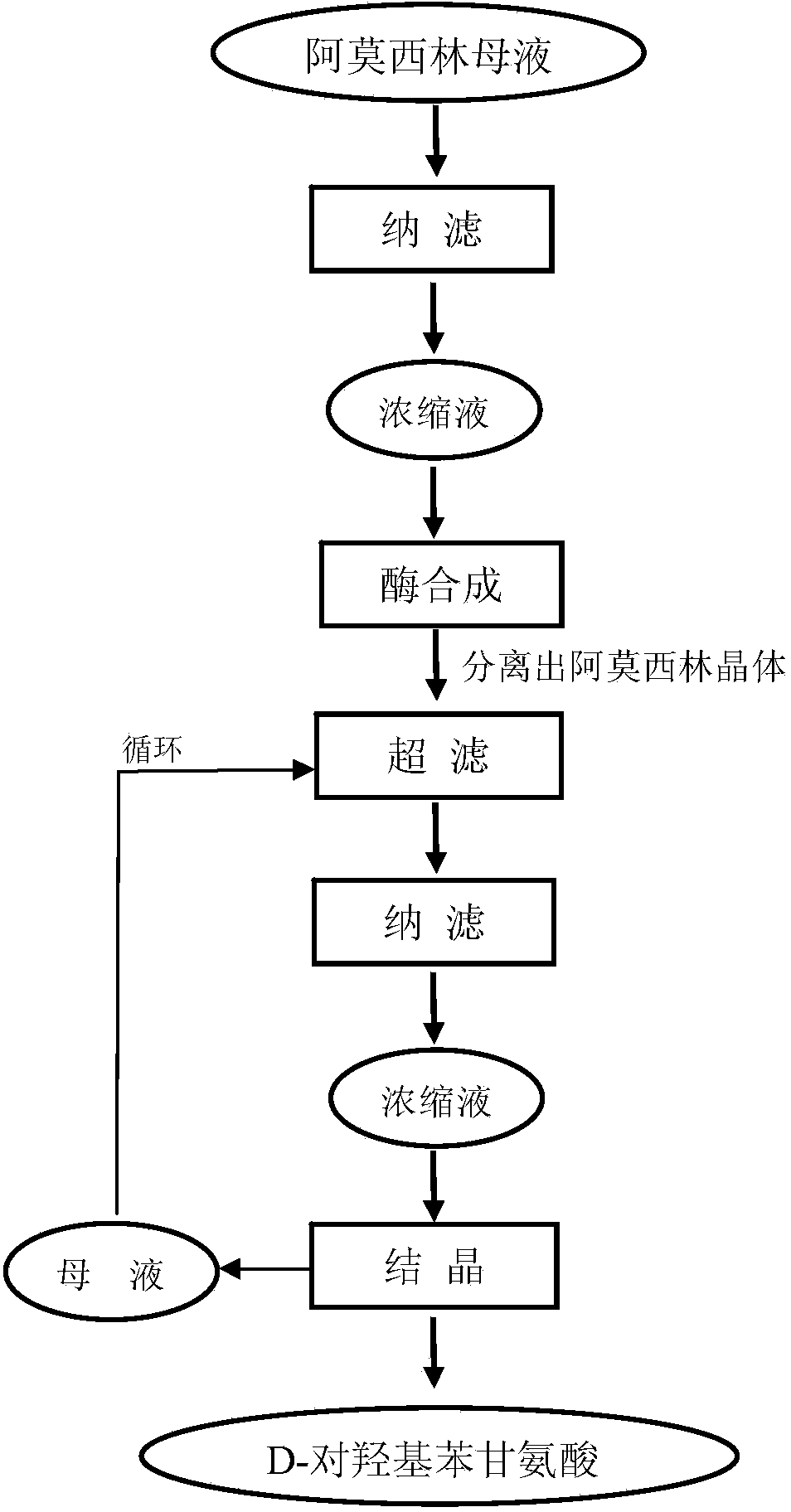
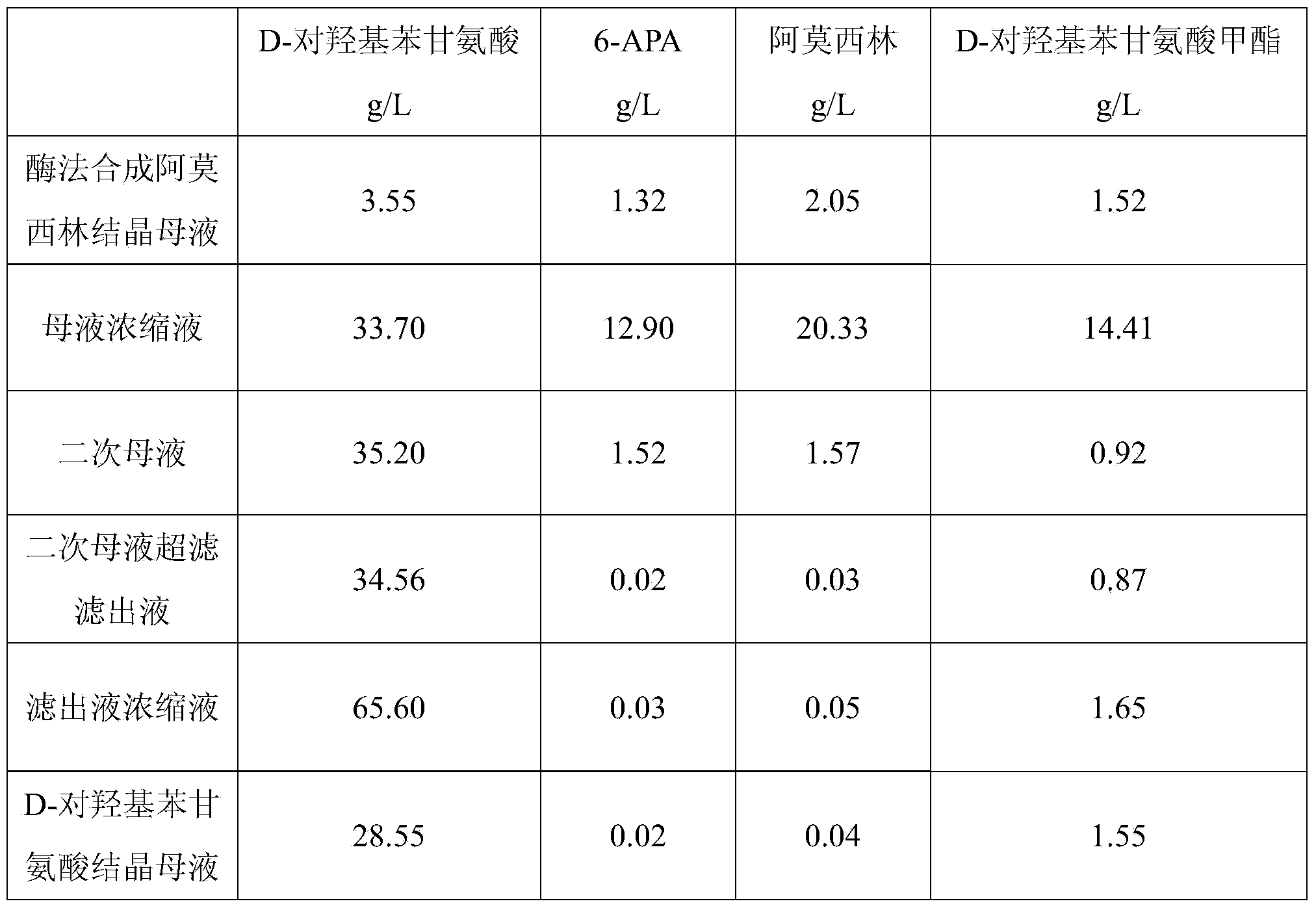
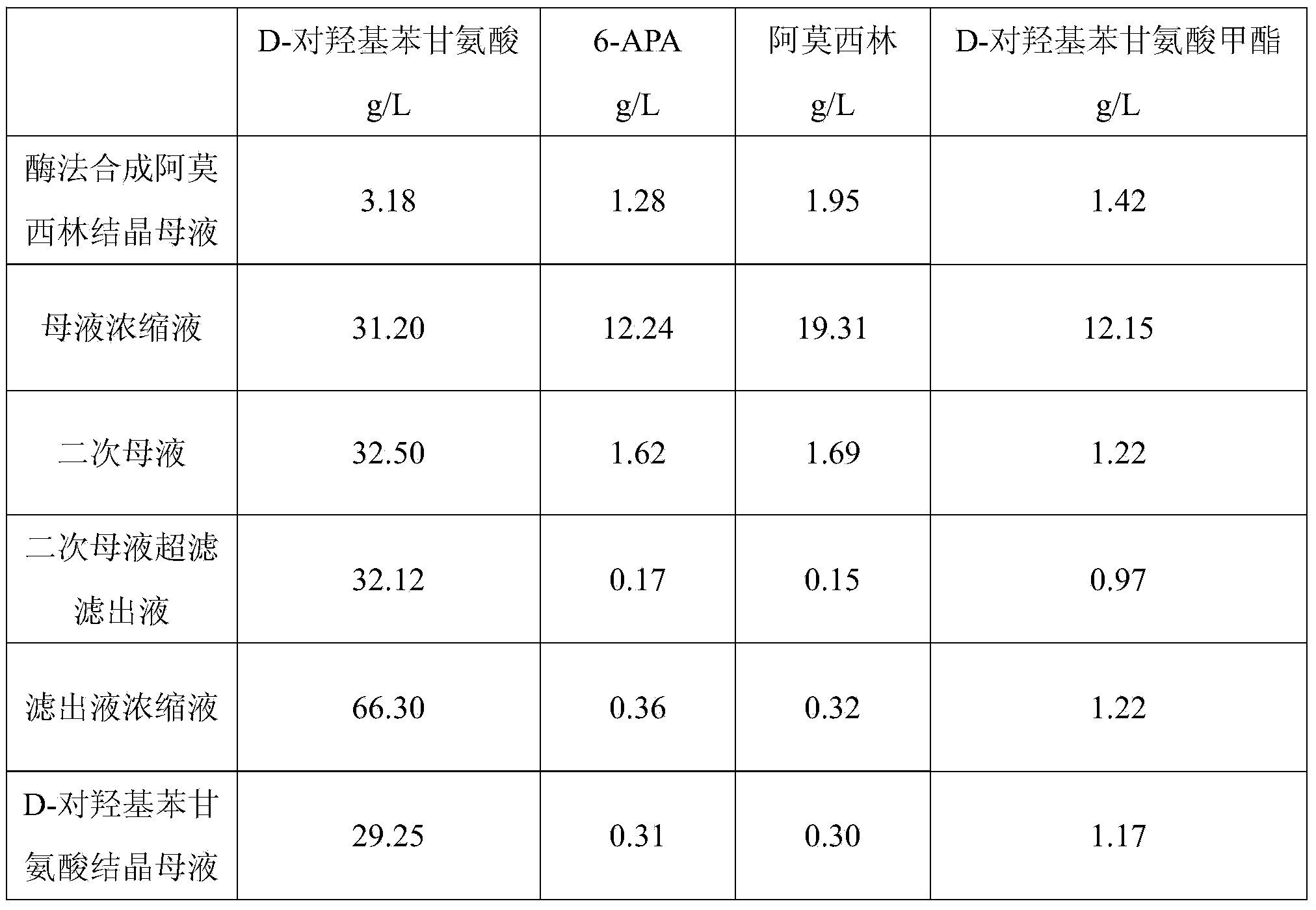
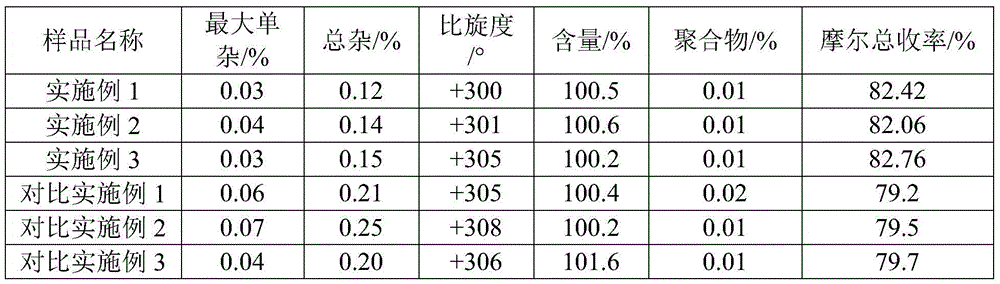
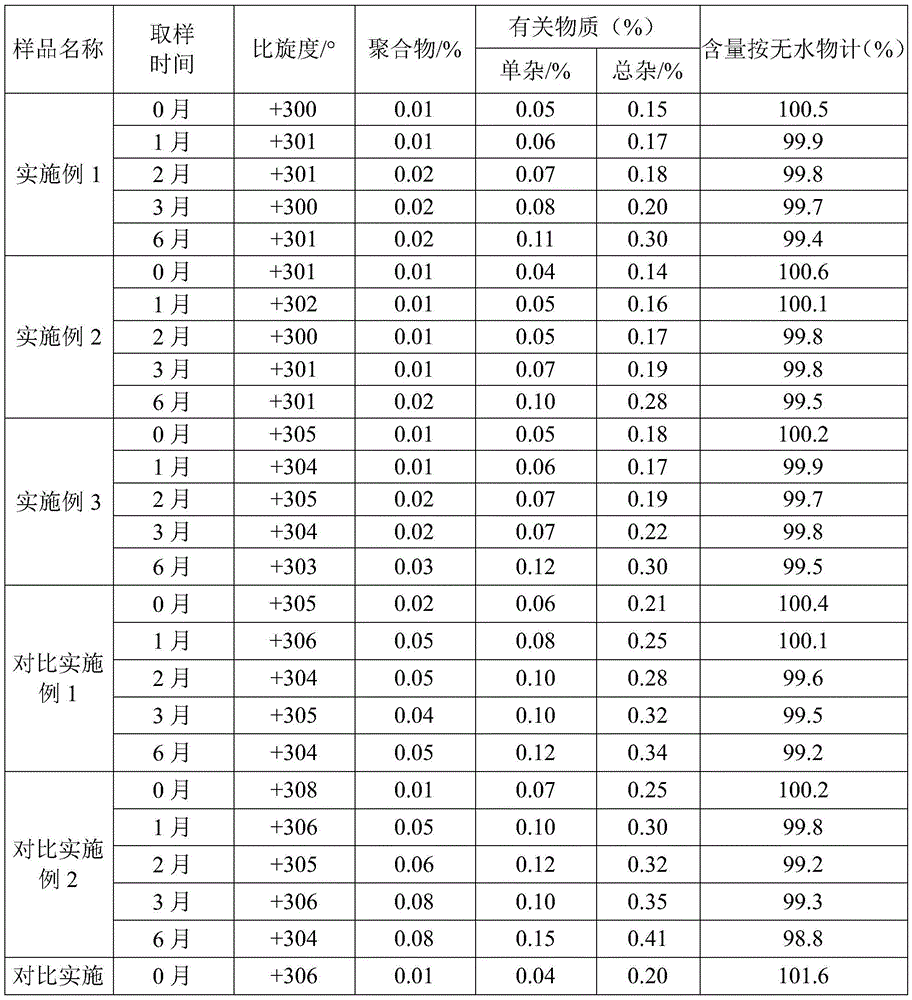
Method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by enzyme process
ActiveCN104357528AImprove product qualityCreate economic growthOrganic compound preparationAmino-carboxyl compound preparationUltrafiltrationChemistry
The invention relates to a method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by an enzyme process. The method comprises the following steps: (1) concentrating the amoxicillin mother liquid, namely adjusting the pH value of the amoxicillin mother liquid prepared by the enzyme process to be 8.0-9.5, and performing nanofiltration and concentration to obtain concentrated mother liquid; (2) synthesizing amoxicillin under enzyme catalysis, namely adjusting the pH value of the concentrated mother liquid to be 5.8-7.0, and converting 6-APA (6-amino penicillanic acid) and D-methyl hydroxyphenyl glycinate into amoxicillin in the presence of immobilized penicillin acylase for synthesis; (3) preparing D-hydroxyphenyl glycine concentrated liquid by ultrafiltration and nanofiltration, namely separating after the enzyme catalysis reaction is ended to obtain amoxicillin crystals and secondary amoxicillin mother liquid, and performing ultrafiltration and nanofiltration on the secondary mother liquid to obtain the D-hydroxyphenyl glycine concentrated liquid; (4) crystallizing D-hydroxyphenyl glycine. According to the method, 6-APA and D-methyl hydroxyphenyl glycinate remained in the mother liquid are consumed through an indirect process of synthesizing amoxicillin under enzyme catalysis, the product quality of D-hydroxyphenyl glycine is improved, and the yield of D-hydroxyphenyl glycine is increased.
Owner:SHANXI WEIQIDA PHARMA IND
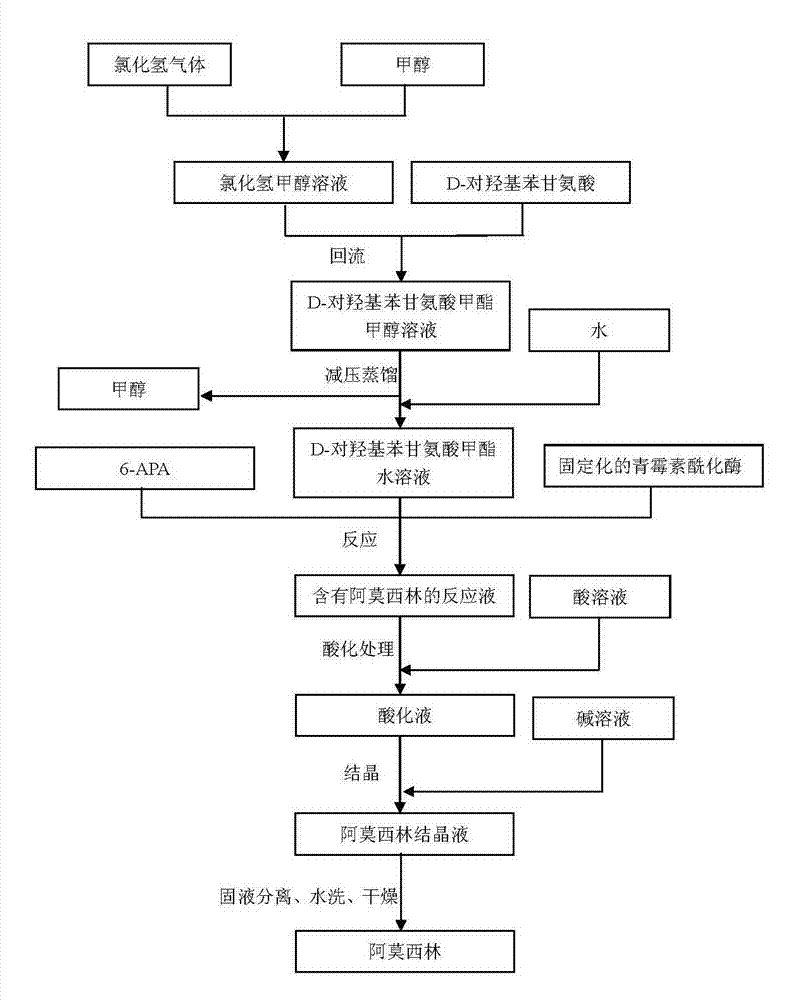
Preparation method of D-para hydroxybenzene glycine methyl ester
InactiveCN103113250AHigh yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsDistillation
The invention provides a preparation method of D-para hydroxybenzene glycine methyl ester. The method comprises the following steps of: firstly, preparing a hydrochloric acid methanol solution; adding D-para hydroxybenzene glycine into the hydrochloric acid methanol solution to perform reflux reaction for 2-4 hours at 65-80 DEG C; performing pressure reduction distillation and removing methanol; and adding water to obtain a D-para hydroxybenzene glycine methyl ester aqueous solution. Based on that, the invention also provides anenzymatic synthesis method of amoxicillin. The method comprises the following steps of: adding 6-APA and immobilized penicillin acylase into the D-para hydroxybenzene glycine methyl ester aqueous solution to react for 1-8 hours at 10-30 DEG C; regulating the pH value of a reaction liquid to 0.8-1.0 by using hydrochloric acid or sulfuric acid aqueous solution; regulating the pH value to 4.5-6.0 by using ammonia water or sodium hydroxide aqueous solution to crystallize for 1-5 hours at 0-30 DEG C; separating solid from liquid; collecting a solid; and washing and drying to obtain amoxicillin. The method is simple in steps, and low in cost; and the obtained -para hydroxybenzene glycine methyl ester is high in yield and less in impurities and can be directly applied to anenzymatic synthesis of amoxicillin.
Owner:NORTH CHINA PHARM GRP SEMISYNTECH CO LTD
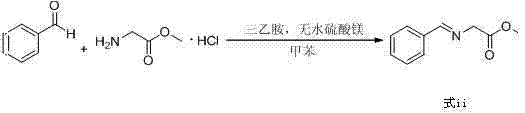
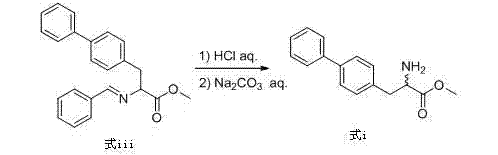
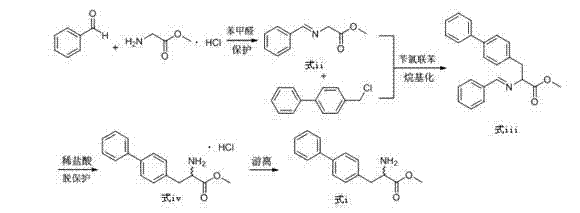
Preparation method of biphenyl alanine derivative
ActiveCN104725256ARaw materials are easy to getLow equipment requirementsOrganic compound preparationAmino-carboxyl compound preparationBenzaldehydeBenzyl chloride
The invention relates to a preparation method of a compound represented by the formula (i); and the compound represented by the formula (i) is prepared by taking glycine methyl ester or hydrochloride thereof as the starting material through benzaldehyde protection, benzyl chloride biphenyl alkylation and deprotection. According to the invention, the starting material is easy to obtain and low in cost; furthermore, an intermediate related in the reaction process is unnecessary to separate and purify; the preparation method is simple to operate and moderate in reaction condition; and therefore, the preparation method is applied to industrialized mass production.
Owner:迪嘉药业集团股份有限公司
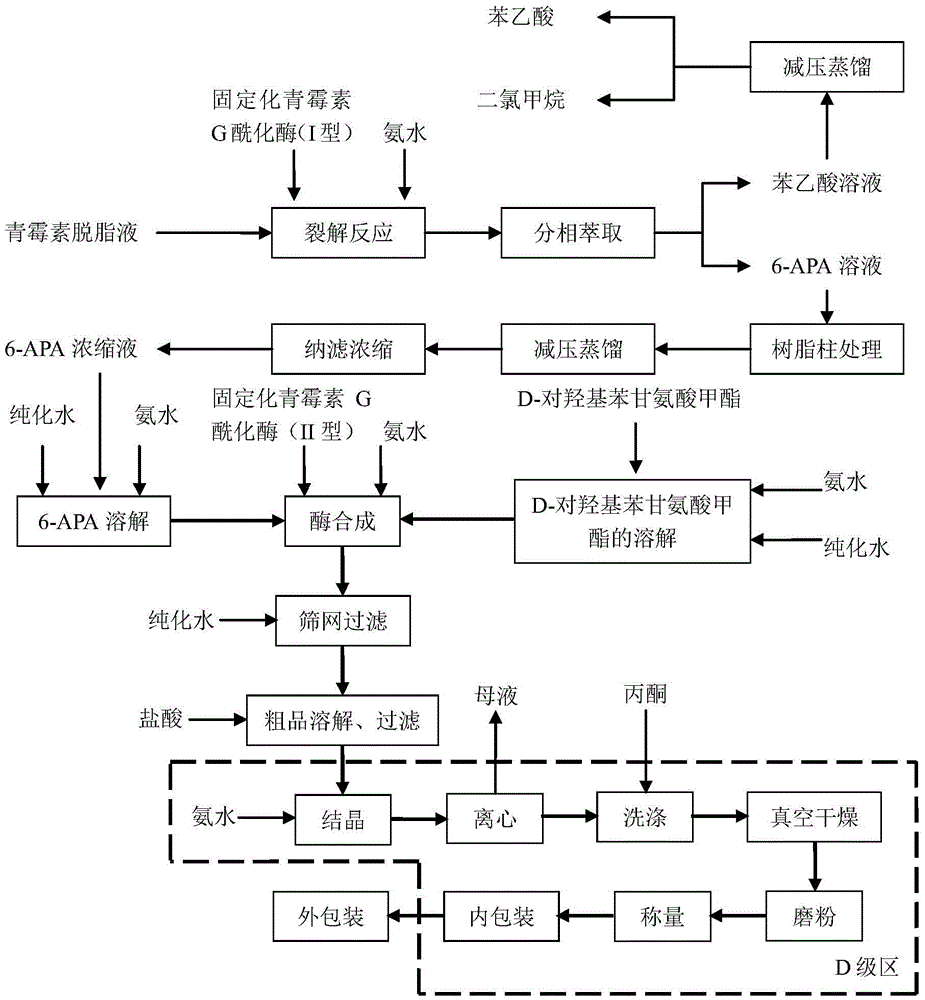
Process for direct preparation of amoxicillin by liquid 6-APA (amino penicillanic acid)
The invention belongs to the technical field of medicine preparation and relates to a process for direct preparation of amoxicillin by liquid 6-APA (amino penicillanic acid). The process includes: taking penicillin degreasing solution as an initial material, sequentially performing cracking reaction, extraction, phase splitting, resin column adsorptive purification, distillation and concentration to obtain a 6-APA solution with the concentration being 80-100g / L, and synthesizing with p-hydroxyphenylglycine methyl ester under a catalytic action of type-II penicillin G acylase to obtain the amoxicillin. Compared with a traditional method, the process has the advantages that subsequent steps of 6-APA crystallization, centrifuging, drying and the like are avoided, investment of fixed assets is reduced, energy loss, equipment loss and cost are reduced, profits are increased, and physical injuries of staffs are reduced. Compared with existing direct amoxicillin preparation methods, the process has the advantages that by adoption of dichloromethane as an extracting agent, total mole yield of amoxicillin is higher relatively, the extracting agent is easy for distillation separation, the content of residual solvents in products is greatly reduced, medication safety is improved, and the process is worthy of popularization in production.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
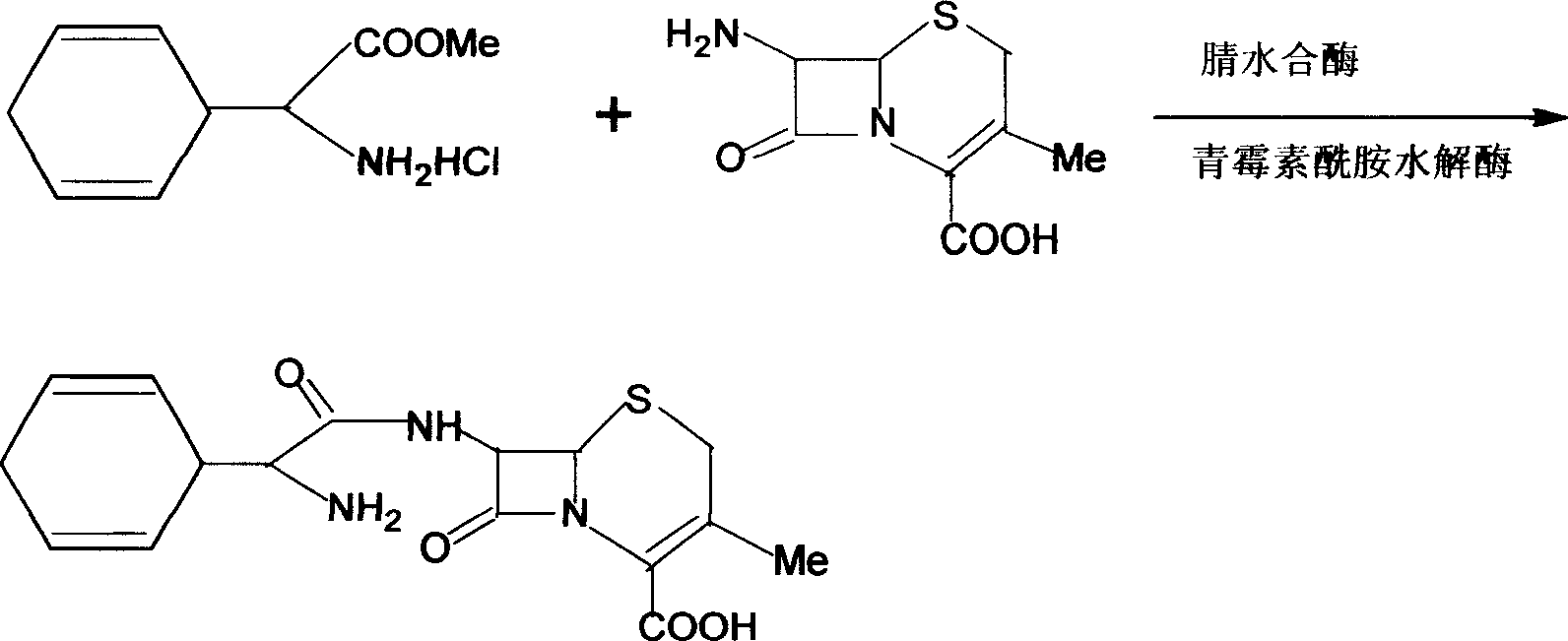
Method of synthesizing cefadroxil by enzyme process
The invention relates to a method of synthesizing cefadroxil by an enzyme process. The method comprises the following steps of: by taking 7-ADCA as an initial raw material, performing a reaction on D-tyrosine methyl ester or D-p-hydroxyl phenylglycine ethyl ester and 7-ADCA in water by directly inputting the solid in the presence of penicillin acylase at 10-25 DEG C; after reaction, separating a cefadroxil coarse product and enzyme reaction mother liquor; further purifying the cefadroxil coarse product to obtain a white cefadroxil product; and adding beta-naphthol or 2,7-dioxynaphthalene into the enzyme reaction mother liquor to obtain a cefadroxil compound. Cefadroxil can be further treated and recovered from the cefadroxil compound, so that the recovery rate of cefadroxil is increased. The product obtained by the method is high in yield and purity. The product is white in appearance, multi-step reaction of a chemical process and various solvents and auxiliary materials are not needed, and green synthesis of cefadroxil is realized.
Owner:苏州盛达药业有限公司 +1
Synthesis method of p-hydroxyphenylglycine methyl ester
ActiveCN102718672AHigh purityReduce pollutionOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsSolid acid
The invention discloses a synthesis method of p-hydroxyphenylglycine methyl ester. The method comprises the steps of: reacting p-hydroxyphenylglycine or salt thereof which is used as an initial raw material with methanol in the presence of solid acid; and treating so as to obtain p-hydroxyphenylglycine methyl ester. The preparation method of p-hydroxyphenylglycine methyl ester is available in raw material, simple and convenient to operate, low in cost, high in yield, high in product purity, and is suitable for commercial production.
Owner:SHANDONG HANXING PHARM TECH CO LTD +2
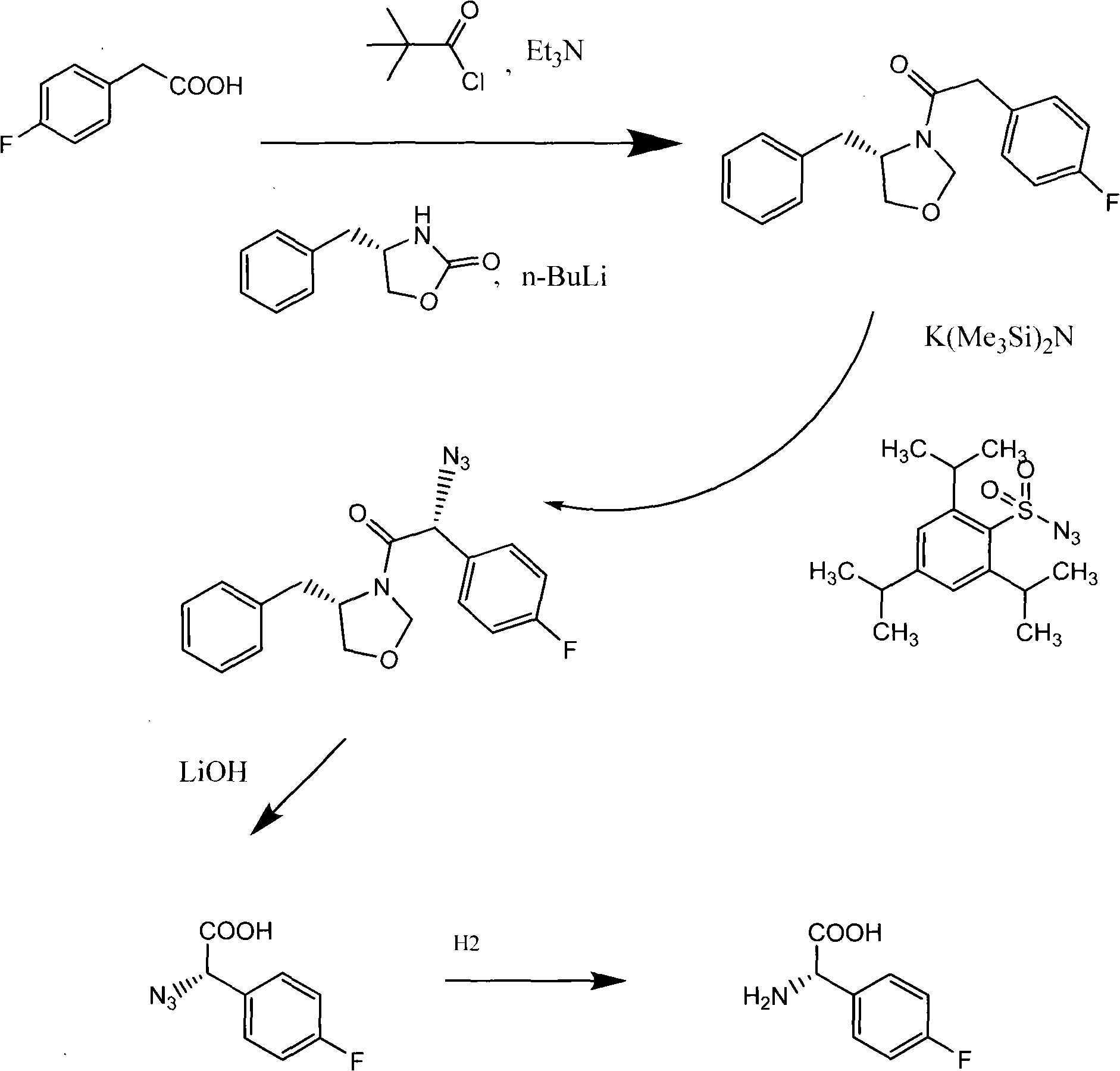
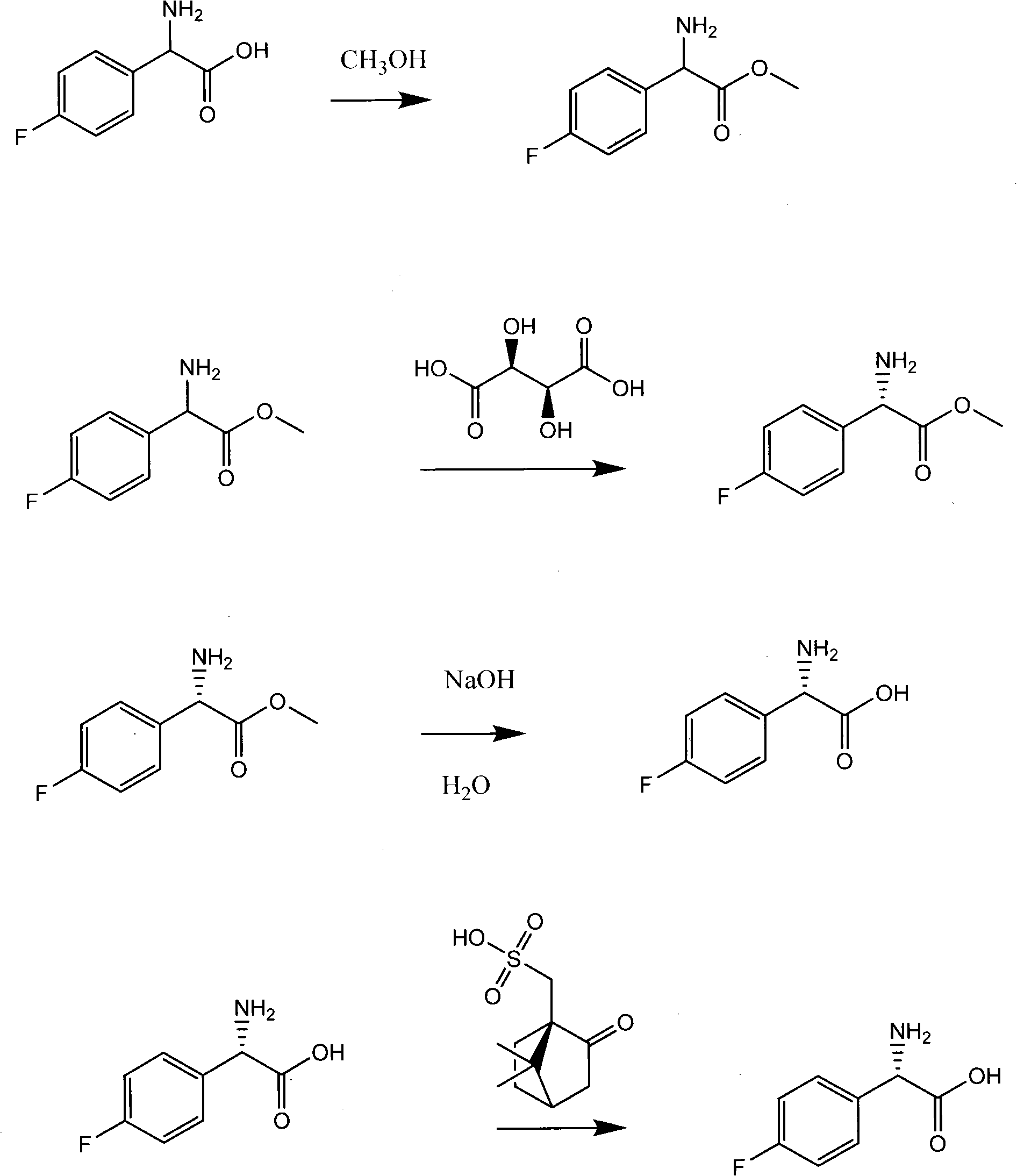
Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative
ActiveCN104788361AThe reaction route has short stepsEasy to operateOrganic chemistryAlkalinitySulfonate
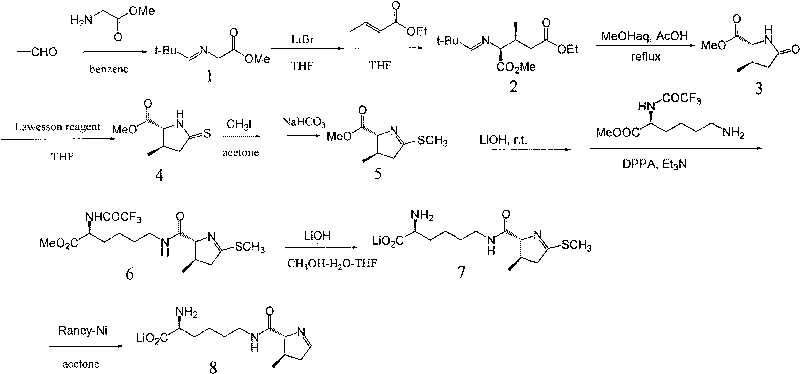
The invention provides a synthetic method for a 5-azaspiro[2.4]heptane-6-formic acid derivative. The synthetic method comprises the following steps: taking 1,1-cyclopropane dimethanol as a starting material, reacting with dichlorosulfoxide, performing oxidization to obtain a sulfonate compound, condensing the sulfonate compound and imine prepared from glycine methyl ester in the presence of potassium tert-butoxide, finishing hydrolysis, cyclization and amino protection with a one-pot procedure by adjusting the acidity and alkalinity of a system to obtain a racemization product, and finally performing resolution to obtain a finished product; the total yield reaches more than 30%; a reaction route has short steps; a used reagent is safe; the method is simple to operate, low in reaction cost, relatively high in yield and suitable for industrialized production.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
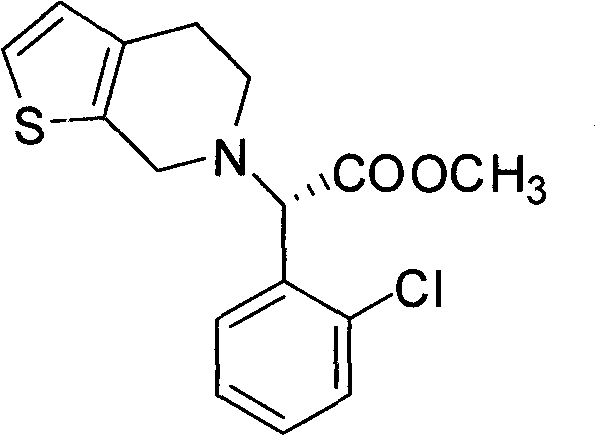
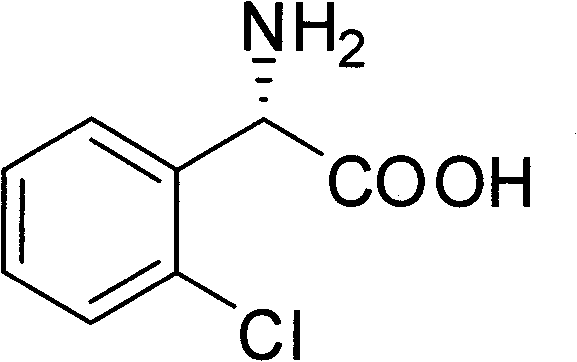
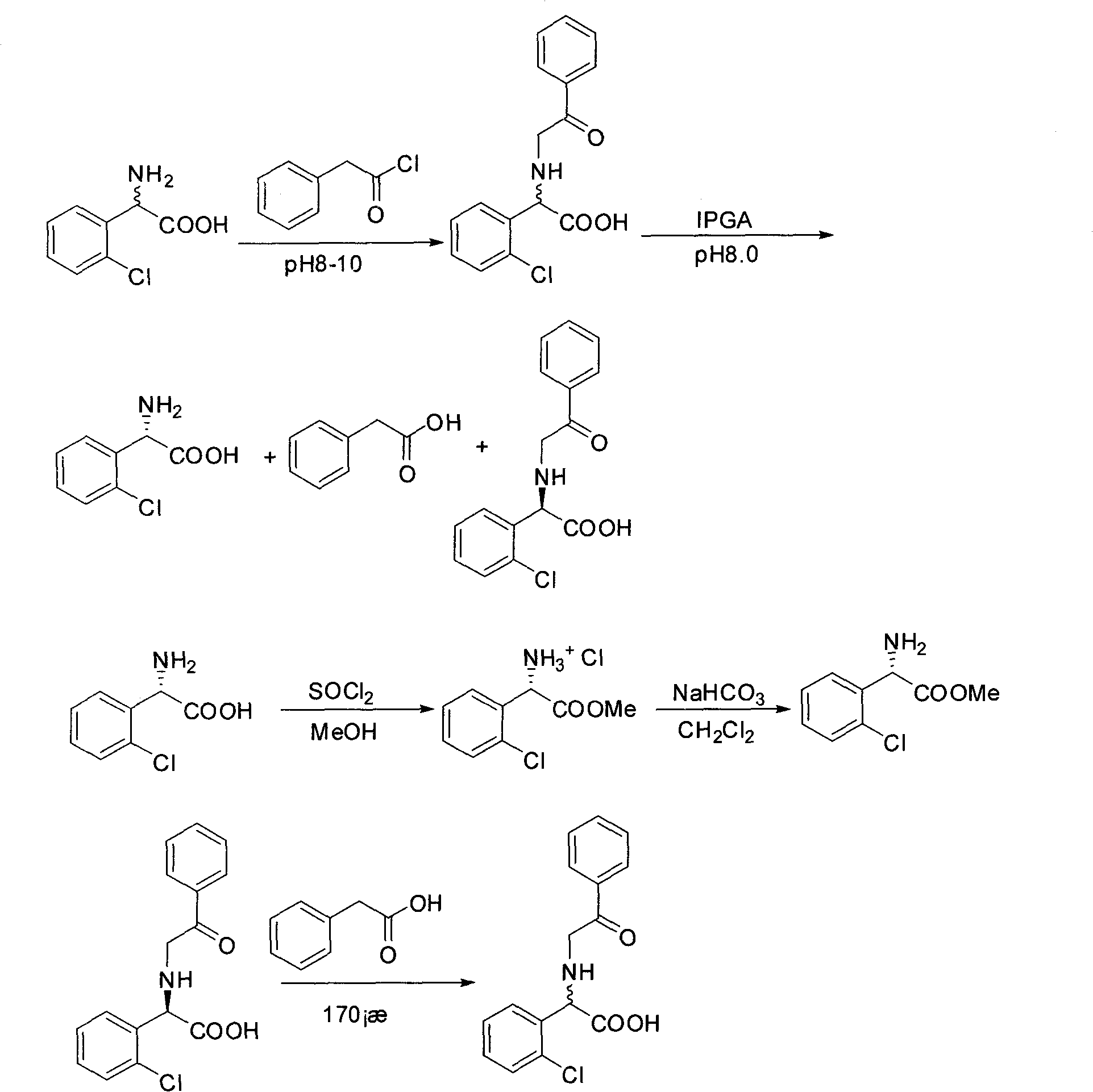
Chemical-enzyme method for preparing (S)-2-chlorophenyl glycine methyl ester clopidogrel chiral intermediate
InactiveCN101864464AHigh optical activityEliminate the recrystallization stepFermentationOrganic acidGlycine methyl ester hydrochloride
The invention provides a chemical-enzyme method for preparing an (S)-2-chlorophenyl glycine methyl ester clopidogrel chiral intermediate. In the method, (R,S)-2-chlorophenyl glycine is used as a raw material, and the (R,S)-2-chlorophenyl glycine is converted to (R,S)-N-phenylacetyl-2-chlorophenyl glycine by a deacylating agent; an immobilized penicillin acylase is used as a biocatalyst to catalyze a reaction for selectively converting the (R,S)-N-phenylacetyl-2-chlorophenyl glycine into (S)-2-chlorophenyl glycine, phenylacetic acid and (R)-N-phenylacetyl-2-chlorophenyl glycine in a water medium correspondingly; the (S)-2-chlorophenyl glycine is converted into (S)-2-chlorophenyl glycine methyl ester hydrochloride, and the (S)-2-chlorophenyl glycine methyl ester hydrochloride is desalinized to form the (S)-2-chlorophenyl glycine methyl ester; and the (R)-N-phenylacetyl-2-chlorophenyl glycine is mutually resolved with an organic acid and is racemized to form the (R,S)-N-phenylacetyl-2-chlorophenyl glycine which is used for resolution circularly. The method has the characteristics of high yield, high optical purity and environmental friendliness.
Owner:CHONGQING CHIRAL BIOCATALYSIS TECH
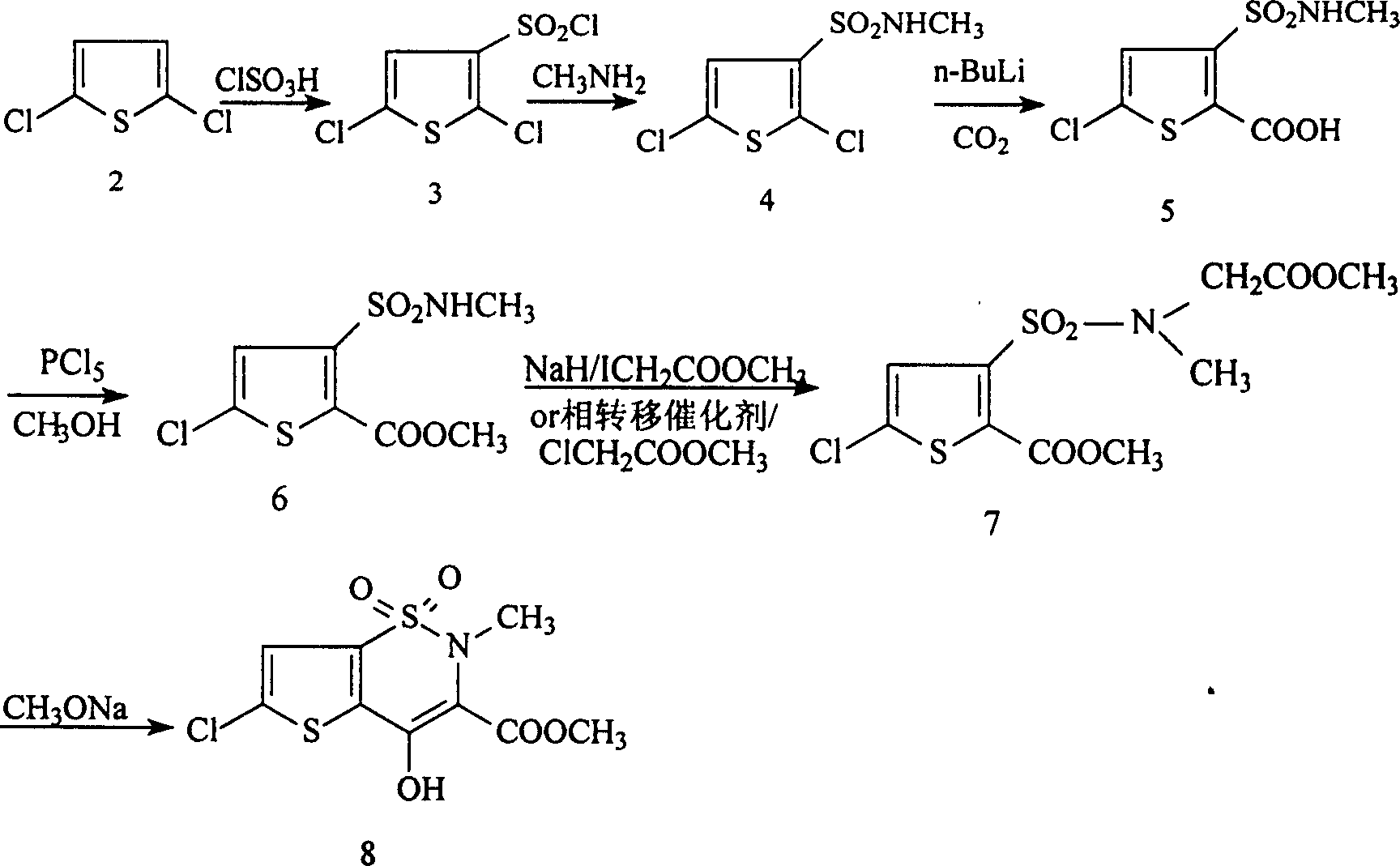
Process for synthesizing lornoxicam intermediate against inflammation and pain
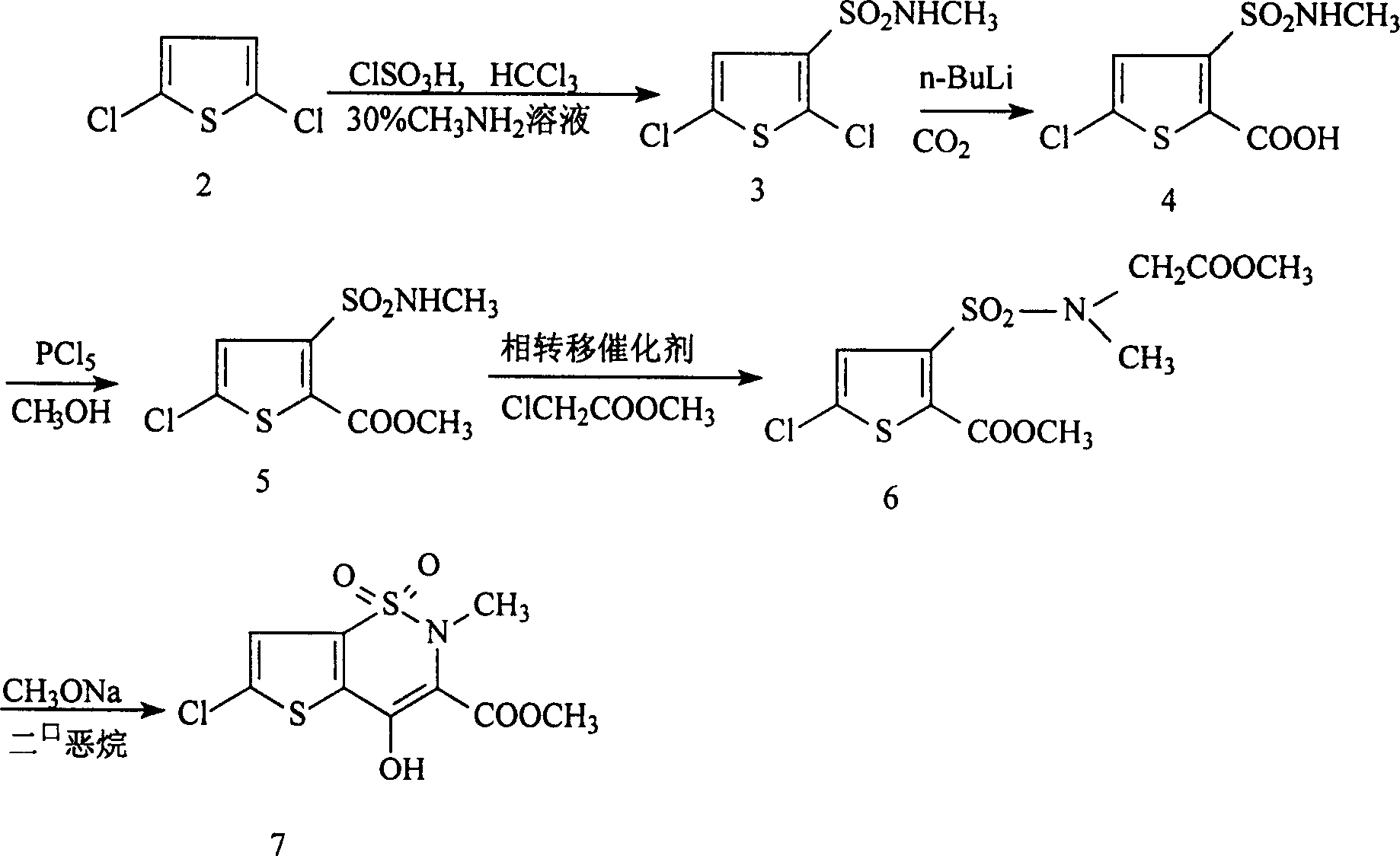
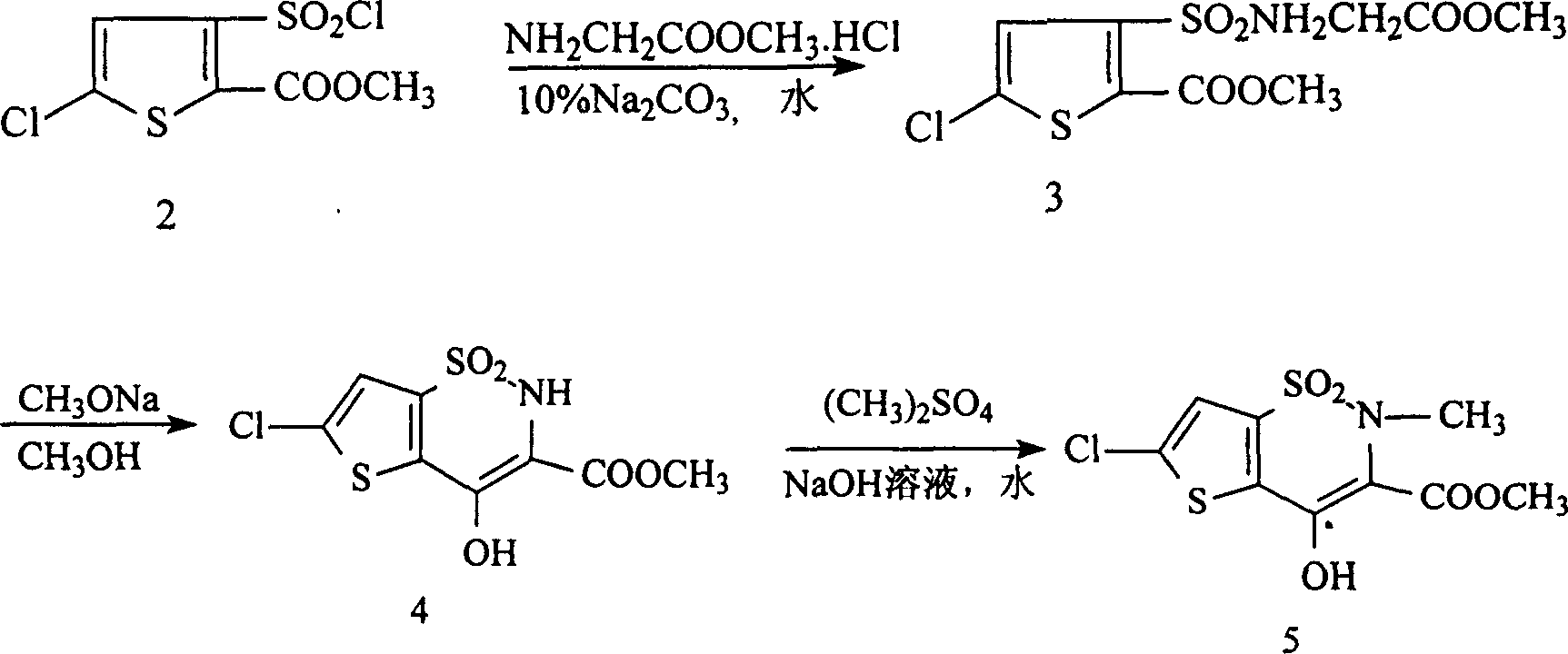
Disclosed is a process for synthesizing lornoxicam intermediate against inflammation and pain, which comprises using 5-chloro-3-thiophene sulfonyl chloride-2-carboxylate as raw material, charging 2-10% of sodium carbonate aqueous solution and 5-30% of glycine methylester hydrochloride simultaneously into water and methanol phases, thermal insulating 6-26 hours at 10-60 deg. C, then filtering and drying to obtain 6-chloro-3-sulfamoyl amido methyl acetate thiophene-2 methyl formate, reacting it with 5-27% of sodium methylate methanol solution 1-15 hours at 30-75 deg. C, filtering to obtain 6-chloro-4-hydroxyl-2H-thione[2,3-e]-1 and 2-thiazine-3-methyl formate-1,1-dioxide, then reacting the filtered substance with dimethyl sulfate in 1-10% aqueous solution of sodium-hydroxide.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
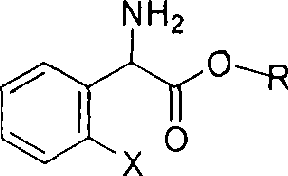
Method for splitting S-(+)-o-chlorobenzene glycine methyl ester
InactiveCN101497575AHigh yieldShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationAlkaneChlorobenzene
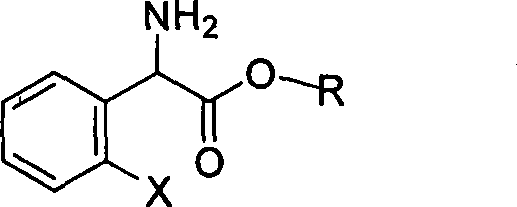
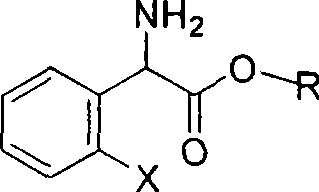
The invention relates to division of a clopidogrel intermediate 2-substituted phenylglycine methyl ester (or acid), which is a medicine for resisting platelet aggregation. The 2-substituted phenylglycine methyl ester (or acid) has the structure shown in a structural formula I. By utilizing the method, the raw materials and the reagents are low-priced and easily obtained, the reaction conditions are mild and the yield is satisfactory, reaching 98 percent. Therefore, the method for dividing the 2-substituted phenylglycine methyl ester (or acid) is easily industrialized. In the structural formula I, R is equal to H, alkyl (C1-C4, benzene)X is equal to a halogen, and for OR1 and SR1, R1 is equal to H or alkane(C1-C3) is equal to a benzene or the benzene for free substitution.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD +1
Cefradine preparing process
The present invention discloses cefradine preparing process. Under the catalysis of enzyme, 7-ADCA and methyl dihydrogen benzene glycinate are reacted in a double water phase system inside an enzyme catalyzed reactor to form cefradine. Cefradine is obtained through separating the upper phase and the lower phase and filtering, and the mother liquid is returned to the enzyme catalyzed reactor for circular reaction. The enzyme catalyzed cefradine synthesizing process has low solvent consumption, reduced environmental pollution, timely separation of the reaction product from side product and high reaction conversion rate.
Owner:ZHEJIANG ANGLIKANG PHARMA
Method for synthesizing bursa of Fabricius bursin
InactiveCN1696151AIncrease productionReduce manufacturing costTripeptidesGlycine methyl esterMedicinal chemistry
A process for synthesizing the high-purity bursin tripeptide inclues such steps as amino acid protection, synthesizing the protected His-Gly methyl bipeptide, removing amino acid protection of the protected His-Gly bipeptide, synthesizing the protected lys-His-Gly, removing amino acid protection of the protected His-Gly bipeptide, synthesizing the protected lys-His-Gly, removing amino acid protection, synthesizing bursin tripeptide, and purifying.
Owner:四川斯特佳生物科技有限公司
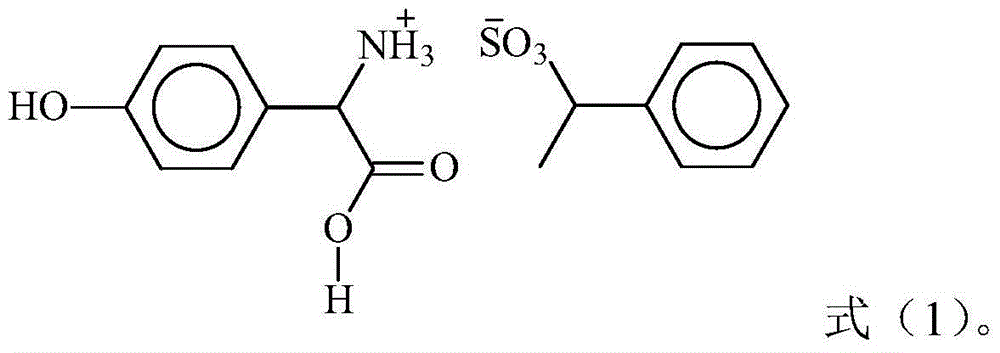
Glycine and application of acid salt thereof in preparation of glyphosate
InactiveCN102161678AGroup 5/15 element organic compoundsGlycine methyl ester hydrochlorideGlycine methyl ester
The invention relates to a glycine and an application of an acid salt thereof in preparation of herbicide-glyphosate, and in particular relates to an application of glycine hydrochloride and glycine methyl ester hydrochloride in preparation of the herbicide-glyphosate.
Owner:李坚
Method for preparing cefprozil in pH responsive regenerative double aqueous phase system
PendingCN106939327AIncreased molar yieldEasy to separateFermentationChemical synthesisCentrifugation
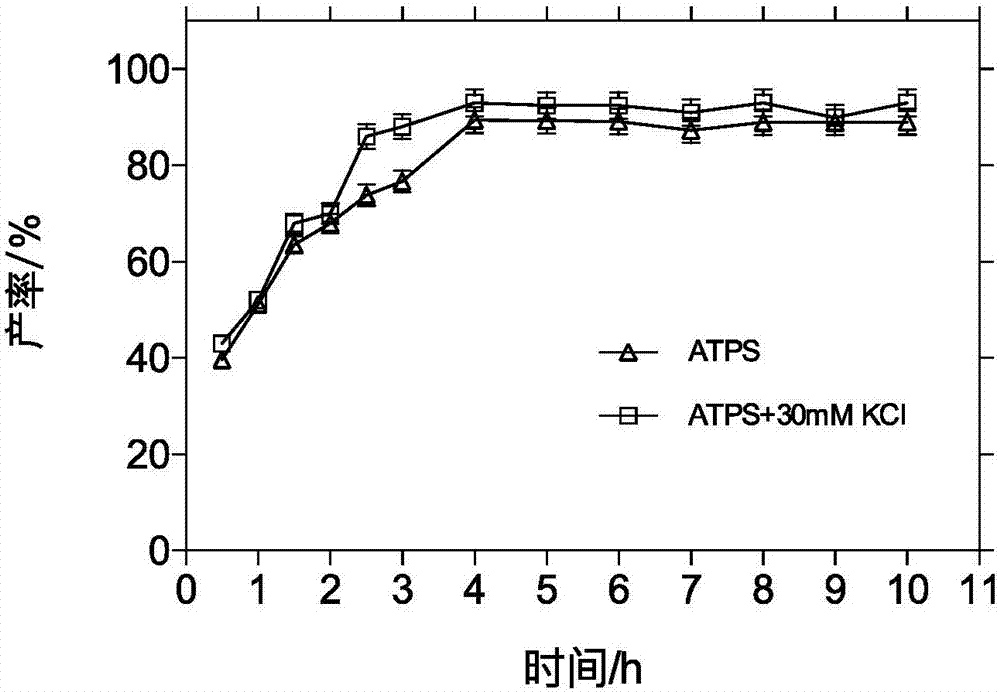
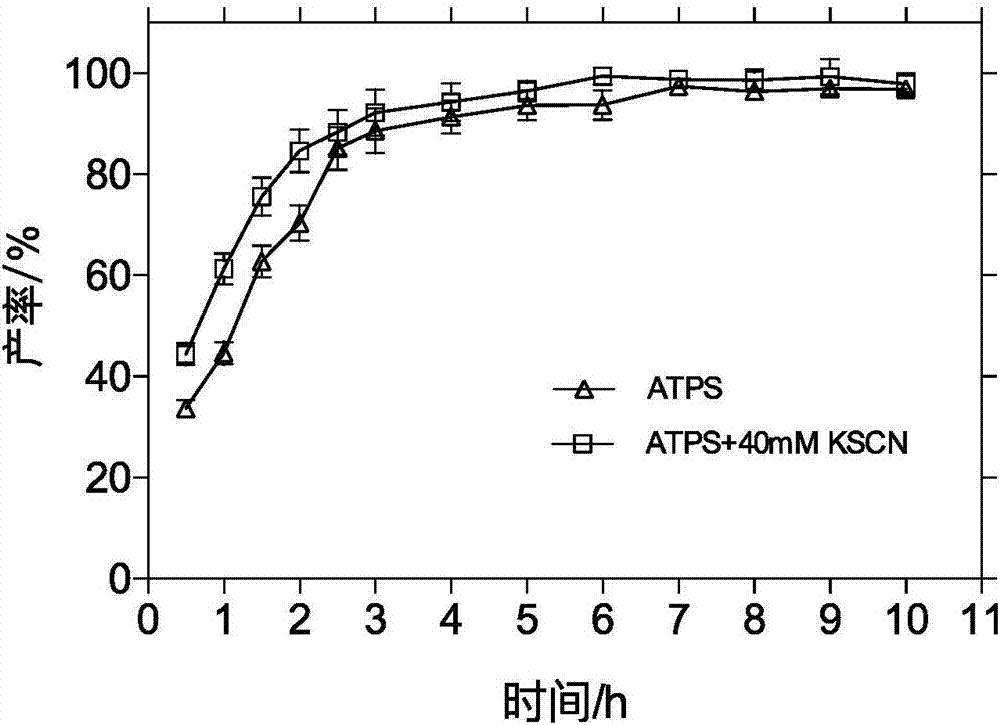
The invention relates to a method for preparing cefprozil in a pH responsive regenerative double aqueous phase system. The method comprises (1) preparing two double-aqueous phase systems, (2) orderly adding 7-APRA, D-p-hydroxyphenylglycine methyl ester hydrochloride into the systems, adjusting solution pH to 5.00-6.50 and controlling a solution temperature in a range of 10-30 DEG C, (3) adding immobilized penicillin acylase into the solution obtained by the step (2), and (4) standing the mixed solution, removing the immobilized penicillin acylase, adjusting the pH of the reaction solution, recovering P<ADBA> / <PMDB> and P<ADB> / P<MDB> polymers of the double-aqueous phase systems, feeding the supernatant to a crystallization section, carrying out crystallization, and carrying out centrifugation, washing and drying to obtain a product. The method reduces a chemical synthesis cost, improves a low product conversion rate of the monohydrolase catalytic reaction, effectively improves a yield, simplifies the operation, reduces a cost and realizes easy recovery of the double-aqueous phase systems.
Owner:EAST CHINA UNIV OF SCI & TECH
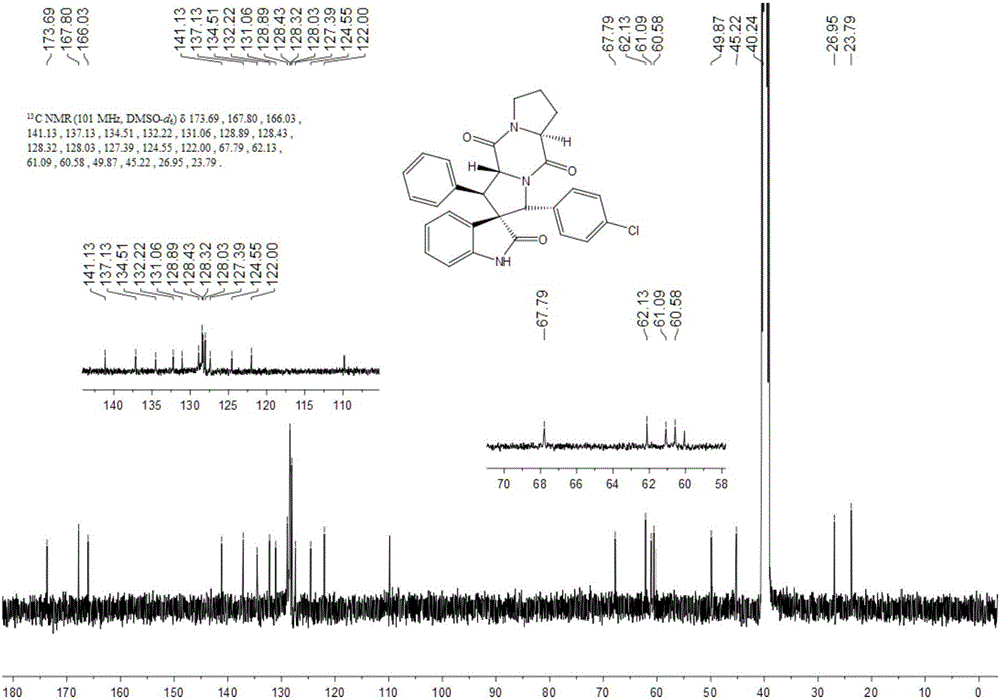
Aromatic substitution spiro indolyl diketopiperazine compound and synthesis method thereof
ActiveCN106478645AGuaranteed singularityHigh conformational specificityOrganic chemistryBulk chemical productionBenzaldehydeCycloaddition
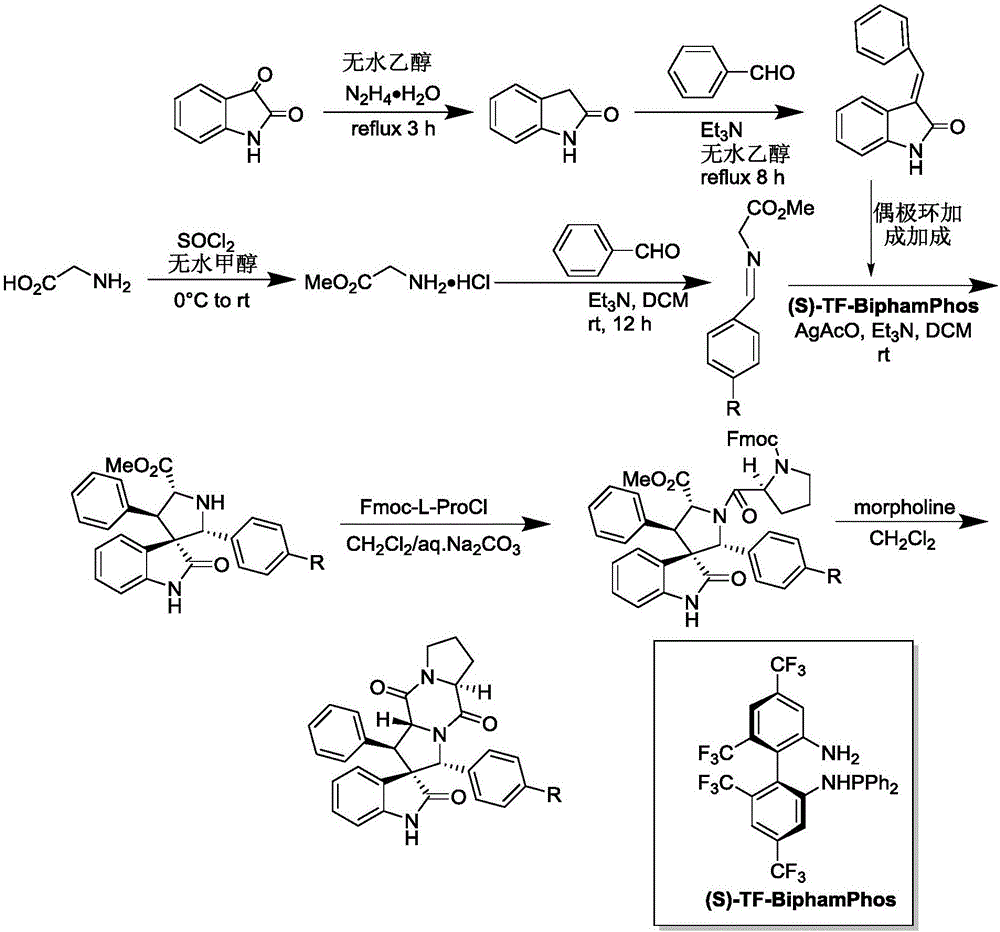
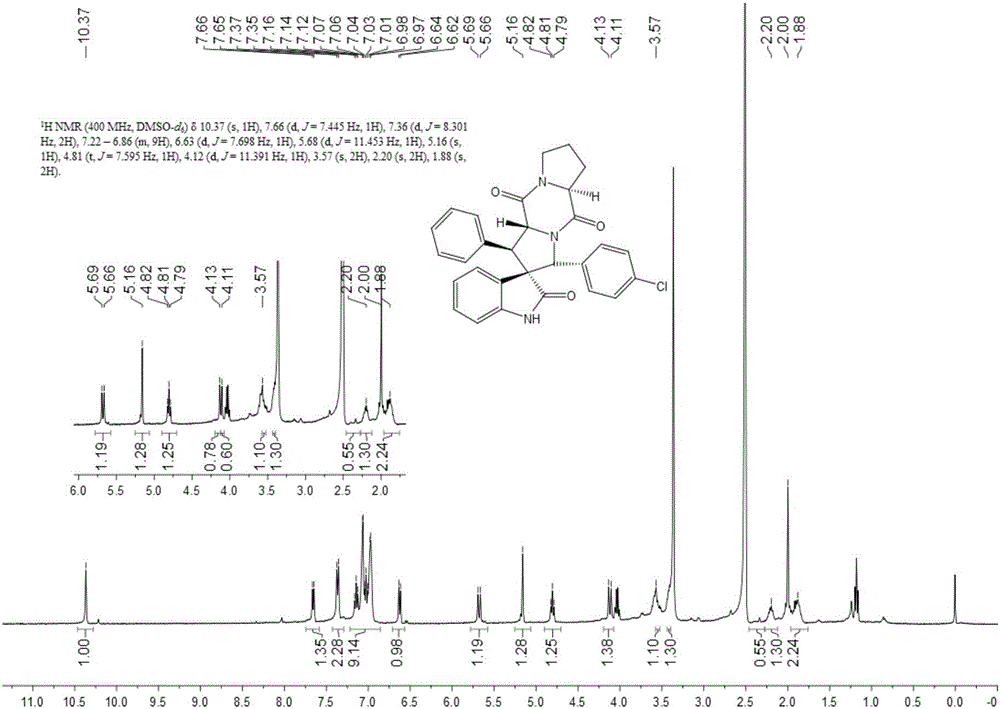
The invention discloses an aromatic substitution spiro indolyl diketopiperazine compound and a synthesis method thereof. Glycine is used as a raw material and subjected to esterification reaction with methyl alcohol and thionyl chloride to obtain methyl glycinate hydrochloride, methyl glycinate hydrochloride and aromatic aldehyde are subjected to condensation reaction to obtain Schiff base, isatin is used as a raw material and subjected to reduction reaction under the action of hydrazine hydrate to obtain indoline-2-ketone, indoline-2-ketone and benzaldehyde are subjected to Knoevenagel reaction under piperidine catalysis to obtain 3-phenylidene-1,3-dihydro-2H-indol-2 ketone, 3-phenylidene-1,3-dihydro-2H-indol-2 ketone and Schiff base are subjected to 1,3-dipole cycloaddition reaction under catalysis of chiral ligand (S)-TF-BiphamPhos / AgoAc to obtain spiro pyrrolidine, and protecting group removal and ring closure are carried out on spiro pyrrolidine and N-(9-fluorene methoxycarbonyl)-L-prolyl chloride through base catalysis to obtain the target product. The method has the advantages of being simple in path, high in yield, high in diastereoselectivity and single in product spatial configuration, and the raw materials are low in price and easy to obtain.
Owner:SHAANXI UNIV OF SCI & TECH
Method for synthesizing D-p-hydroxyphenylglycine methyl ester
ActiveCN104892444AHigh yieldOrganic compound preparationAmino-carboxyl compound preparationHydrogenEsterification reaction
The invention relates to the field of compound synthesis and discloses a method for synthesizing D-p-hydroxyphenylglycine methyl ester. The method comprises steps as follows: (1), in the presence of thionyl chloride, D-p-hydroxyphenylglycine resolving agent salt and methanol have an esterification reaction, and D-p-hydroxyphenylglycine methyl ester resolving agent salt is obtained; (2), the D-p-hydroxyphenylglycine methyl ester resolving agent salt and alkaline metal hydroxide are dropwise added to a D-p-hydroxyphenylglycine methyl ester aqueous solution at the temperature of 10-15 DEG C, the pH (potential of hydrogen) value of a system is controlled in the range from 6.5 to 7 in the dropwise adding process, after dropwise adding of the D-p-hydroxyphenylglycine methyl ester resolving agent salt is completed, alkaline metal hydroxide is continuously dropwise added until the pH value of the system ranges from 7.5 to 8, a crystal is grown at the temperature of 10-15 DEG C and under the condition of the pH value being 7.5-8, an obtained crystalline liquid is filtered, and a D-p-hydroxyphenylglycine methyl ester crystal and a mother liquor are obtained. With adoption of the method provided by the invention, D-p-hydroxyphenylglycine methyl ester with a higher yield can be produced.
Owner:山西双雁生物科技有限公司
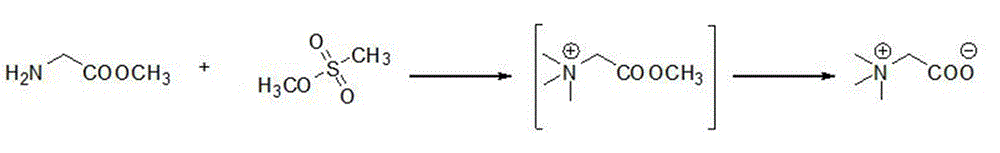
Preparation method of betaine
ActiveCN105130830AHigh yieldEasy to operateOrganic compound preparationAmino-carboxyl compound preparationEnvironmental resistanceSodium bicarbonate
The invention discloses a preparation method of betaine. Specifically the method comprises the following steps: uniformly mixing glycine methyl ester, acetonitrile and sodium bicarbonate, dropwise adding methyl methanesulfonate, and stirring to enable the materials to react for 16 to 32h; adding a sodium hydroxide solution into reactant, rising the temperature to 50 DEG C, stirring to enable the materials to react for 20 h, then distilling to remove solvent, obtaining filtrate through filtering, removing foreign ions through adsorption, distilling the filtrate at ordinary pressure, collecting precipitated solid, performing vacuum drying, and then obtaining the betaine. All the raw materials related involved in the synthetic method adopted can be purchased in the market, and the operation of all the steps is simple, and the product cost can be effectively reduced; the condition of each step in the reaction is mild, and the safety is high; toxic and harmful substances are not produced, so the method is green and environmentally friendly; the yield of reaction products is high, and the method is easy for large-scale popularization and application.
Owner:宜兴市天石饲料有限公司
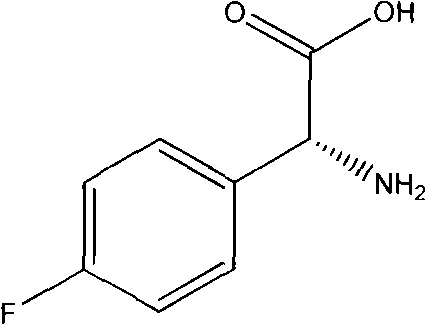
Preparation method of L(+)-p-fluorophenyl glycine
ActiveCN101565380AHigh yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationGlycine methyl esterOrganic chemistry
The invention relates to a preparation method of L(+)-p-fluorophenyl glycine (d-p-fluorophenyl glycine), in particular to a method capable of separating racemized p-fluorophenyl glycine methyl ester and turning the racemized l-p-fluorophenyl glycine methyl ester generated in the separating process into racemized l-p-fluorophenyl glycine methyl acid. The invention also relates to a refining method of the L(+)-p-fluorophenyl glycine.
Owner:JIANGSU ALPHA PHARM CO LTD
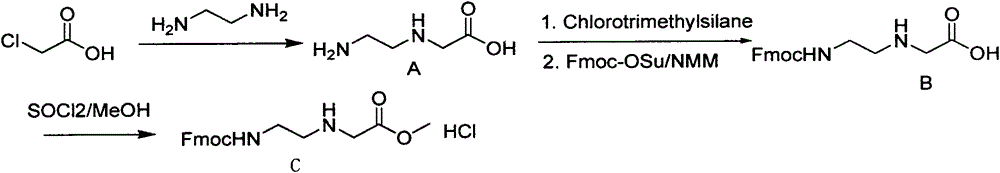
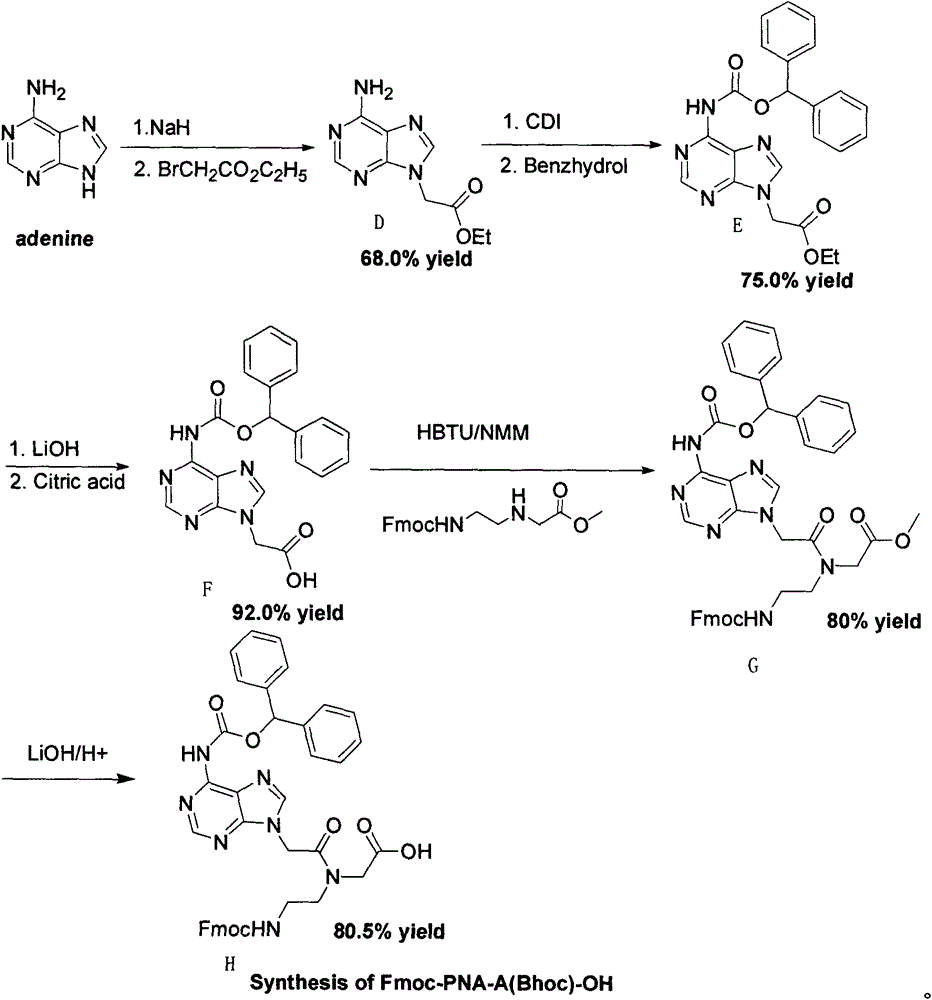
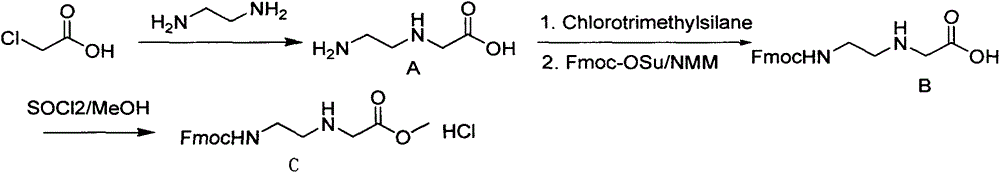
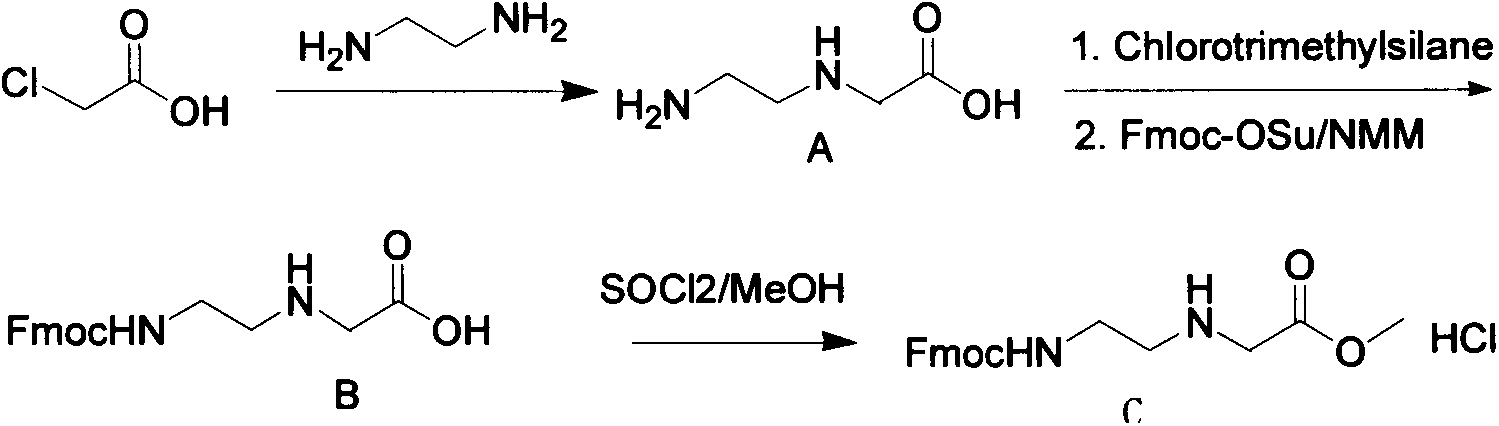
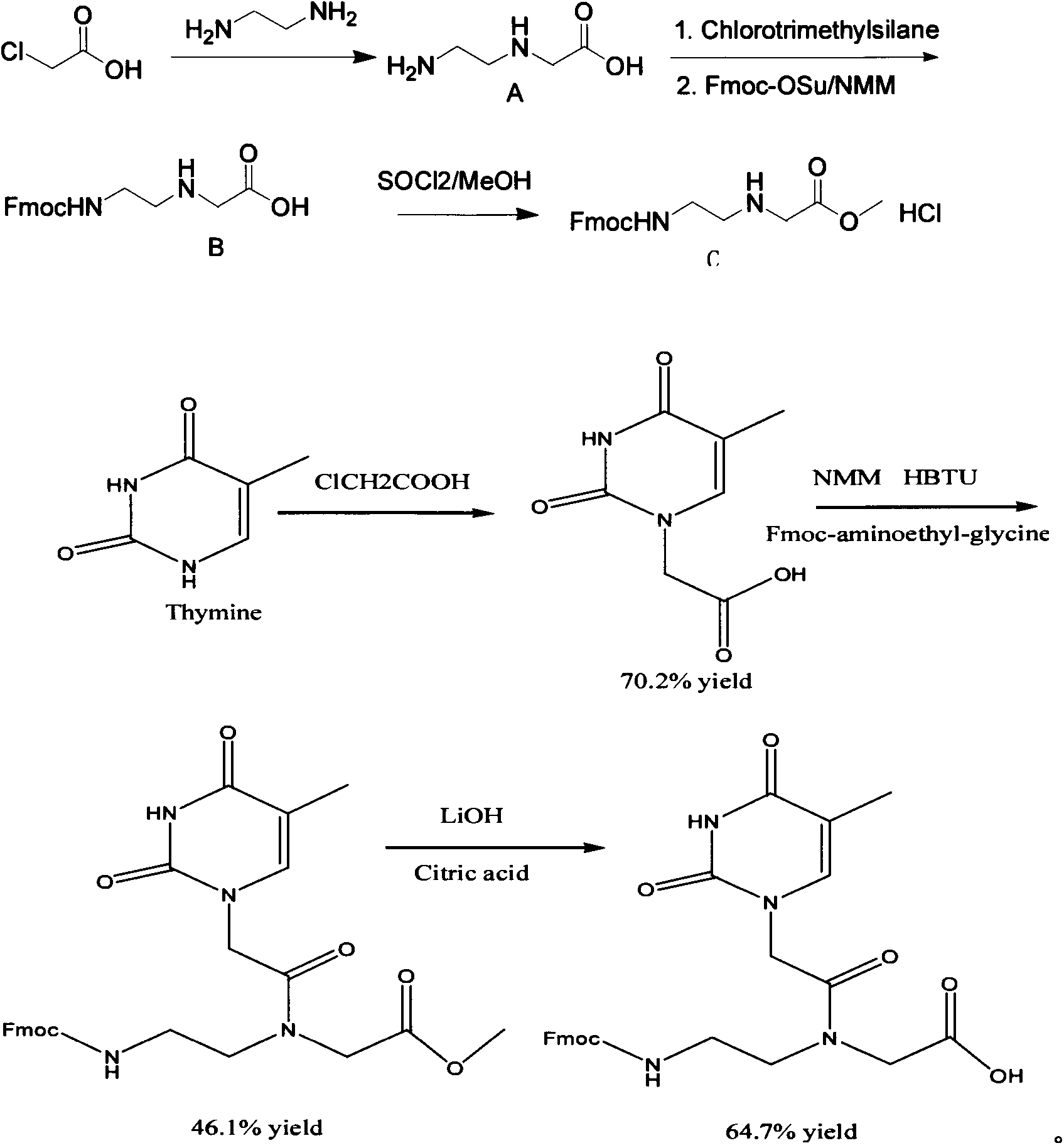
Feather weight PNA (pentose nucleic acid) synthesis method
The invention discloses a feather weight PNA (pentose nucleic acid) synthesis method. The method comprises the following steps: reacting ethylene diamine with chloroacetic acid, and recrystallizing with DMSO to obtain N-(2-aminoethyl)glycine; by taking the N-(2-aminoethyl)glycine as a reactant, adding raw materials, and reacting to obtain N-(2-Fmoc-aminoethyl)glycine methyl ester hydrochloride; by taking adenine as a reactant, adding raw materials, and reacting to obtain 9-ehtyl acetate adenine; by taking the 9-ehtyl acetate adenine and carbonyl diimidazole as reactants, adding raw materials, and reacting to obtain 6-N-(benzhydryloxycarbonyl)adenine-9-acetic acid; by taking the 6-N-(benzhydryloxycarbonyl)adenine-9-acetic acid as a reactant, adding raw materials, and reacting to obtain the PNA. The feather weight PNA synthesis method belongs to large-scale production technology, does material preparation for the large-scale research of the PNA, as well as technological preparation for the future large-scale application.
Owner:SUZHOU VIVOTIDE BIOTECH
Recycling process of amoxicillin in enzymatically synthesized amoxicillin mother liquor
The invention discloses a recycling process of amoxicillin in enzymatically synthesized amoxicillin mother liquor. The process comprises the following steps: concentrating the amoxicillin mother liquor through the adoption of nanofiltration membrane high-power concentration equipment, feeding the concentrated liquor in a reaction tank, sufficiently reacting the 6-APA in the concentrated liquor with methyl D-(-)-4-hydroxy-phenylglycinate under the effect of penicillin G acylase so as to generate the amoxicillin. The recycling process disclosed by the invention has the advantages that through the nanofiltration concentration, not only the amoxicillin in the mother liquor is recycled, but also the 6-APA contained in the mother liquor participates the enzymatic synthesis reaction to be translated into the amoxicillin, the aim of extremely recycling the amoxicillin in the mother liquor is achieved, and the recycling process has more economy.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Synthesis method of new ligand for efficient catalysis of CuAAC reaction
InactiveCN107629013AThe effect of accelerating the reaction is obviousOrganic chemistrySynthesis methodsRoom temperature
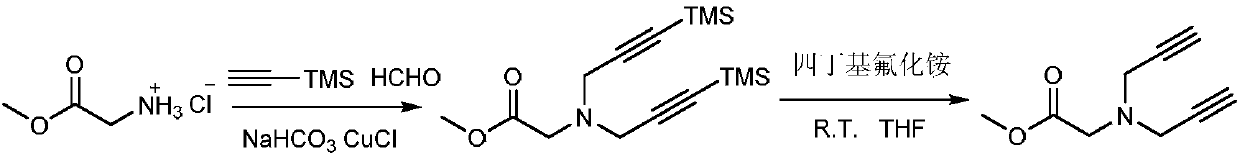
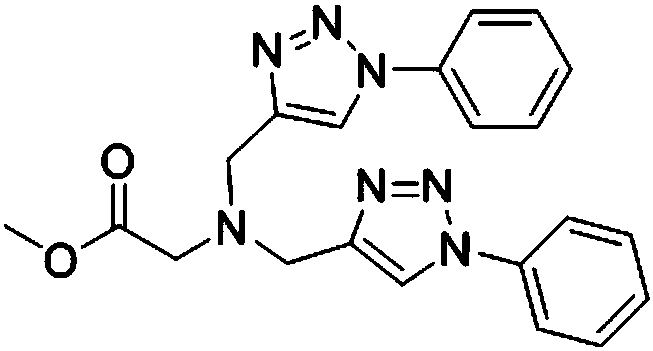
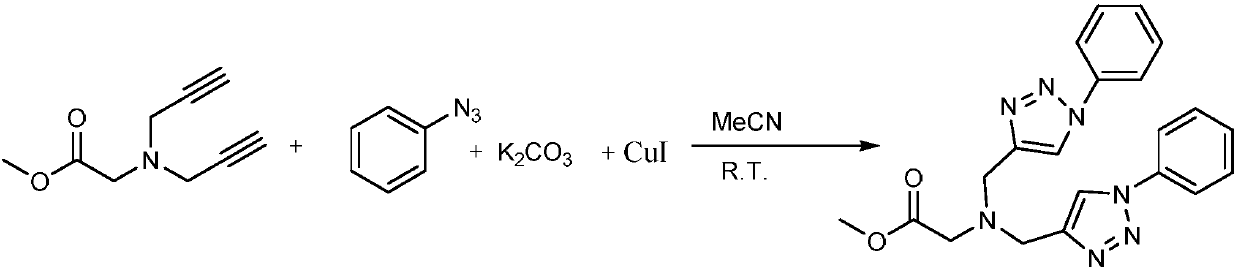
Belonging to the technical field of synthesis of functional structural molecules, the invention discloses a synthesis method of a new ligand for efficient catalysis of CuAAC reaction. The key point ofthe technical scheme adopted by the invention lies in that: the synthesis method of the new ligand for efficient catalysis of CuAAC reaction includes: firstly synthesizing glycine methyl ester diyne,then taking glycine methyl ester diyne, an azido compound, K2CO3 and copper iodide as the raw materials, using acetonitrile and water as the solvent, carrying out stirring reaction at room temperature, and performing post-treatment at the end of reaction, thus obtaining the new ligand glycine methyl ester 1, 2, 3-ditriazole ring opening compound. The invention provides a variety of novel, efficient and easily available ligand catalysts for click reaction, as a ligand, the compound synthesized by the method provided by the invention has obvious effect of accelerating reaction and has a very practical application.
Owner:HENAN NORMAL UNIV
Novel process for synthesizing rebamipide
The invention discloses a novel process for synthesizing rebamipide. The novel process comprises the following steps: adopting glycine methyl ester as a starting raw material, then performing amidation and chlorination to obtain chloroimide intermediate, enabling the chloroimide intermediate to react with bromomethylquinolone, and hydrolyzing to obtain the rebamipide. The novel process has the advantages that the starting raw material is low in price and easy to obtain, the reaction yield is high, the industrialization is easy to realize and the like.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
2-sulfohydantoin as well as preparation method and application thereof
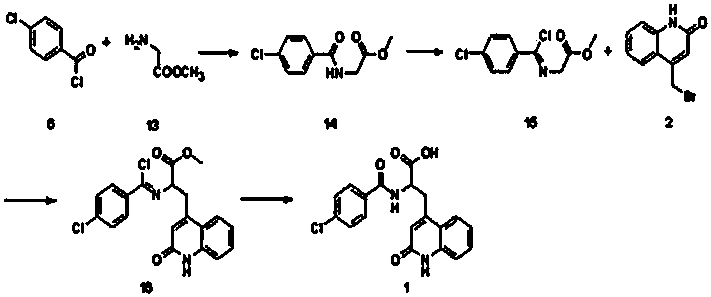
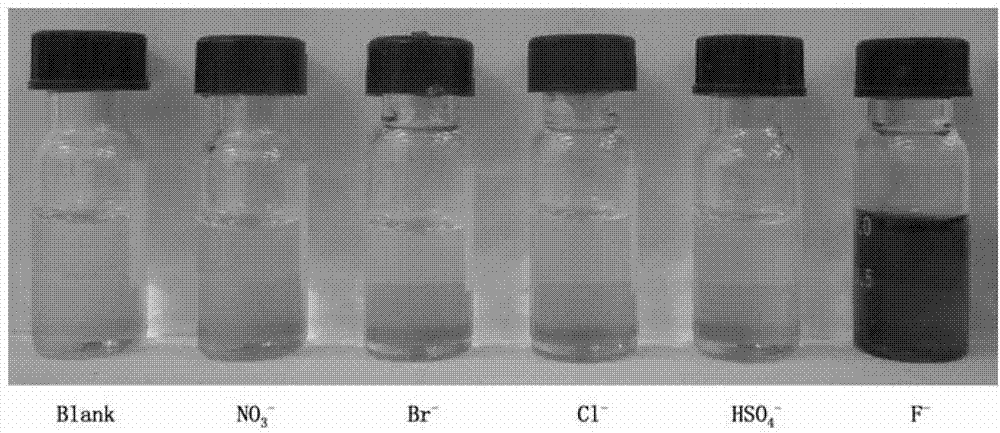
InactiveCN102924383AEasy to carryQuick checkOrganic chemistryMaterial analysis by observing effect on chemical indicatorChemical structurePhenylglycine methyl ester
The invention relates to a sulfur-containing heterocyclic compound. The compound is 2-sulfohydantoin; a chemical structure of the 2-sulfohydantoin is shown in a formula (I) in the specification; and in the formula (I), R is one of C1-12 alkyl, p-nitrophenyl, p-cyano phenyl, p-methoxyphenyl, p-butyl phenyl, 3,5-di(trifluoromethyl)phenyl and phenyl. The 2-sulfohydantoin is obtained by reacting isothiocyanate with para hydroxy phenylglycine methyl ester, has an anion recognition performance, and can be used for detecting fluorinion.
Owner:SOUTHERN MEDICAL UNIVERSITY
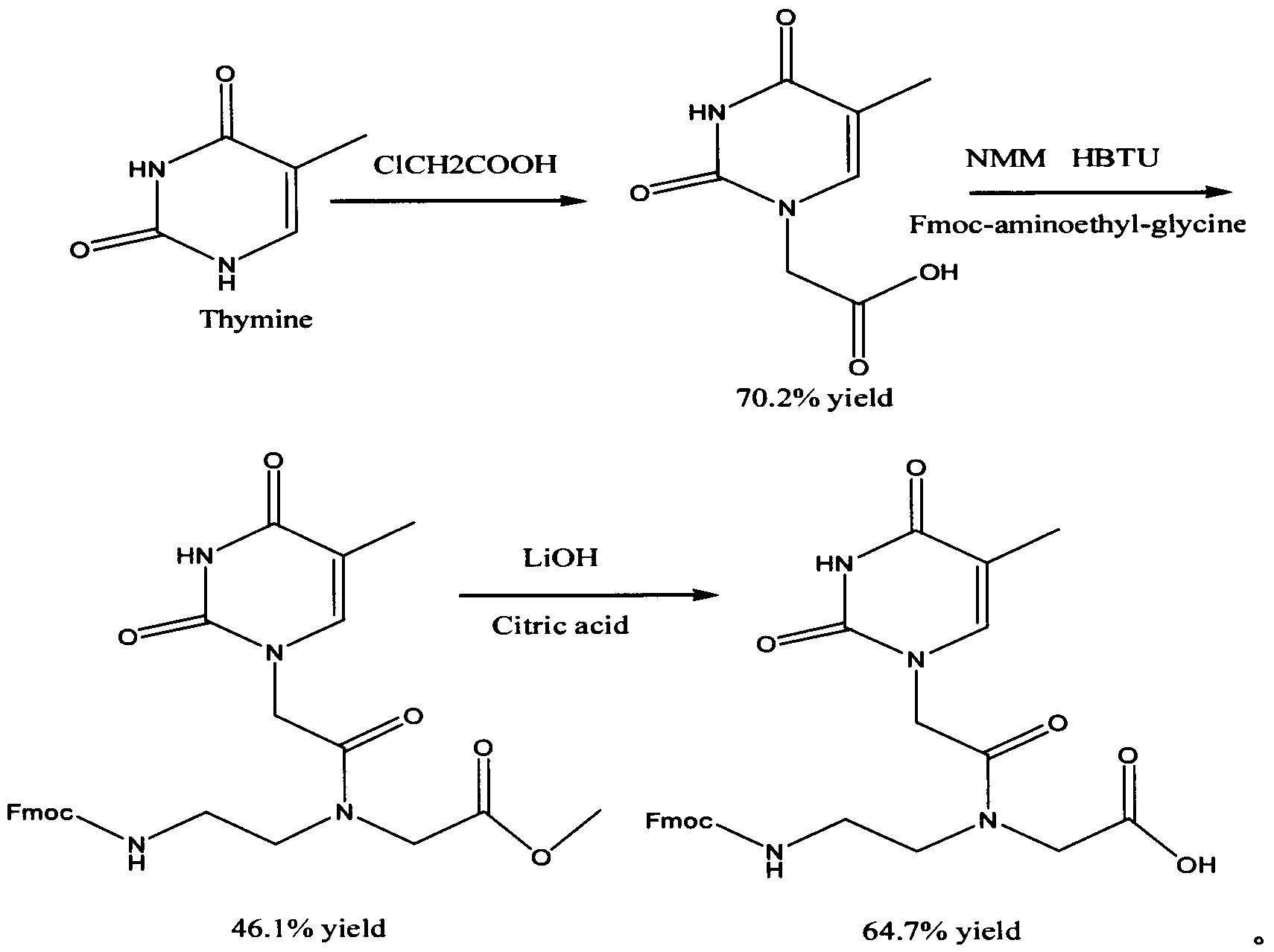
Feather weight synthesis method of Fmoc-PNA-T-OH
The invention discloses a feather weight synthesis method of Fmoc-PNA-T-OH. The feather weight synthesis method comprises the following steps: reacting ethanediamine serving as a raw material with haloacetic acid, and recrystallizing by using DMSO (dimethylsulfoxide) so as to obtain N-(2-aminoethyl) glycine; reacting the N-(2-aminoethyl) glycine serving as a raw material with Fmoc-osu so as to obtain N-(2-Fmoc-aminoethyl) glycine, and then reacting with methyl alcohol so as to obtain N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride; using thymine as a raw material, adding raw materials, and reacting so as to obtain 1-carbethoxy thymine; reacting the 1-carbethoxy thymine serving as the raw material with the N-(2-Fmoc-aminoethyl) glycine methyl ester so as to obtain Fmoc-PNA-T-OMe; and hydrolyzing the Fmoc-PNA-T-OMe so as to obtain the product.
Owner:SUZHOU VIVOTIDE BIOTECH
Chemical complete synthesis method for 22nd natural amino acid-pyrrolysine
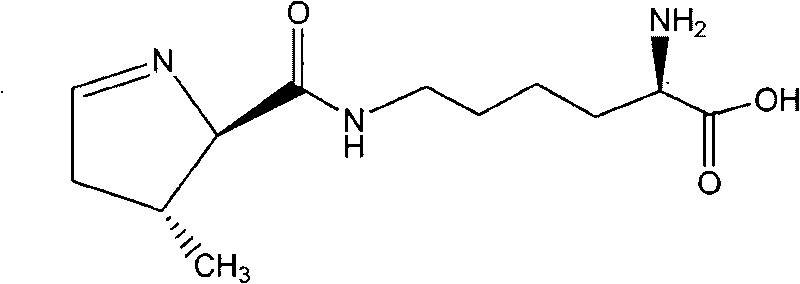
InactiveCN101709047AWide variety of sourcesLow priceOrganic chemistryBulk chemical productionPyrrolysineSynthesis methods
The invention relates to a chemical complete synthesis method for 22nd natural amino acid-pyrrolysine. 2,2-dimethyl propionaldehyde reacts with glycine methyl ester hydrochloride to form (E)-2-(2,2-dimethyl propylidene-amino) methylacetate, and the product generates Michael addition with olefine aldehyde by the catalysis of DBU and then carries out hydrolysis to obtain a pyrrole ring. After being vulcanized and methylated, the pyrrole ring is condensed with a derivative of lysine, and finally sulfomethyl is removed to obtain the pyrrolysine. The invention has cheap and easily acquired chemical raw materials, wide source, low cost, mild and easily controlled reaction condition, simple reaction device and low operation cost; by adopting various protecting groups, all intermediate compounds are more stable and are difficult for modification; and all reactions are mature and are in fixed quantity, and the yield is higher.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for synthesizing amoxicillin and generating byproduct of sodium phenylacetate solution by using semi-direct method
InactiveCN107988306AHigh yieldImprove product qualityOrganic compound preparationCarboxylic acid salt preparationPhenylacetic acidBenzylpenicillin potassium
The invention relates to a method for synthesizing amoxicillin and generating a byproduct of a sodium phenylacetate solution by using a semi-direct method and belongs to the technical field of medicine preparation. The method comprises the following steps: splitting benzylpenicillin potassium into 6-APA (Amino Penicillanic Acid) and phenylacetic acid under the action of penicillin acylase, extracting a splitting solution with dichloromethane to separate 6-APA from the phenylacetic acid, putting ammonia water into an extraction water phase, adjusting the pH value of a material liquid to be neutral, removing the dichloromethane, putting the solid 6-APA into the obtained 6-APA dissolving liquid, and synthesizing the amoxicillin together with D-methyl p-hydroxyphenylglycinate under the actionof amoxicillin synthetase. A sodium phenylacetate solution can be prepared by alkalizing the dichloromethane obtained by extracting the splitting solution, the sodium phenylacetate solution can be directly applied to fermentation production of penicillin, the step of recycling the phenylacetic acid is avoided, and the production cost is reduced.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Preparation method of methyl D-4-hydroxy-phenylglycinate and hydrochloride thereof
InactiveCN103641729AHigh purityReduce pollutionOrganic compound preparationAmino-carboxyl compound preparationPollutionMethanol
The invention discloses a preparation method of D-4-hydroxy-phenylglycinate and hydrochloride thereof. D-4-hydroxyphenylglycine is used as an initial raw material. The methyl D-4-hydroxy-phenylglycinate or the hydrochloride thereof is prepared by reacting the D-4-hydroxyphenylglycine with methanol or a methanol solution of hydrogen chloride under the existence of trimethylchlorosilane and by post-treatment. The preparation method has characteristics of easily available raw materials, simple operation, high yield, high product purity and little environment pollution, and is suitable for industrial production.
Owner:河北爱弗特精细化工有限责任公司
Catalyst and method for preparing glycine methyl ester and glycine from methyl glycolate by using bimetallic glass fiber layered eutectic
ActiveCN111495373ALow costSimple process routeOrganic compound preparationCatalystsGlass fiberPtru catalyst
The invention belongs to the technical field of catalysts, and particularly relates to a catalyst and a method for preparing glycine methyl ester and glycine from methyl glycolate by using a bimetallic glass fiber layered eutectic. The invention relates to a catalyst for preparing glycine methyl ester and glycine from methyl glycolate by using a bimetallic glass fiber layered eutectic, which is characterized by comprising the following components in parts by weight: based on 100 parts of the total weight, 5-20 parts of metal cobalt, 1-5 parts of metal nickel and the balance of vitrified silicon dioxide carrier are prepared. The invention has the advantages of advanced technical route, no toxicity of raw materials, no emission of three wastes and zero pollution of the process.
Owner:SHAANXI YANCHANG PETROLEUM GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative](https://images-eureka.patsnap.com/patent_img/5a858dde-8c3b-4e46-ae81-05b747950675/BDA0000703241120000012.PNG)
![Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative](https://images-eureka.patsnap.com/patent_img/5a858dde-8c3b-4e46-ae81-05b747950675/BDA0000703241120000021.PNG)
![Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative](https://images-eureka.patsnap.com/patent_img/5a858dde-8c3b-4e46-ae81-05b747950675/BDA0000703241120000022.PNG)