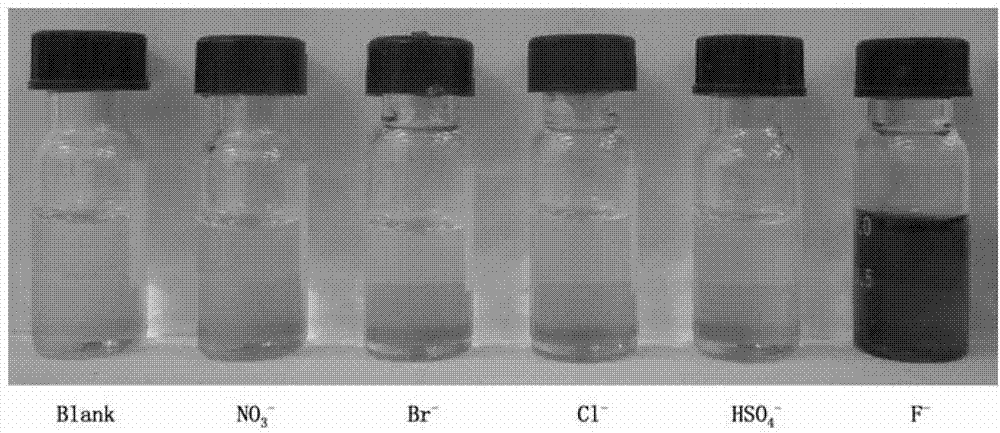
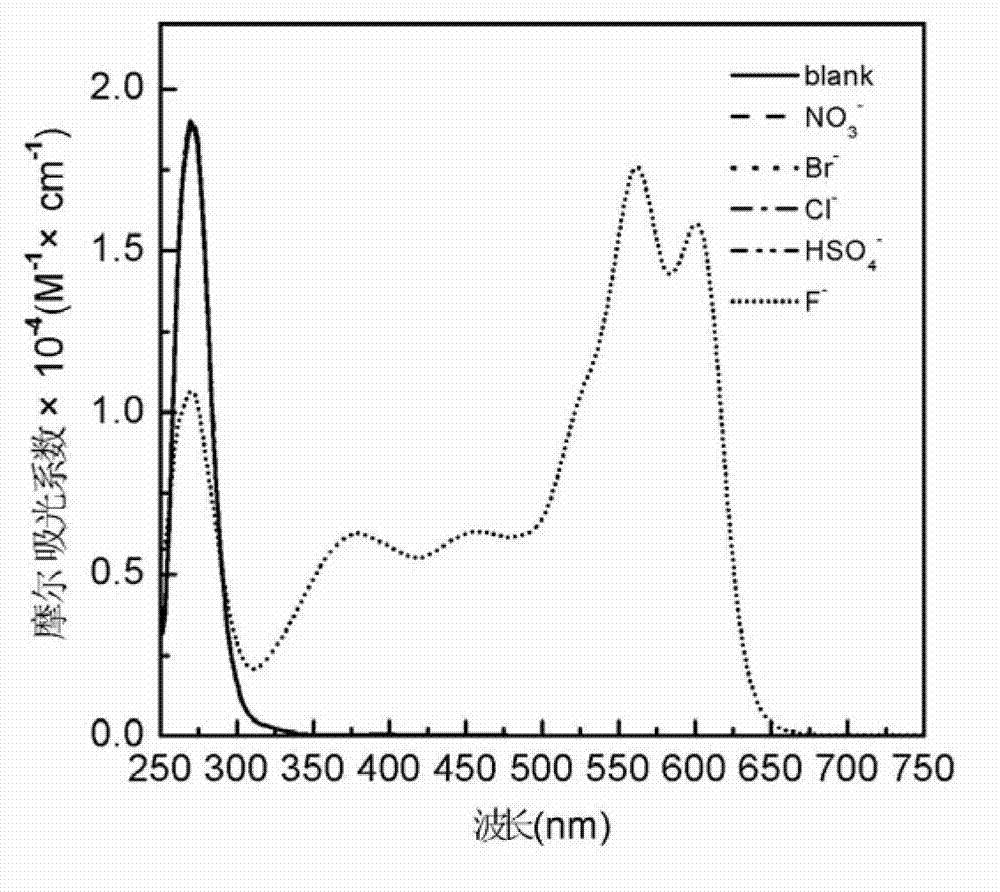
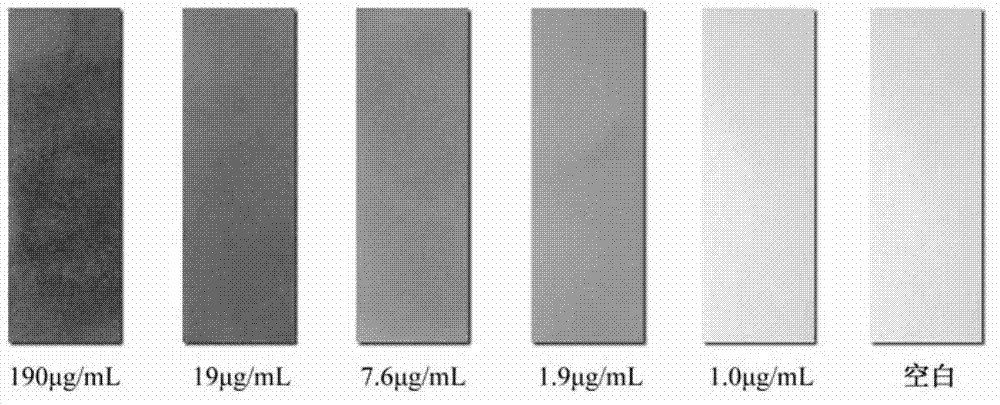
2-sulfohydantoin as well as preparation method and application thereof
A technology of thiohydantoin and p-nitrophenyl, which is applied in the field of sulfur-substituted heterocyclic compounds and heterocyclic compounds, can solve problems that have not been seen, and achieve the effect of easy portability and fast detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Compound Preparation
[0033] 3.62 g of 4-hydroxyphenylglycine methyl ester and 3.82 g of n-butyl isothiocyanate were added to a 100 mL flask, and then 30 mL of tetrahydrofuran was added. After reacting at room temperature for 12 hours, the solvent was distilled off under reduced pressure, and the residual solid was purified by a silica gel column with the composition of ethyl acetate:n-hexane=1:1 (v / v) to obtain 4.44 g of white solid. Yield 84%.
[0034] 2. Compound Characterization
[0035] Mp 291-292°C.
[0036] 1 H NMR (400MHz,THF-d 8 ):δ0.83~0.87(m,3H,CH3),1.09~1.24(m,4H,CH2CH2),3.42~3.56(m,2H,CH2),5.28(s,1H,CH),6.70~6.73( d,2H,Ar),7.53~7.55(d,2H,Ar),8.59(s,1H,OH),10.09(s,1H,NH).
[0037] 13 C NMR (100MHz,THF-d 8 ): δ13.08, 19.69, 29.24, 40.38, 61.44, 114.66, 121.75, 128.46, 158.54, 171.76, 182.36.
[0038] IR (cm -1 ,KBr):3263,3014,2362,2106,1588,1525,1409,1334,1251,1179,1013,930,843,735,698.
[0039] Anal. Calcd for C 13 h 16 N 2 o 2 S:C,59.07;H...
Embodiment 2
[0063] 4-(4’-Hydroxyphenyl)1-(4’-nitrophenyl)-2-thiohydantoin
[0064] 1. Preparation of 4-(4'-hydroxyphenyl)1-(4'-nitrophenyl)-2-thiohydantoin
[0065] Add 3.62 g (20 mmol) of 4-hydroxyphenylglycine methyl ester and 3.60 g (20 mmol) of p-nitrophenyl isothiocyanate into a 100 mL flask, and then add 40 mL of tetrahydrofuran. After reacting at room temperature for 10 hours, the solvent was distilled off under reduced pressure, and the residual solid was purified with a silica gel column under the condition that the eluent composition was ethyl acetate:n-hexane=1:2 (v / v) to obtain 5.40 g of white solid. Yield 82%.
[0066] 2. Characterization of 4-(4’-hydroxyphenyl)1-(4’-nitrophenyl)-2-thiohydantoin
[0067] 1 H NMR (400MHz, DMSO-d 6 ):δ5.47(s,1H,CH),6.70~6.73(d,2H,Ar),6.92~6.93(d,2H,Ar),7.64~7.66(d,2H,Ar),8.02~8.04( d, 2H Ar), 8.62 (s, 1H, OH), 10.21 (s, 1H, NH).
[0068] 13 C NMR (100MHz, DMSO-d 6 ): δ67.34, 114.66, 121.75, 123.71, 127.88, 128.46, 131.33, 147.55, 157.54...
Embodiment 3
[0077] 4-(4’-Hydroxyphenyl)1-(4’-cyanophenyl)-2-thiohydantoin
[0078] 1. Preparation of 4-(4'-hydroxyphenyl)1-(4'-cyanophenyl)-2-thiohydantoin
[0079] Add 3.62 g (20 mmol) of 4-hydroxyphenylglycine methyl ester and 3.20 g (20 mmol) of p-cyanophenyl isothiocyanate into a 100 mL flask, and then add 50 mL of tetrahydrofuran. After reacting at room temperature for 24 hours, the solvent was distilled off under reduced pressure, and the residual solid was purified by a silica gel column with the composition of ethyl acetate:n-hexane=1:3 (v / v) to obtain 5.32 g of white solid. Yield 86%.
[0080] 2. Characterization of 4-(4’-hydroxyphenyl)1-(4’-cyanophenyl)-2-thiohydantoin
[0081] 1 H NMR (400MHz, DMSO-d 6 ):δ5.52(s,1H,CH),6.67~6.69(d,2H,Ar),6.87~6.90(d,2H,Ar),7.53~7.55(d,2H,Ar),7.99~8.01( d, 2H, Ar), 8.55 (s, 1H, OH), 9.31 (s, 1H, NH).
[0082] 13 C NMR (100MHz, DMSO-d 6 ): δ63.44, 114.61, 122.15, 123.33, 127.92, 128.36, 133.48, 146.15, 157.04, 172.78, 180.22.
[0083] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com