Patents
Literature
34 results about "P-hydroxyphenylglycine methyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
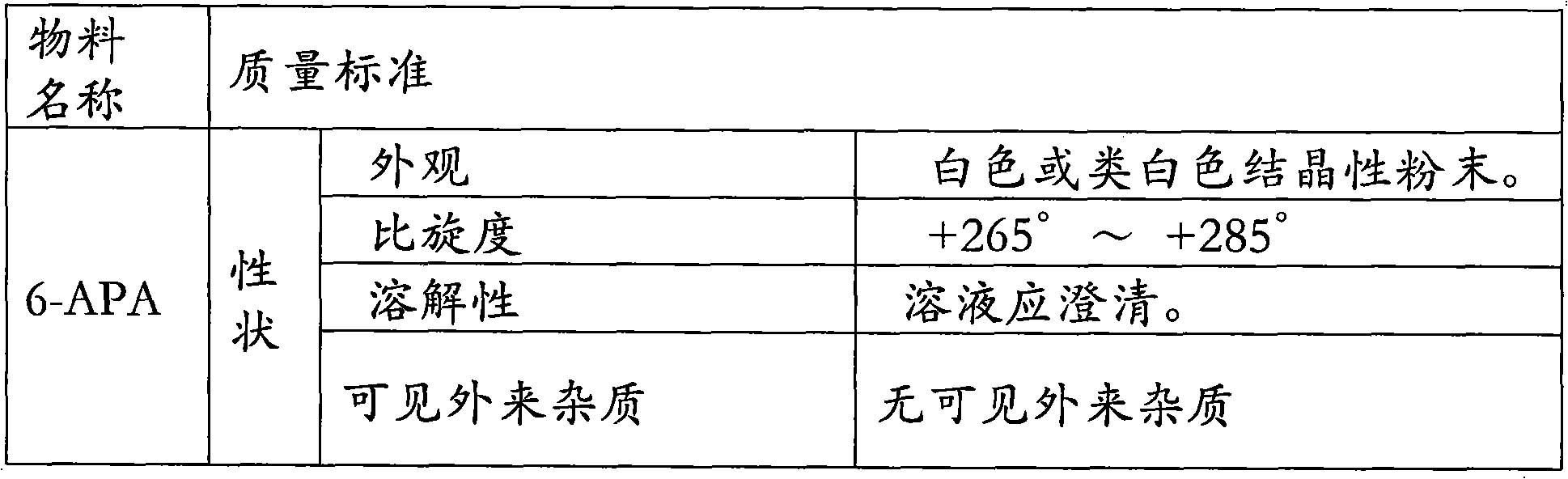
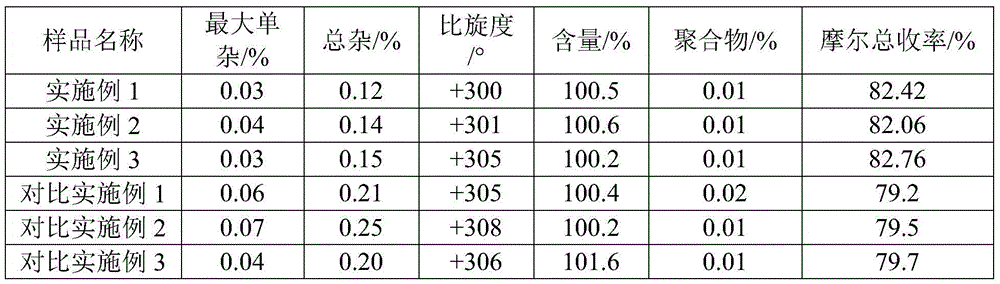
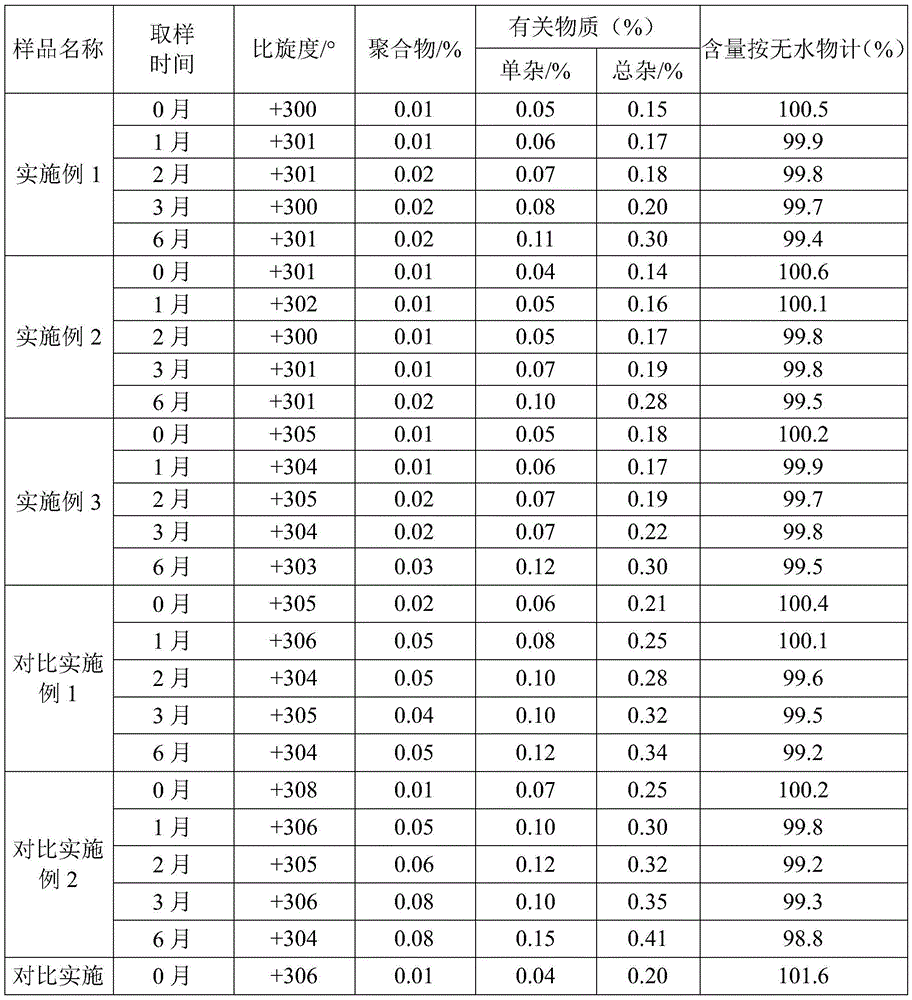
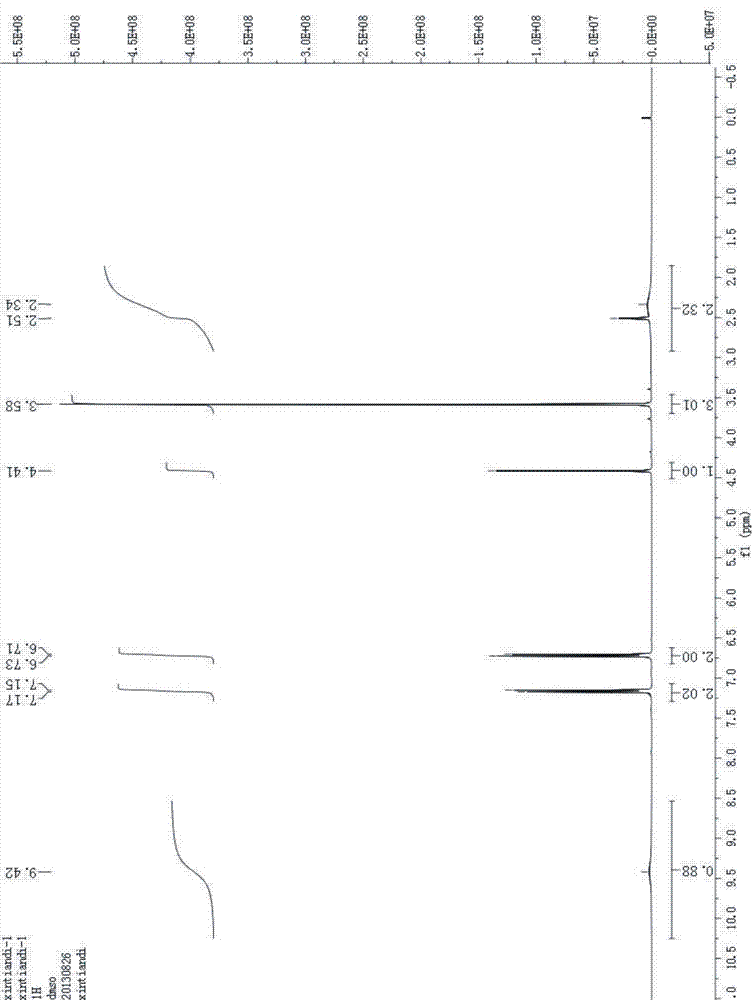
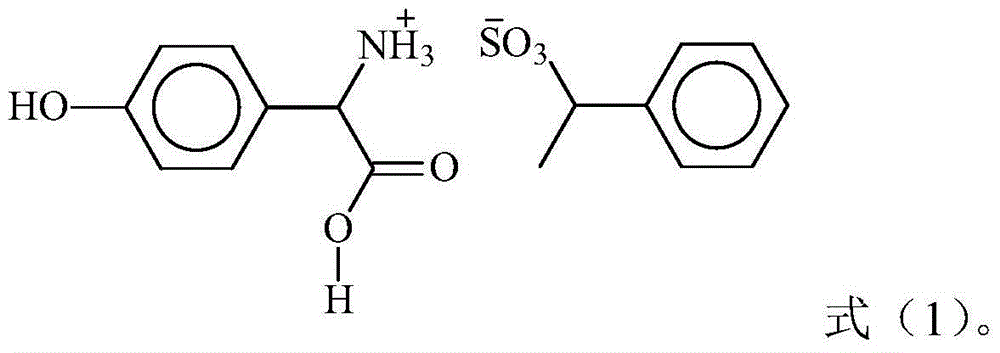
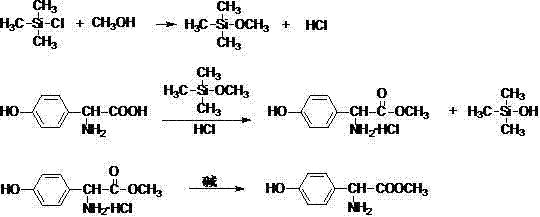
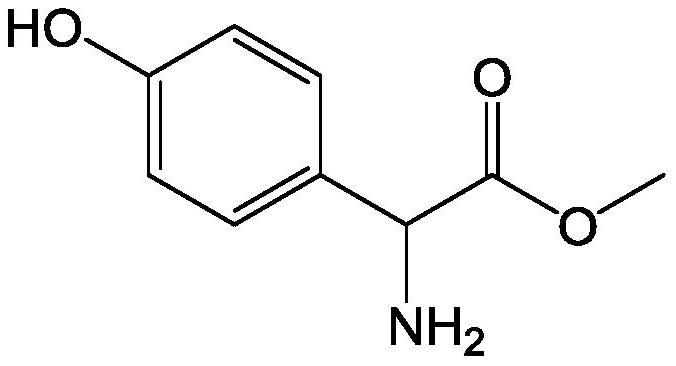
L-p-Hydroxyphenylglycine methyl ester hydrochloride 97% Synonym: (S)-Amino-(4-hydroxyphenyl) acetic acid methyl ester hydrochloride CAS Number 127369-30-6. Empirical Formula (Hill Notation) C 9 H 11 NO 3 · HCl . Molecular Weight 217.65 . MDL number MFCD01862153
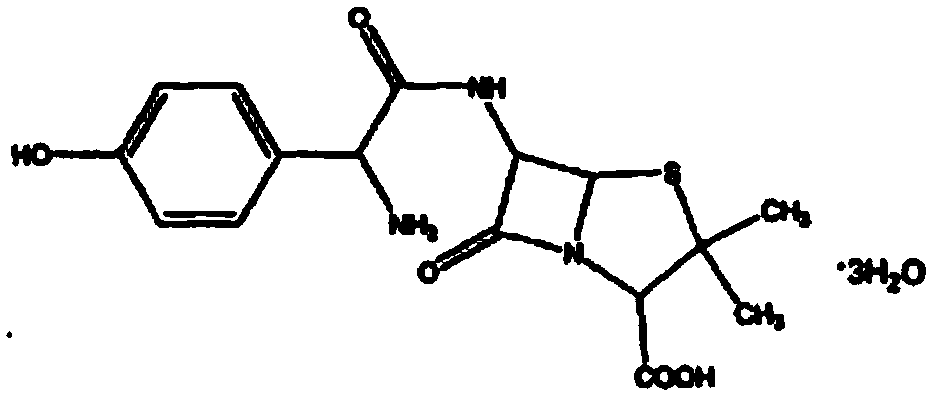
Improved method for preparing amoxicillin by enzymic method
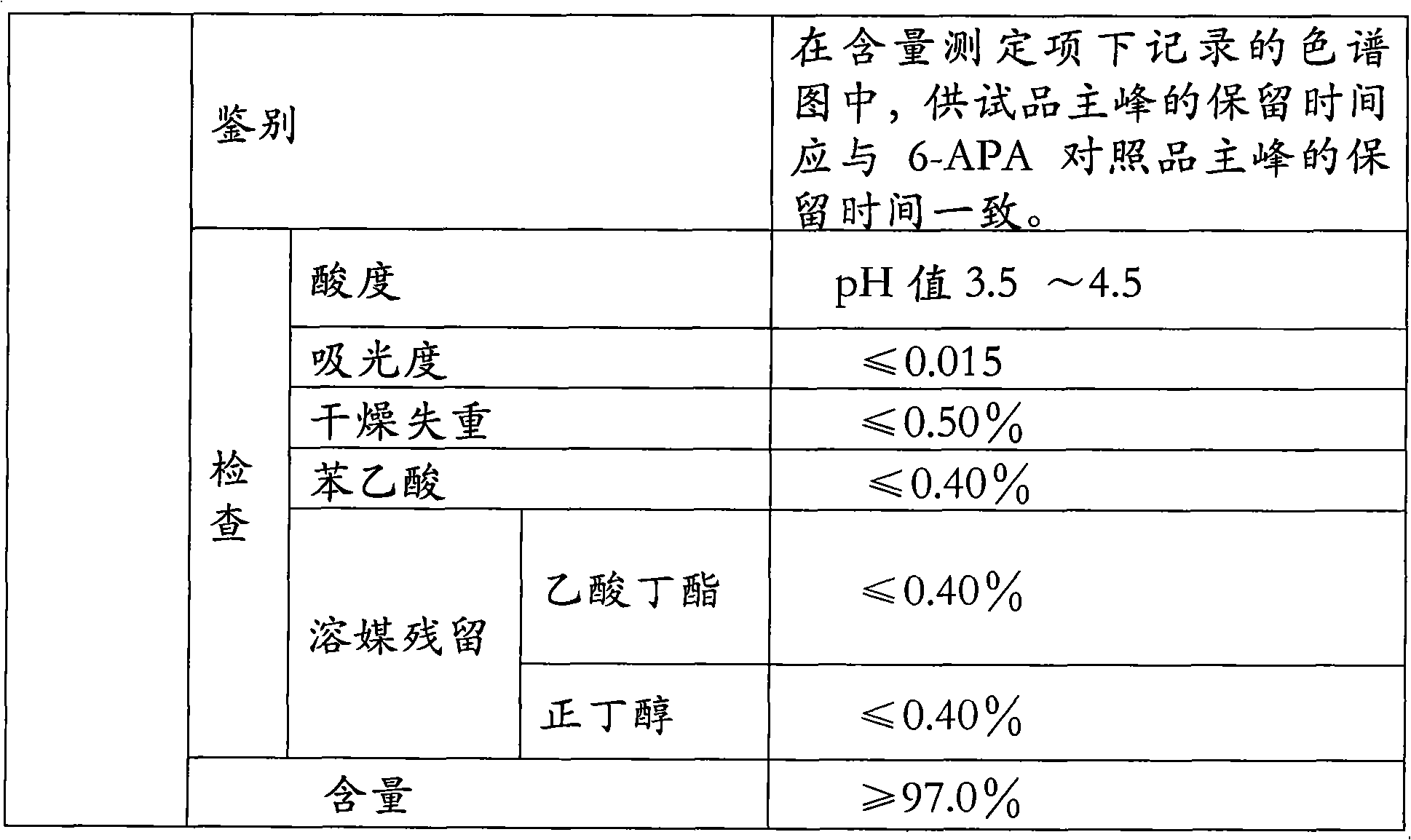
The invention relates to the field of pharmacy, and provides an improved method for preparing amoxicillin by an enzymic method, and a product obtained by the improved method for preparing amoxicillin by the enzymic method. The method comprises the following steps of: 1) dissolving 6-aminopenicillanic acid (6-APA) at the temperature of between 10 and 20 DEG C by using water or / and aqueous solution of ammonia which has the pH value of 7.0 to 8.0, and adding D-p-Hydroxyphenylglycine methyl ester hydrochlorid and penicillin G acyltransferase; 2) adjusting the pH value of a solution obtained in the step 1) to be 6.0 to 6.5, and reacting at the temperature of between 21 and 30 DEG C until the content of 6-APA is less than 5mg / ml to obtain a solution of an amoxicillin product; and 3) separating the penicillin G acyltransferase from the solution of the amoxicillin product, adjusting by using hydrochloric acid until the solution of the amoxicillin product is clarified, adding the aqueous solution of ammonia, adjusting the pH value to be 5.5 to 6.5, and crystallizing at the temperature of between 0 and 5 DEG C to obtain amoxicillin. By the improved method for preparing amoxicillin by the enzymic method, the quality of the amoxicillin product is greatly improved, and the medication safety of the amoxicillin product is further improved.
Owner:UNITED LAB INNER MONGOLIA CO LTD
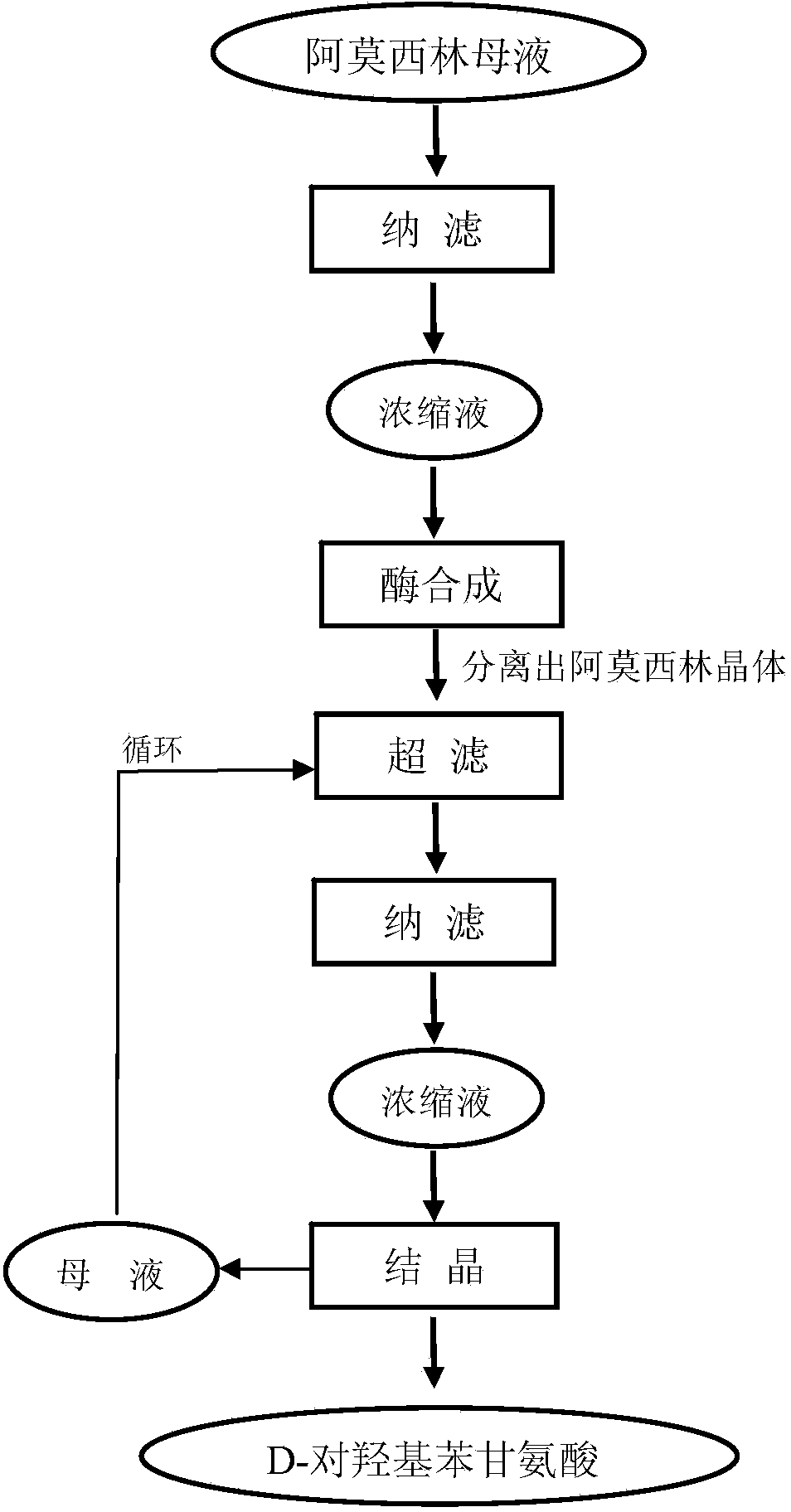
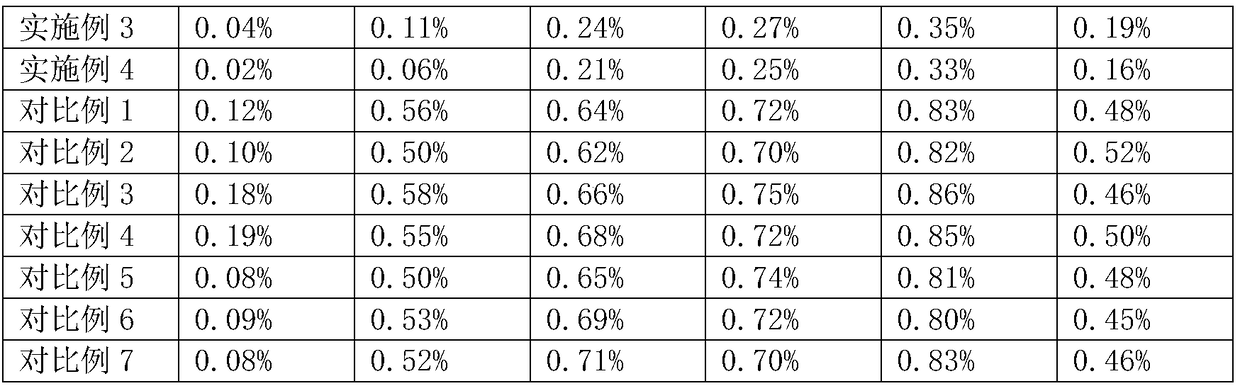
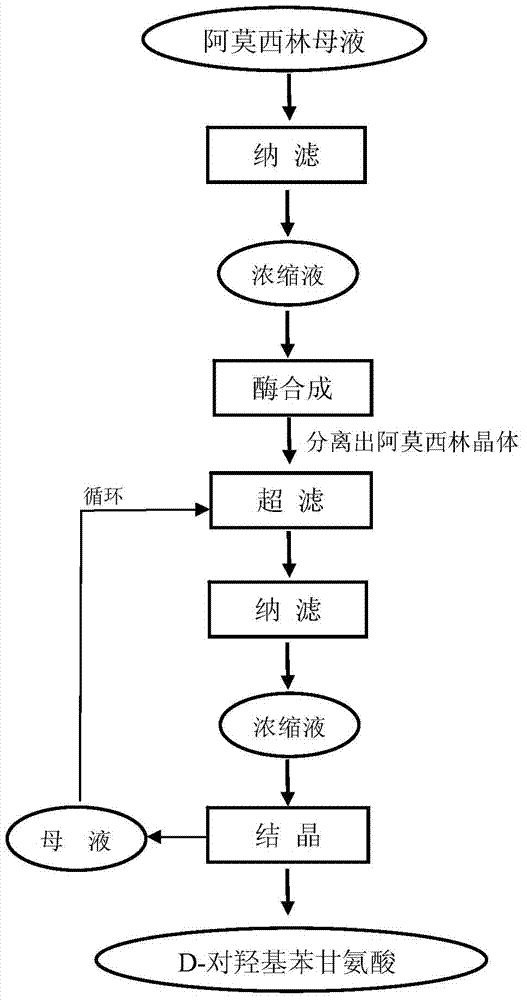
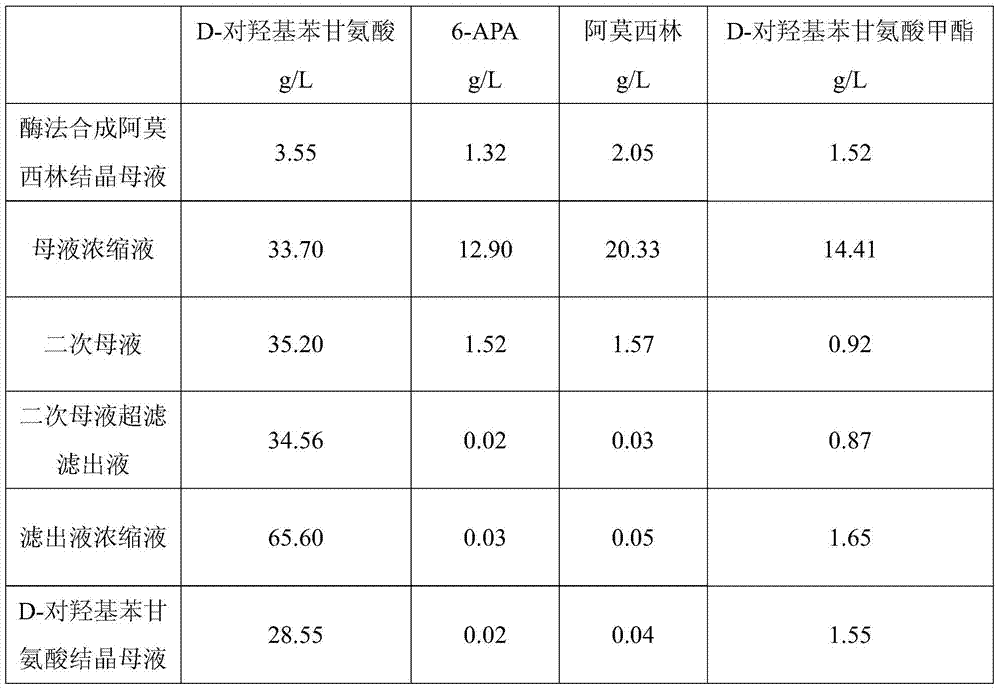
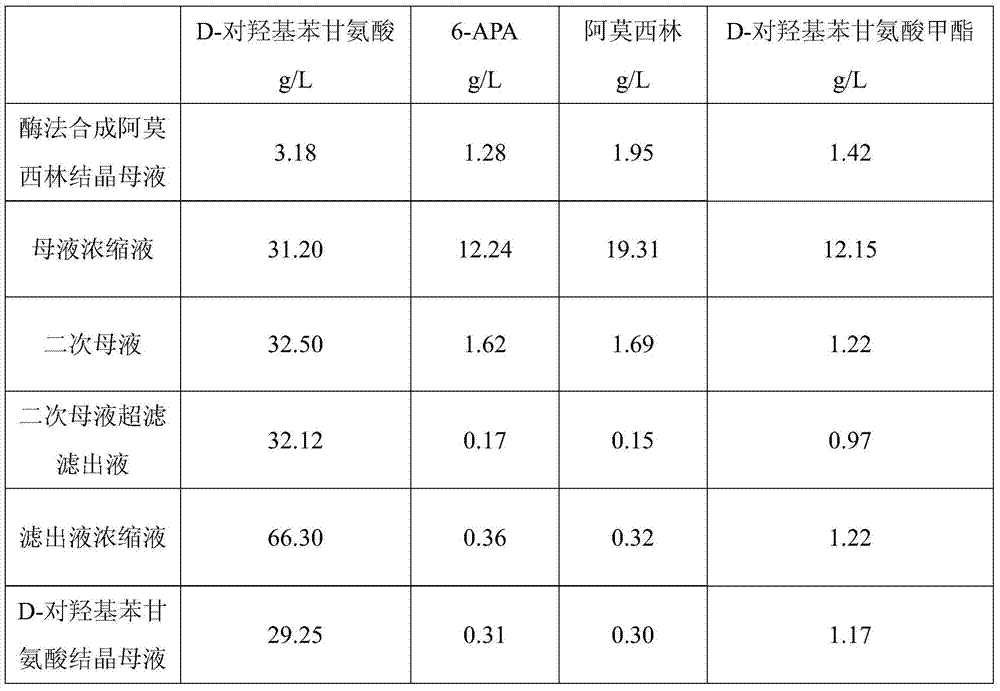
Method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by enzyme process
ActiveCN104357528AImprove product qualityCreate economic growthOrganic compound preparationAmino-carboxyl compound preparationUltrafiltrationChemistry
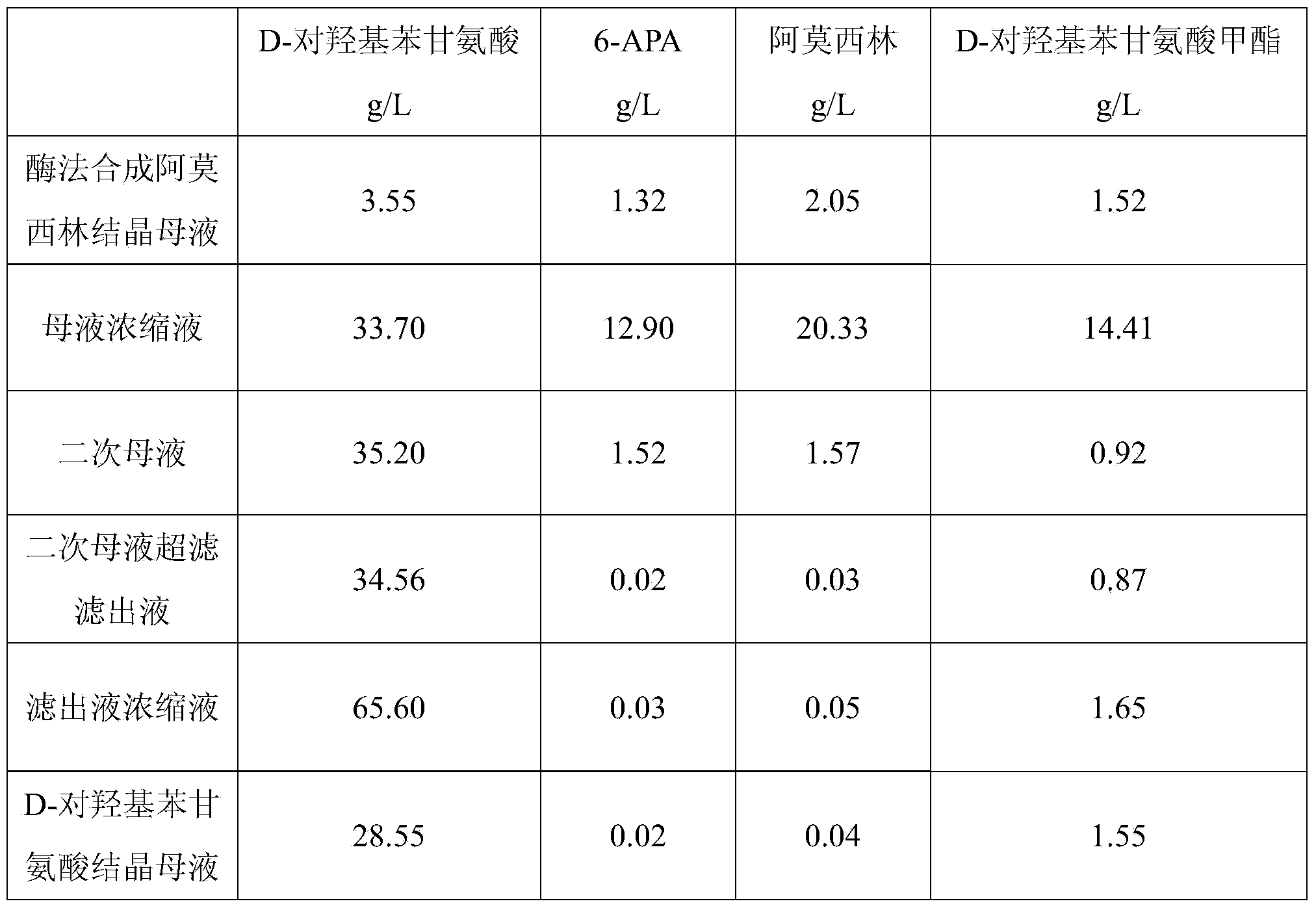
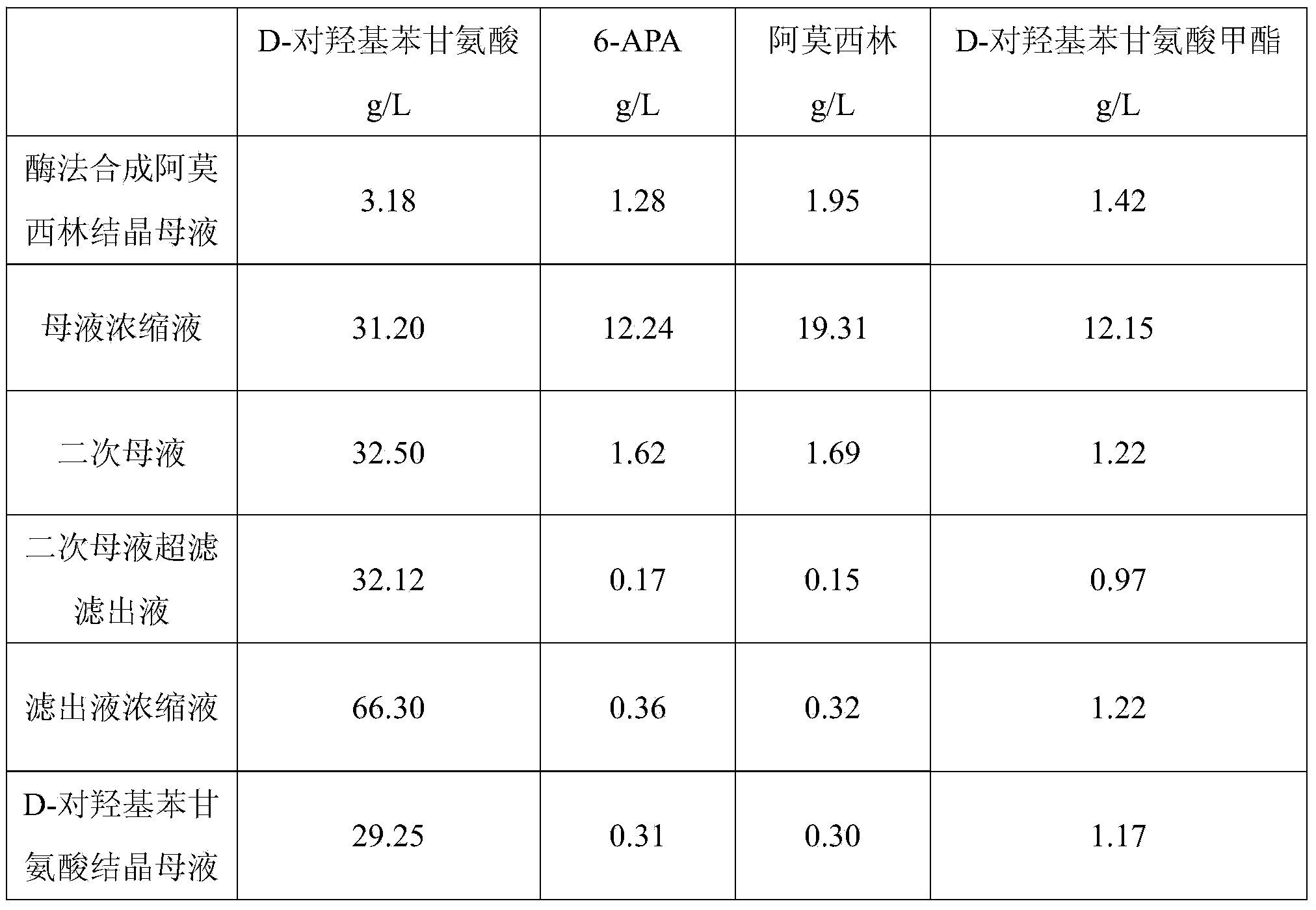
The invention relates to a method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by an enzyme process. The method comprises the following steps: (1) concentrating the amoxicillin mother liquid, namely adjusting the pH value of the amoxicillin mother liquid prepared by the enzyme process to be 8.0-9.5, and performing nanofiltration and concentration to obtain concentrated mother liquid; (2) synthesizing amoxicillin under enzyme catalysis, namely adjusting the pH value of the concentrated mother liquid to be 5.8-7.0, and converting 6-APA (6-amino penicillanic acid) and D-methyl hydroxyphenyl glycinate into amoxicillin in the presence of immobilized penicillin acylase for synthesis; (3) preparing D-hydroxyphenyl glycine concentrated liquid by ultrafiltration and nanofiltration, namely separating after the enzyme catalysis reaction is ended to obtain amoxicillin crystals and secondary amoxicillin mother liquid, and performing ultrafiltration and nanofiltration on the secondary mother liquid to obtain the D-hydroxyphenyl glycine concentrated liquid; (4) crystallizing D-hydroxyphenyl glycine. According to the method, 6-APA and D-methyl hydroxyphenyl glycinate remained in the mother liquid are consumed through an indirect process of synthesizing amoxicillin under enzyme catalysis, the product quality of D-hydroxyphenyl glycine is improved, and the yield of D-hydroxyphenyl glycine is increased.
Owner:SHANXI WEIQIDA PHARMA IND
Preparation method of D-para hydroxybenzene glycine methyl ester
InactiveCN103113250AHigh yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsDistillation
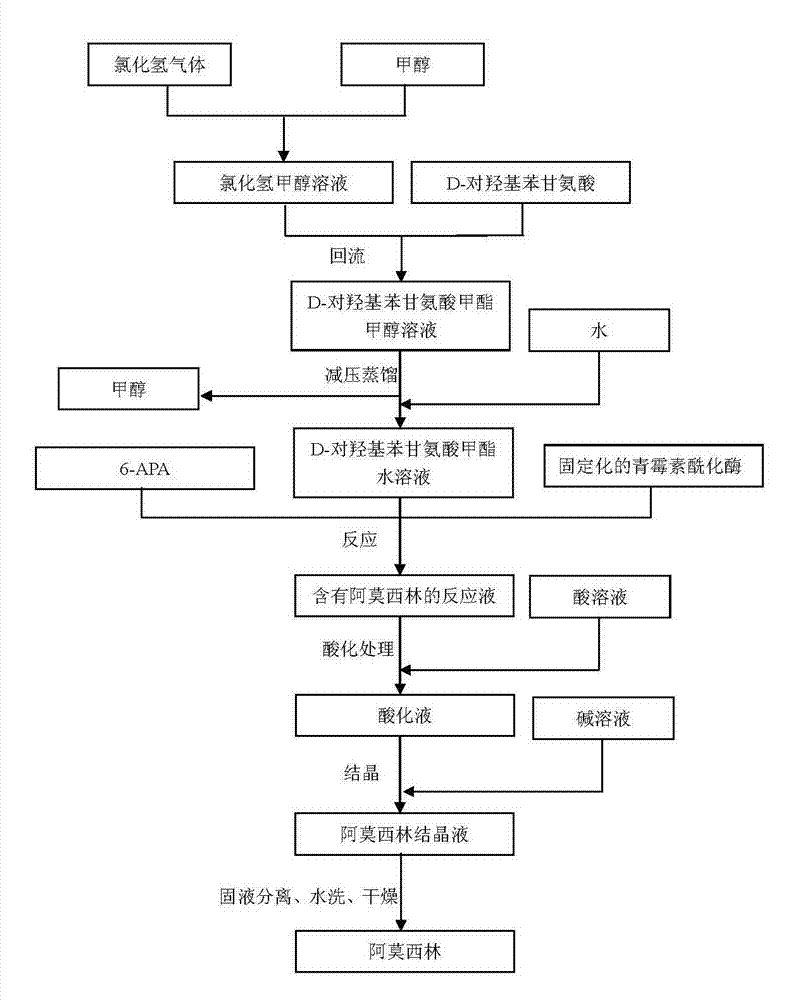
The invention provides a preparation method of D-para hydroxybenzene glycine methyl ester. The method comprises the following steps of: firstly, preparing a hydrochloric acid methanol solution; adding D-para hydroxybenzene glycine into the hydrochloric acid methanol solution to perform reflux reaction for 2-4 hours at 65-80 DEG C; performing pressure reduction distillation and removing methanol; and adding water to obtain a D-para hydroxybenzene glycine methyl ester aqueous solution. Based on that, the invention also provides anenzymatic synthesis method of amoxicillin. The method comprises the following steps of: adding 6-APA and immobilized penicillin acylase into the D-para hydroxybenzene glycine methyl ester aqueous solution to react for 1-8 hours at 10-30 DEG C; regulating the pH value of a reaction liquid to 0.8-1.0 by using hydrochloric acid or sulfuric acid aqueous solution; regulating the pH value to 4.5-6.0 by using ammonia water or sodium hydroxide aqueous solution to crystallize for 1-5 hours at 0-30 DEG C; separating solid from liquid; collecting a solid; and washing and drying to obtain amoxicillin. The method is simple in steps, and low in cost; and the obtained -para hydroxybenzene glycine methyl ester is high in yield and less in impurities and can be directly applied to anenzymatic synthesis of amoxicillin.
Owner:NORTH CHINA PHARM GRP SEMISYNTECH CO LTD
Process for direct preparation of amoxicillin by liquid 6-APA (amino penicillanic acid)
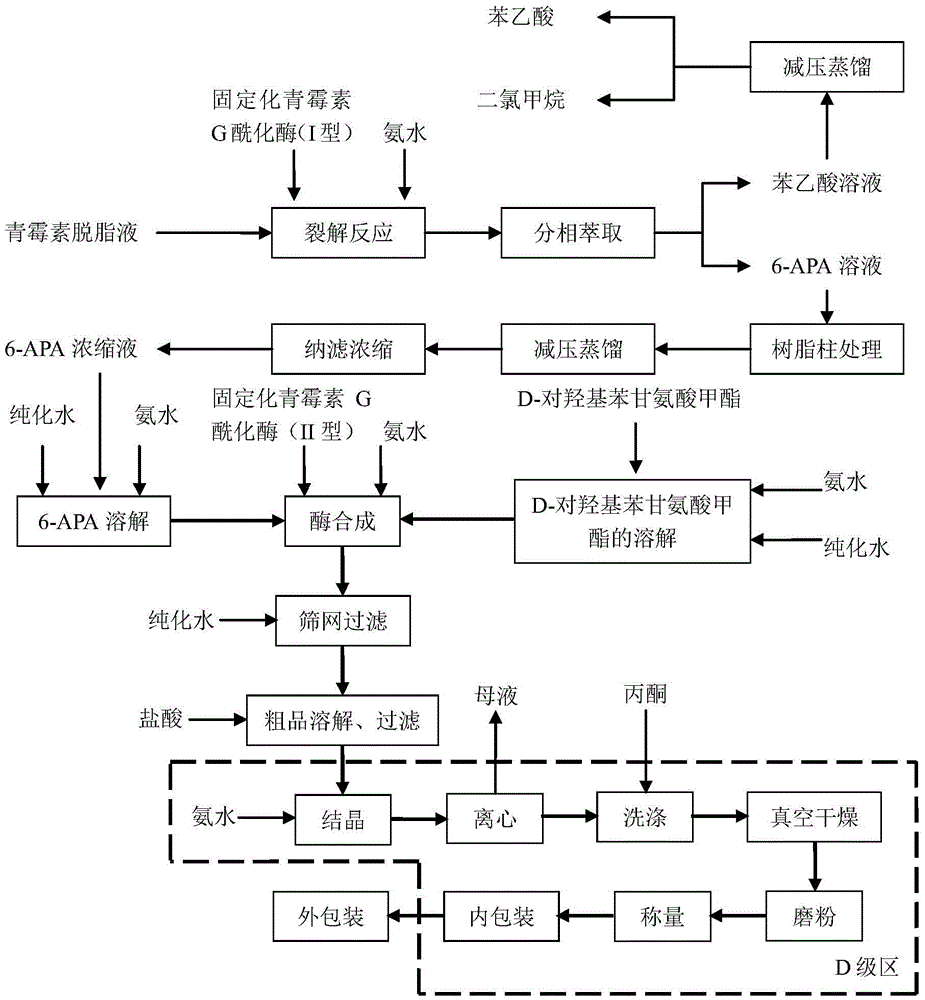
The invention belongs to the technical field of medicine preparation and relates to a process for direct preparation of amoxicillin by liquid 6-APA (amino penicillanic acid). The process includes: taking penicillin degreasing solution as an initial material, sequentially performing cracking reaction, extraction, phase splitting, resin column adsorptive purification, distillation and concentration to obtain a 6-APA solution with the concentration being 80-100g / L, and synthesizing with p-hydroxyphenylglycine methyl ester under a catalytic action of type-II penicillin G acylase to obtain the amoxicillin. Compared with a traditional method, the process has the advantages that subsequent steps of 6-APA crystallization, centrifuging, drying and the like are avoided, investment of fixed assets is reduced, energy loss, equipment loss and cost are reduced, profits are increased, and physical injuries of staffs are reduced. Compared with existing direct amoxicillin preparation methods, the process has the advantages that by adoption of dichloromethane as an extracting agent, total mole yield of amoxicillin is higher relatively, the extracting agent is easy for distillation separation, the content of residual solvents in products is greatly reduced, medication safety is improved, and the process is worthy of popularization in production.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Method of synthesizing cefadroxil by enzyme process
The invention relates to a method of synthesizing cefadroxil by an enzyme process. The method comprises the following steps of: by taking 7-ADCA as an initial raw material, performing a reaction on D-tyrosine methyl ester or D-p-hydroxyl phenylglycine ethyl ester and 7-ADCA in water by directly inputting the solid in the presence of penicillin acylase at 10-25 DEG C; after reaction, separating a cefadroxil coarse product and enzyme reaction mother liquor; further purifying the cefadroxil coarse product to obtain a white cefadroxil product; and adding beta-naphthol or 2,7-dioxynaphthalene into the enzyme reaction mother liquor to obtain a cefadroxil compound. Cefadroxil can be further treated and recovered from the cefadroxil compound, so that the recovery rate of cefadroxil is increased. The product obtained by the method is high in yield and purity. The product is white in appearance, multi-step reaction of a chemical process and various solvents and auxiliary materials are not needed, and green synthesis of cefadroxil is realized.
Owner:苏州盛达药业有限公司 +1
Synthesis method of p-hydroxyphenylglycine methyl ester
ActiveCN102718672AHigh purityReduce pollutionOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsSolid acid
The invention discloses a synthesis method of p-hydroxyphenylglycine methyl ester. The method comprises the steps of: reacting p-hydroxyphenylglycine or salt thereof which is used as an initial raw material with methanol in the presence of solid acid; and treating so as to obtain p-hydroxyphenylglycine methyl ester. The preparation method of p-hydroxyphenylglycine methyl ester is available in raw material, simple and convenient to operate, low in cost, high in yield, high in product purity, and is suitable for commercial production.
Owner:SHANDONG HANXING PHARM TECH CO LTD +2
Synthetic method of D-p-hydroxyphenylglycine methyl ester
InactiveCN104744281AAvoid the risk of hydrolysisReduce processOrganic compound preparationAmino-carboxyl compound preparationGlycineBiochemical engineering
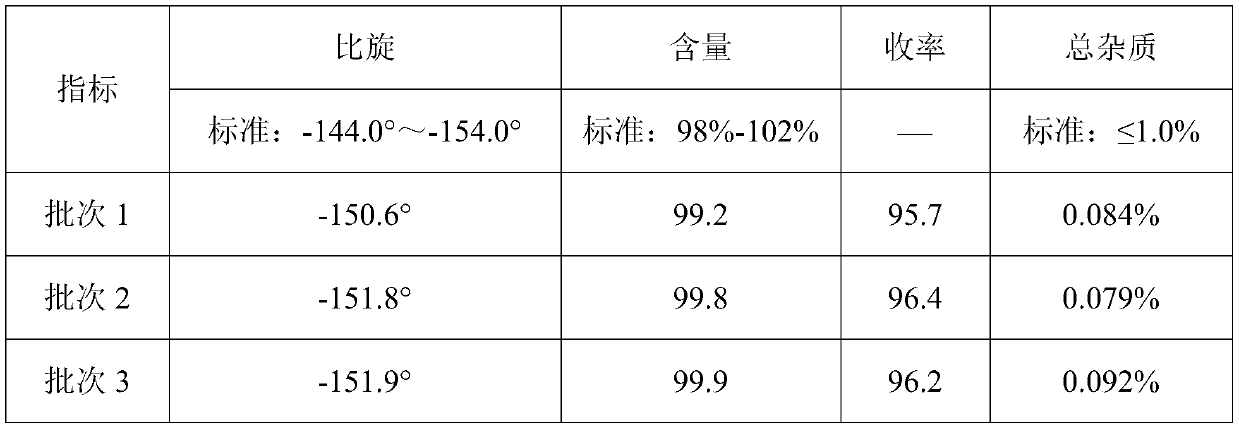
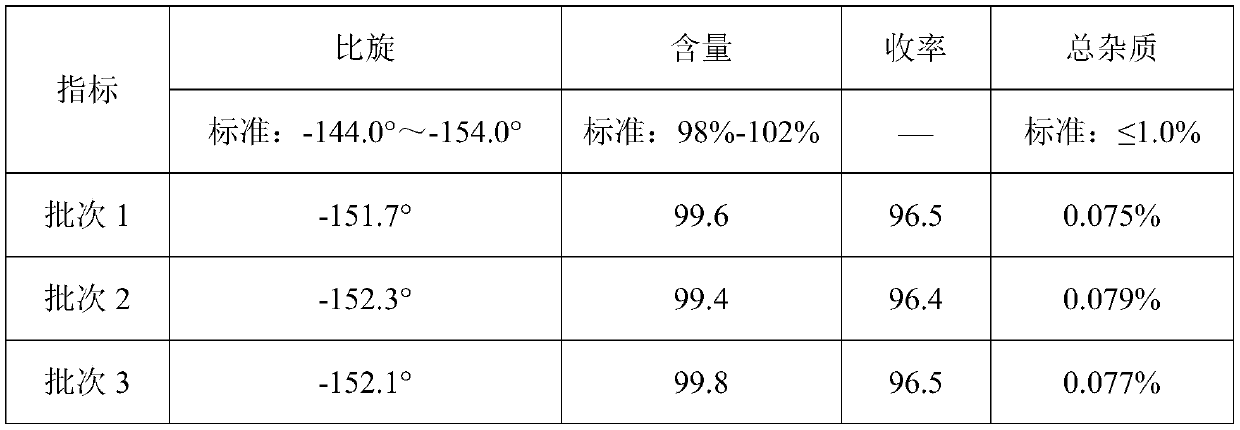
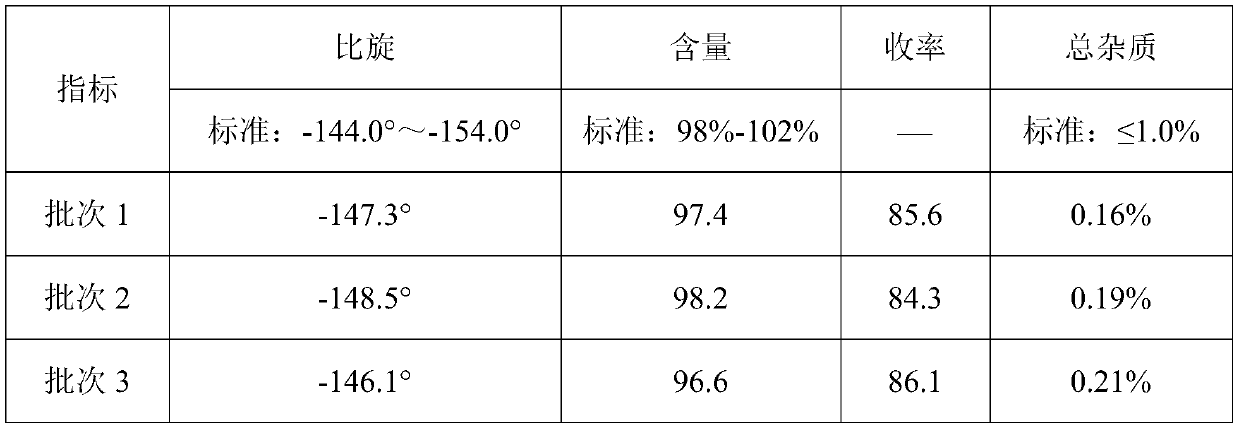
The invention relates to the medicine field, and especially relates to a synthetic method of D-p-hydroxyphenylglycine methyl ester. The invention provides a synthetic method by directly using d-p-hydroxyphenylglycine as an initial raw material without splitting and separation, a target compound D-p-hydroxyphenylglycine methyl ester can be obtained through one-pot synthesis, a technology process is shortened, and hydrolysis risk of ester in a long production process can be avoided. According to the invention, yield can reach more than 96%, the ee value of D-p-hydroxyphenylglycine methyl ester can reach more than 99%, and the quality index is excellent.
Owner:HENAN NEWLAND PHARMA
Preparation method of D-p-hydroxyphenylglycine methyl ester
PendingCN111153821AGuaranteed yieldGuaranteed quality indicatorsOrganic compound preparationOrganic chemistry methodsMethanolEster sulfate
Owner:SHANXI WEIQIDA PHARMA IND
Method for preparing cefprozil in pH responsive regenerative double aqueous phase system
PendingCN106939327AIncreased molar yieldEasy to separateFermentationChemical synthesisCentrifugation
The invention relates to a method for preparing cefprozil in a pH responsive regenerative double aqueous phase system. The method comprises (1) preparing two double-aqueous phase systems, (2) orderly adding 7-APRA, D-p-hydroxyphenylglycine methyl ester hydrochloride into the systems, adjusting solution pH to 5.00-6.50 and controlling a solution temperature in a range of 10-30 DEG C, (3) adding immobilized penicillin acylase into the solution obtained by the step (2), and (4) standing the mixed solution, removing the immobilized penicillin acylase, adjusting the pH of the reaction solution, recovering P<ADBA> / <PMDB> and P<ADB> / P<MDB> polymers of the double-aqueous phase systems, feeding the supernatant to a crystallization section, carrying out crystallization, and carrying out centrifugation, washing and drying to obtain a product. The method reduces a chemical synthesis cost, improves a low product conversion rate of the monohydrolase catalytic reaction, effectively improves a yield, simplifies the operation, reduces a cost and realizes easy recovery of the double-aqueous phase systems.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for synthesizing D-p-hydroxyphenylglycine methyl ester
ActiveCN104892444AHigh yieldOrganic compound preparationAmino-carboxyl compound preparationHydrogenEsterification reaction
The invention relates to the field of compound synthesis and discloses a method for synthesizing D-p-hydroxyphenylglycine methyl ester. The method comprises steps as follows: (1), in the presence of thionyl chloride, D-p-hydroxyphenylglycine resolving agent salt and methanol have an esterification reaction, and D-p-hydroxyphenylglycine methyl ester resolving agent salt is obtained; (2), the D-p-hydroxyphenylglycine methyl ester resolving agent salt and alkaline metal hydroxide are dropwise added to a D-p-hydroxyphenylglycine methyl ester aqueous solution at the temperature of 10-15 DEG C, the pH (potential of hydrogen) value of a system is controlled in the range from 6.5 to 7 in the dropwise adding process, after dropwise adding of the D-p-hydroxyphenylglycine methyl ester resolving agent salt is completed, alkaline metal hydroxide is continuously dropwise added until the pH value of the system ranges from 7.5 to 8, a crystal is grown at the temperature of 10-15 DEG C and under the condition of the pH value being 7.5-8, an obtained crystalline liquid is filtered, and a D-p-hydroxyphenylglycine methyl ester crystal and a mother liquor are obtained. With adoption of the method provided by the invention, D-p-hydroxyphenylglycine methyl ester with a higher yield can be produced.
Owner:山西双雁生物科技有限公司
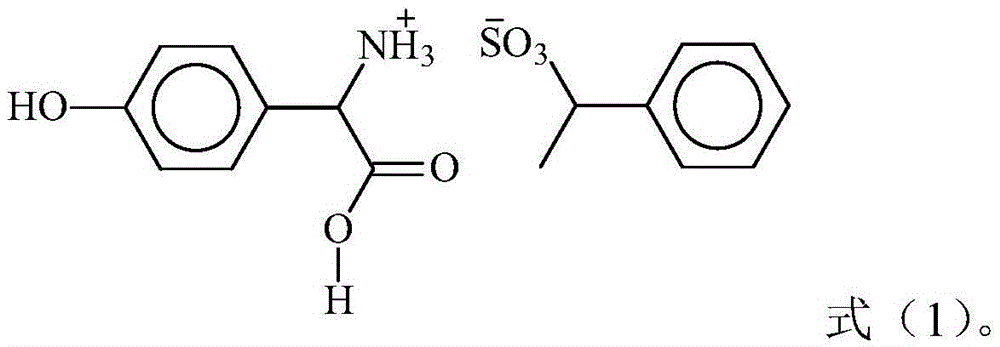
2-sulfohydantoin as well as preparation method and application thereof
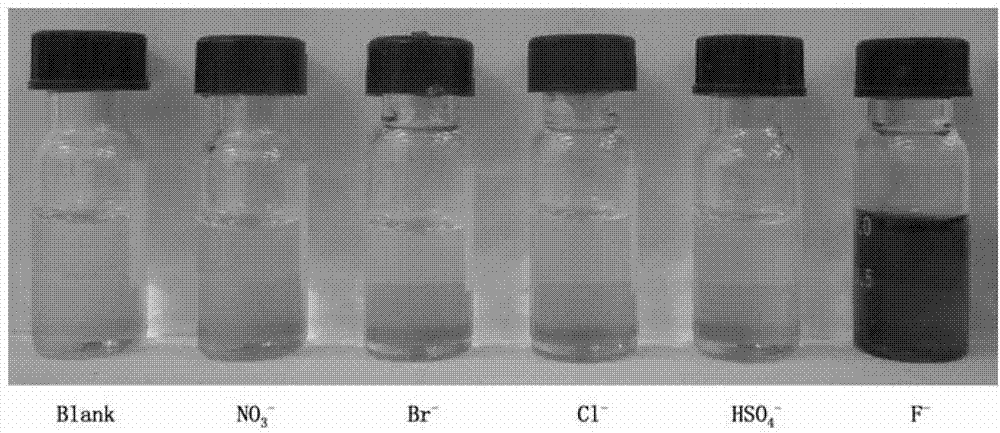
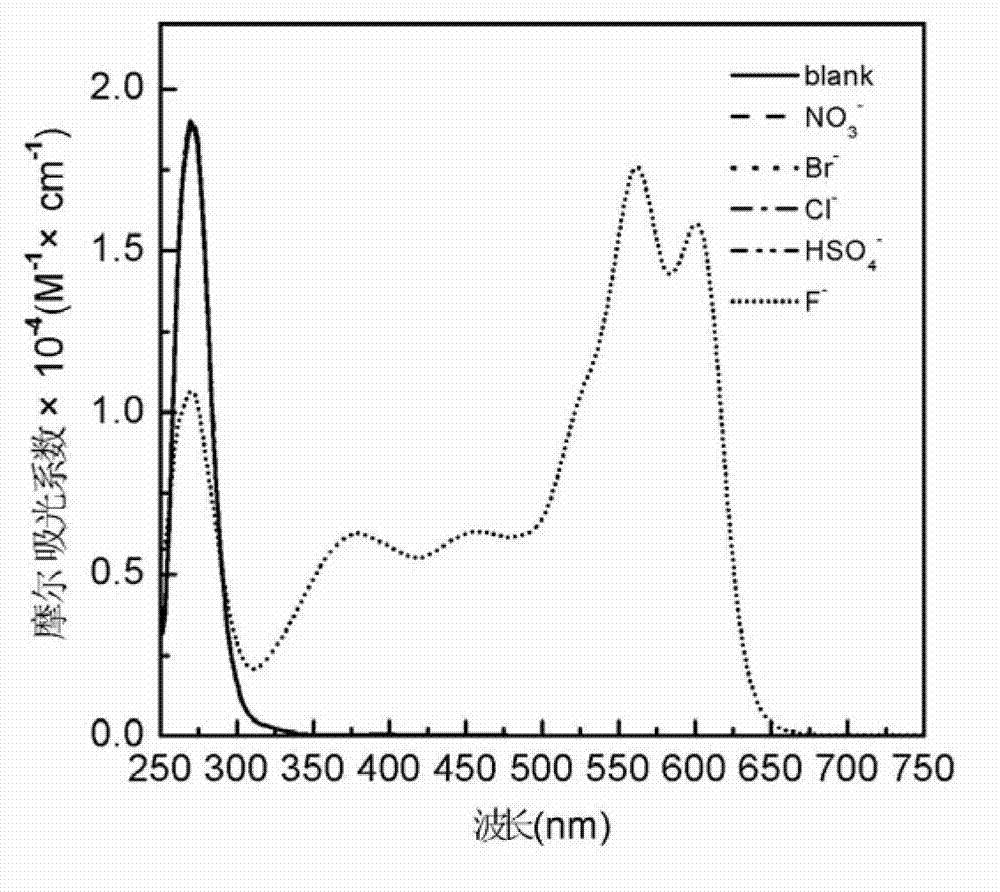
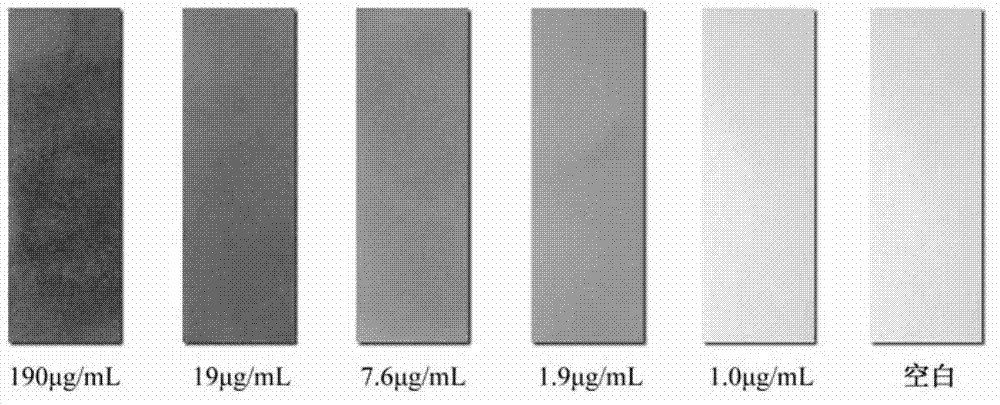
InactiveCN102924383AEasy to carryQuick checkOrganic chemistryMaterial analysis by observing effect on chemical indicatorChemical structurePhenylglycine methyl ester
The invention relates to a sulfur-containing heterocyclic compound. The compound is 2-sulfohydantoin; a chemical structure of the 2-sulfohydantoin is shown in a formula (I) in the specification; and in the formula (I), R is one of C1-12 alkyl, p-nitrophenyl, p-cyano phenyl, p-methoxyphenyl, p-butyl phenyl, 3,5-di(trifluoromethyl)phenyl and phenyl. The 2-sulfohydantoin is obtained by reacting isothiocyanate with para hydroxy phenylglycine methyl ester, has an anion recognition performance, and can be used for detecting fluorinion.
Owner:SOUTHERN MEDICAL UNIVERSITY
Method for synthesizing amoxicillin and generating byproduct of sodium phenylacetate solution by using semi-direct method
InactiveCN107988306AHigh yieldImprove product qualityOrganic compound preparationCarboxylic acid salt preparationPhenylacetic acidBenzylpenicillin potassium
The invention relates to a method for synthesizing amoxicillin and generating a byproduct of a sodium phenylacetate solution by using a semi-direct method and belongs to the technical field of medicine preparation. The method comprises the following steps: splitting benzylpenicillin potassium into 6-APA (Amino Penicillanic Acid) and phenylacetic acid under the action of penicillin acylase, extracting a splitting solution with dichloromethane to separate 6-APA from the phenylacetic acid, putting ammonia water into an extraction water phase, adjusting the pH value of a material liquid to be neutral, removing the dichloromethane, putting the solid 6-APA into the obtained 6-APA dissolving liquid, and synthesizing the amoxicillin together with D-methyl p-hydroxyphenylglycinate under the actionof amoxicillin synthetase. A sodium phenylacetate solution can be prepared by alkalizing the dichloromethane obtained by extracting the splitting solution, the sodium phenylacetate solution can be directly applied to fermentation production of penicillin, the step of recycling the phenylacetic acid is avoided, and the production cost is reduced.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Preparation method of methyl D-4-hydroxy-phenylglycinate and hydrochloride thereof
InactiveCN103641729AHigh purityReduce pollutionOrganic compound preparationAmino-carboxyl compound preparationPollutionMethanol
The invention discloses a preparation method of D-4-hydroxy-phenylglycinate and hydrochloride thereof. D-4-hydroxyphenylglycine is used as an initial raw material. The methyl D-4-hydroxy-phenylglycinate or the hydrochloride thereof is prepared by reacting the D-4-hydroxyphenylglycine with methanol or a methanol solution of hydrogen chloride under the existence of trimethylchlorosilane and by post-treatment. The preparation method has characteristics of easily available raw materials, simple operation, high yield, high product purity and little environment pollution, and is suitable for industrial production.
Owner:河北爱弗特精细化工有限责任公司
Synthetic method of p-hydroxyphenylglycine methyl ester
PendingCN112830882AReduce consumptionPrevent oxidationOrganic compound preparationOrganic chemistry methodsActivated carbonGlycine
The invention provides a synthetic method of p-hydroxyphenylglycine methyl ester. The synthetic method comprises the following steps: (1) adding D-p-hydroxyphenylglycine into methanol, conducting uniform stirring, and then dropwise adding thionyl chloride at 30-50 DEG C to obtain a methanol solution of D-p-hydroxyphenylglycine methyl ester; (2) decompressing the methanol solution of D-p-hydroxyphenylglycine methyl ester to obtain methanol under the condition that a vacuum degree is less than or equal to 0.090 MPa, then adding water, performing stirring, and carrying out decolorizing with activated carbon under an acidic condition to obtain an aqueous solution of the D-p-hydroxyphenylglycine methyl ester; and (3) dropwise adding 15-20wt% of ammonia water into the aqueous solution of D-p-hydroxyphenylglycine methyl ester to adjust a pH value to 9-10, wherein the dropwise adding time of the ammonia water is 3-6 hours, subjecting D-p-hydroxyphenylglycine methyl ester to crystallization and precipitation, and finally, carrying out centrifugal separation to obtain a D-p-hydroxyphenylglycine methyl ester product. The D-p-hydroxyphenylglycine methyl ester prepared by the method disclosed by the invention is high in recovery rate and high in purity.
Owner:河南绿园药业有限公司
Preparation method of methyl D-4-hydroxy-phenylglycinate and hydrochloride thereof
InactiveCN103641729BHigh purityReduce pollutionOrganic compound preparationAmino-carboxyl compound preparationTrimethylsilyl chlorideP-hydroxyphenylglycine
The invention discloses a preparation method of D-4-hydroxy-phenylglycinate and hydrochloride thereof. D-4-hydroxyphenylglycine is used as an initial raw material. The methyl D-4-hydroxy-phenylglycinate or the hydrochloride thereof is prepared by reacting the D-4-hydroxyphenylglycine with methanol or a methanol solution of hydrogen chloride under the existence of trimethylchlorosilane and by post-treatment. The preparation method has characteristics of easily available raw materials, simple operation, high yield, high product purity and little environment pollution, and is suitable for industrial production.
Owner:河北爱弗特精细化工有限责任公司
A kind of synthetic method of amoxicillin production intermediate
ActiveCN114105795BOvercoming serious pollution defectsHigh yieldPhysical/chemical process catalystsOrganic compound preparationO-Phosphoric AcidPtru catalyst
The present invention relates to a kind of synthesis method of amoxicillin production intermediate, use DL-p-hydroxyphenylglycine and methanol as raw materials, and use solid phosphoric acid as catalyst to synthesize D-p-hydroxyphenylglycine methyl ester, including preparation of solid phosphoric acid catalyst, DL-p-hydroxyphenylglycine Preparation of p-hydroxyphenylglycine methyl ester, hydrolysis of solid phosphoric acid catalyst, crystallization of D-p-hydroxyphenylglycine methyl ester and racemization of crystallization mother liquor in 5 parts. In the present invention, the solid phosphoric acid catalyst can be hydrolyzed into phosphoric acid after the esterification is completed, and forms a phosphoric acid double salt with DL-p-hydroxyphenylglycine methyl ester. ‑Resolving agent for methyl p-hydroxyphenylglycine. In the present invention, the synthesis and resolution of DL-p-hydroxyphenylglycine methyl ester is carried out in methanol aqueous solution, and the crystallization mother liquor is recycled, which greatly reduces the generation of waste liquid, and is a clean production process of D-p-hydroxyphenylglycine methyl ester.
Owner:TIANJIN HANRUI PHARMA +1
A method for synthesizing d-p-hydroxyphenylglycine methyl ester
ActiveCN104892444BHigh yieldOrganic compound preparationAmino-carboxyl compound preparationP-hydroxyphenylglycineEsterification reaction
The present invention relates to the field of compound synthesis and discloses a method for synthesizing D-para-hydroxyphenylglycine methyl ester. The method comprises the steps of: (1) in the presence of thionyl chloride, carrying out an esterification reaction of D-para-hydroxyphenylglycine resolving agent salt and methanol to obtain a D-para-hydroxyphenylglycine methyl ester resolving agent salt; (2) dropwise adding the D-para-hydroxyphenylglycine methyl ester resolving agent salt and an alkaline metal hydroxide to an aqueous D-para-hydroxyphenylglycine methyl ester solution at 10-15°C, controlling the pH value of the system at 6.5-7 during the dropwise addition, after completing the dropwise addition of the D-para-hydroxyphenylglycine methyl ester resolving agent salt, continuing the dropwise addition of the alkaline metal hydroxide until the pH value of the system is 7.5-8, then growing crystals under the conditions of a temperature of 10-15°C and the pH value of 7.5-8, and subsequently filtering the resultant crystallization liquid to obtain D-para-hydroxyphenylglycine methyl ester crystals and a mother liquor. With the adoption of the method provided by the present invention, D-para-hydroxyphenylglycine methyl ester can be produced with a higher yield.
Owner:山西双雁生物科技有限公司
Method for synthetizing amoxicillin through enzymatic method
The invention discloses a method for synthetizing amoxicillin through an enzymatic method. The method comprises the following steps: (1) immobilized penicillin G acylase is washed through distilled water and then soaked through hydrochloric acid; (2) Methyl p-hydroxyphenylglycinate and 6-APA are evenly mixed and then soaked through hydrochloric acid, and then purified water is added; (3) the immobilized penicillin G acylase, the Methyl p-hydroxyphenylglycinate and the 6-APA are mixed and then react to obtain an amoxicillin suspension; and (4) the amoxicillin suspension is cooled, hydrochloricacid is dripped till the solution is clear, gain growing is conducted after suction filtration, washing and drying are conducted, and then the amoxicillin product is obtained. In the reaction process,the immobilized penicillin G acylase is soaked by the hydrochloric acid for a short time, thus the reaction speed is increased, the reaction time is shortened, the yield of the product is increased,and the purity of the product is improved; and sucrose and histidine with the mass ratio being 3 to 1 are added in a reaction system, and thus the stability of the product is improved.
Owner:GUANGZHOU LIXIN PHARM CO LTD +1
A kind of synthetic method of p-hydroxyphenylglycine methyl ester
ActiveCN102718672BHigh purityReduce pollutionOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsP-hydroxyphenylglycine
The invention discloses a synthesis method of p-hydroxyphenylglycine methyl ester. The method comprises the steps of: reacting p-hydroxyphenylglycine or salt thereof which is used as an initial raw material with methanol in the presence of solid acid; and treating so as to obtain p-hydroxyphenylglycine methyl ester. The preparation method of p-hydroxyphenylglycine methyl ester is available in raw material, simple and convenient to operate, low in cost, high in yield, high in product purity, and is suitable for commercial production.
Owner:SHANDONG HANXING PHARM TECH CO LTD +2
Method for Comprehensive Recovery of Active Components in Enzymatic Amoxicillin Mother Liquor
ActiveCN104357528BImprove product qualityCreate economic growthOrganic compound preparationAmino-carboxyl compound preparationUltrafiltrationBULK ACTIVE INGREDIENT
The invention relates to a method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by an enzyme process. The method comprises the following steps: (1) concentrating the amoxicillin mother liquid, namely adjusting the pH value of the amoxicillin mother liquid prepared by the enzyme process to be 8.0-9.5, and performing nanofiltration and concentration to obtain concentrated mother liquid; (2) synthesizing amoxicillin under enzyme catalysis, namely adjusting the pH value of the concentrated mother liquid to be 5.8-7.0, and converting 6-APA (6-amino penicillanic acid) and D-methyl hydroxyphenyl glycinate into amoxicillin in the presence of immobilized penicillin acylase for synthesis; (3) preparing D-hydroxyphenyl glycine concentrated liquid by ultrafiltration and nanofiltration, namely separating after the enzyme catalysis reaction is ended to obtain amoxicillin crystals and secondary amoxicillin mother liquid, and performing ultrafiltration and nanofiltration on the secondary mother liquid to obtain the D-hydroxyphenyl glycine concentrated liquid; (4) crystallizing D-hydroxyphenyl glycine. According to the method, 6-APA and D-methyl hydroxyphenyl glycinate remained in the mother liquid are consumed through an indirect process of synthesizing amoxicillin under enzyme catalysis, the product quality of D-hydroxyphenyl glycine is improved, and the yield of D-hydroxyphenyl glycine is increased.
Owner:SHANXI WEIQIDA PHARMA IND
A method for enzymatically synthesizing cefprozil
ActiveCN105368910BAchieve green synthesisEasy to addFermentationGlycine ethyl esterP-hydroxyphenylglycine
The invention relates to a method for synthesizing cefprozil through an enzymatic method. According to the method, a cefprozil parent nucleus, D-para hydroxybenzene glycine methyl ester and / or D-para hydroxybenzene glycine ethyl ester, water and penicillin acylase react at the temperature of 10 DEG C-25 DEG C to obtain a crude cefprozil product and enzyme reaction mother liquor, the temperature is controlled to be 0 DEG C-15 DEG C, a pH value is controlled to be 0.2-0.8, N,N-dimethyl formamide is added, the mixture is stirred uniformly, a seed crystal is added for crystal growing, the pH value is adjusted to be 5.5-6.5, and the temperature is controlled to be 10 DEG C-30 DEG C to obtain a cefprozil N,N-dimethyl formamide compound; the temperature is controlled to be 0 DEG C-25 DEG C, water and the cefprozil N,N-dimethyl formamide compound are added into a reactor, the mixture is stirred for crystal transformation for 1-4.5 h, and then filtering, washing, swabbing-off and drying are carried out to obtain a finished cefprozil product. The cefprozil is high in product yield and high in purity, appearance is white, the preparing method is simple and easy to implement, the condition is mild, and the method is more suitable for industrial production.
Owner:苏州盛达药业有限公司
Synthetic method of amoxicillin production intermediate
ActiveCN114105795AOvercoming serious pollution defectsHigh yieldPhysical/chemical process catalystsOrganic compound preparationO-Phosphoric AcidPtru catalyst
The invention relates to a synthesis method of an amoxicillin production intermediate, which comprises the following steps of: synthesizing D-p-hydroxyphenylglycine methyl ester by taking DL-p-hydroxyphenylglycine and methanol as raw materials and solid phosphoric acid as a catalyst; comprising the following five parts: preparation of a solid phosphoric acid catalyst, preparation of DL-p-hydroxyphenylglycine methyl ester, hydrolysis of the solid phosphoric acid catalyst, crystallization of D-p-hydroxyphenylglycine methyl ester and racemization of crystallization mother liquor. The solid phosphoric acid catalyst can be hydrolyzed into phosphoric acid after esterification is completed, phosphoric acid and DL-p-hydroxyphenylglycine methyl ester form phosphoric acid double salt, and phosphoric acid serves as an esterification catalyst for synthesis of DL-p-hydroxyphenylglycine methyl ester and also serves as a resolving agent of DL-p-hydroxyphenylglycine methyl ester. The synthesis and resolution of the DL-p-hydroxyphenylglycine methyl ester are carried out in the methanol aqueous solution, and the crystallization mother liquor is recycled, so that the generation of waste liquid is greatly reduced, and the method is a clean production process of the D-p-hydroxyphenylglycine methyl ester.
Owner:TIANJIN HANRUI PHARMA +1
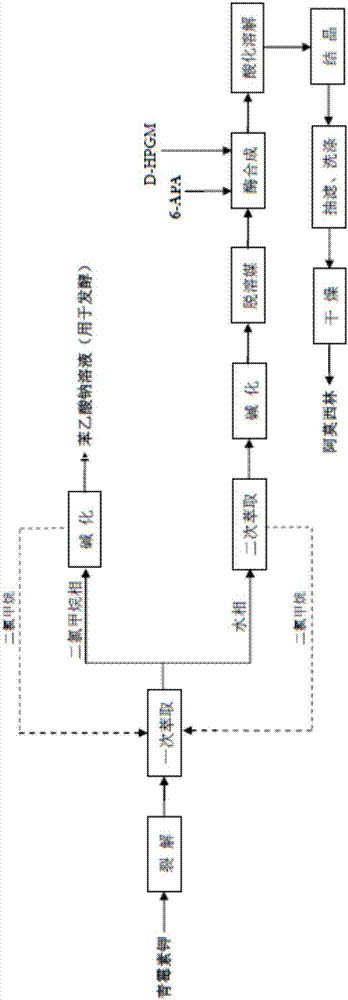
Method for preparing amoxicillin or ampicillin through full water phase
ActiveCN105132513BHigh vitality coefficientEasy to synthesizeFermentationPenicillin VKHigh concentration
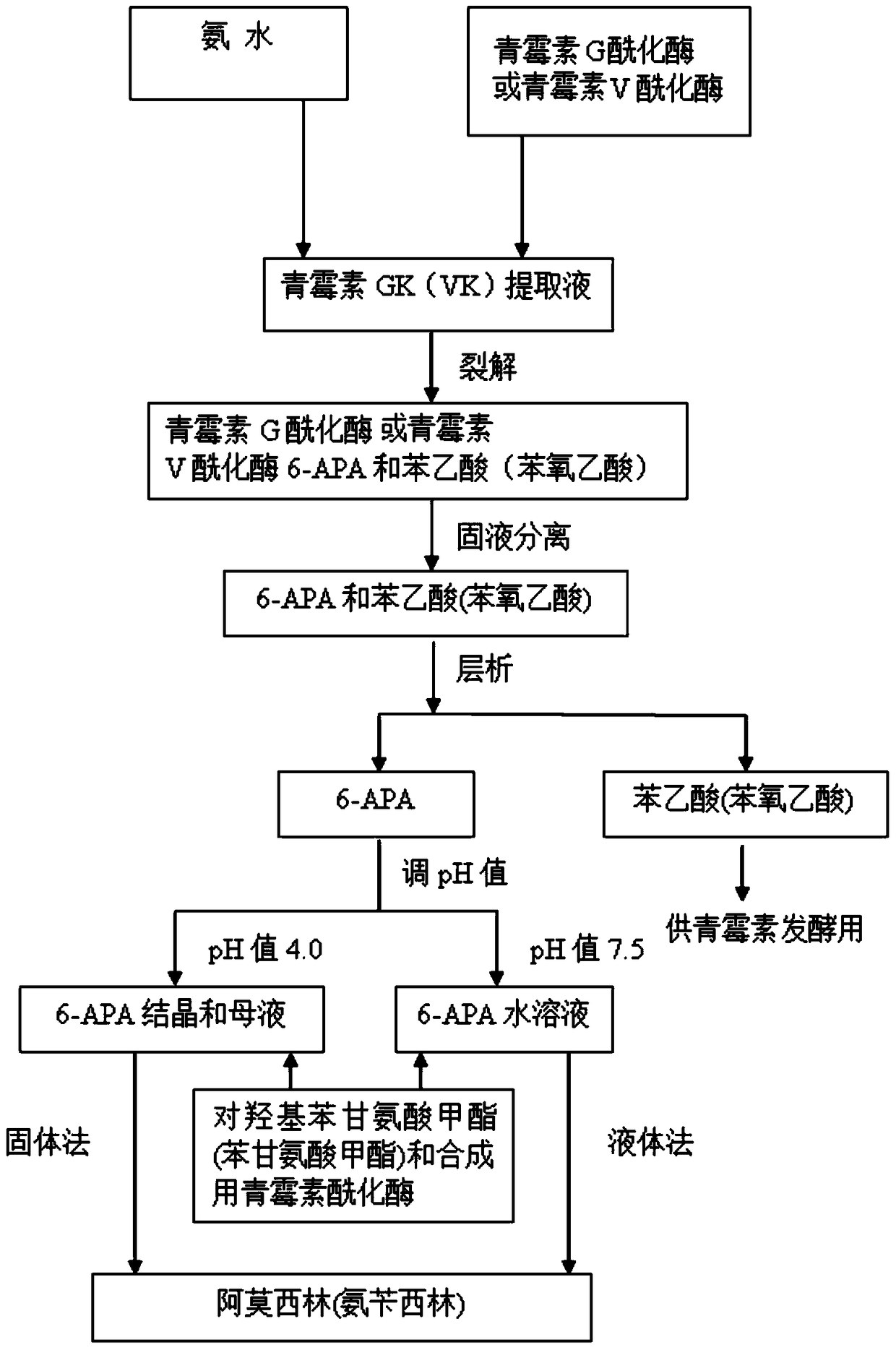
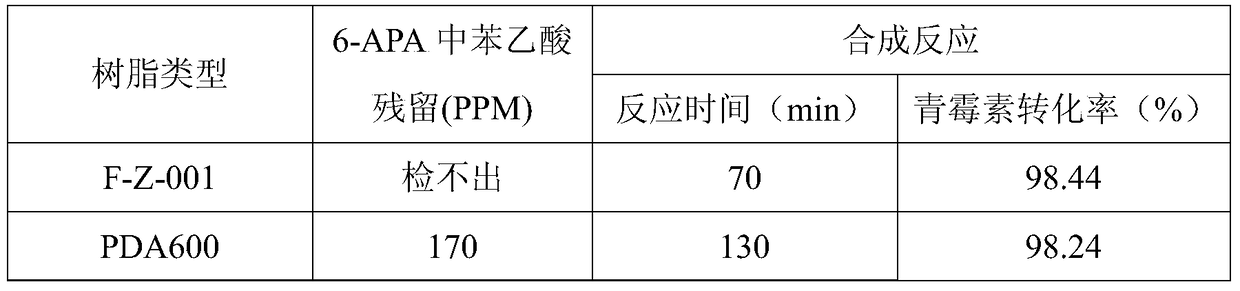
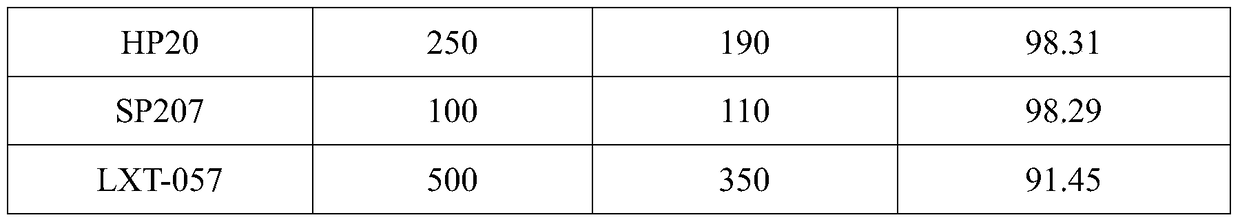
The invention discloses a method for preparing amoxicillin or ampicillin in a full-water-phase through mode. The method includes the steps that a high-concentration penicillin GK or penicillin VK extracting solution is used as a raw material, immobilized penicillin G acylase or penicillin V acylase is used as an enzyme catalyst, a high-concentration 6-APA solution or crystal is obtained through full-water-phase operations such as catalytic cracking, separating, acidization, filtering, chromatography and concentration by nanofiltration, and then the amoxicillin or the ampicillin is synthesized by the solution or crystal and HPGME or phenylglycine methyl ester under the catalytic action of the immobilized penicillin synthetase. According to the method, water-phase reacting is conducted in the whole process, no organic solvent is used, and environmental pollution is reduced; besides, the special immobilized penicillin G acylase and the penicillin V acylase are used for cracking the high-concentration penicillin GK or VK extracting solution, and special macroporous absorption resin is used for separating and cracking products, so that the processing steps are greatly simplified, the production cost is lowered, the product yield is increased, and the industrial production requirement is met.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
A kind of crystallization preparation method of L-p-hydroxyphenylglycine methyl ester
ActiveCN109400491BHigh crystallinitySimple filterOrganic compound preparationOrganic chemistry methodsPhysical chemistryHydroxy group
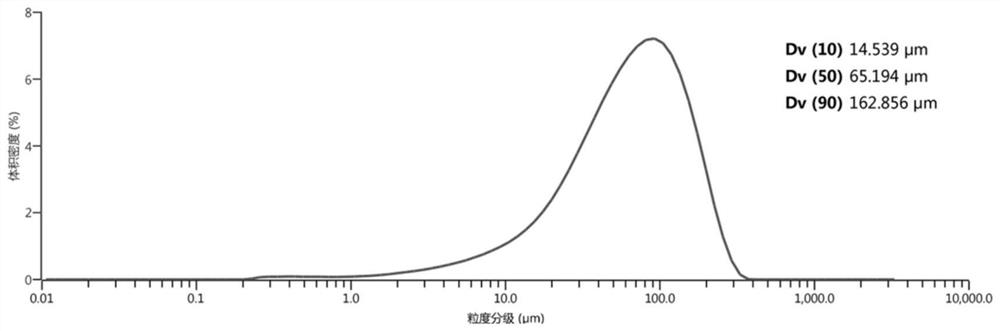
The invention relates to a preparation method for a crystal of L-p-hydroxyphenylglycine methyl ester. The preparation method comprises the following steps: (1) adding L-p-hydroxyphenylglycine methyl ester hydrochloride into a container filled with water, keeping solid-liquid ratio of solution within 0.08-0.15g / ml, stirring and dissolving under constant temperature at 25-40 DEG C, continuously stirring and then filtering and decolorizing; (2) pouring a filtrate into a crystallizer, stirring under constant temperature at 25-40 DEG C, adding alkali liquor into the crystallizer till pH of solutionis 5.0-5.8, adding a L-p-hydroxyphenylglycine methyl ester seed crystal and growing crystal for 1-3h; (3) continuing to add alkali liquor till pH of solution is 8.2-8.4, continuously cooling to 0-5 DEG C and growing crystal for 1-3h; (4) performing suction filtration, washing filter cake with water, and drying the acquired crystal product. The product acquired according to the invention is high in purity, large in grain size and high in liquidity; labor intensity of filtering and drying is low; the preparation method is easy for industrialization.
Owner:WEIHAI OCEAN VOCATIONAL COLLEGE
Synthesis method of cefpiramide side chain acid
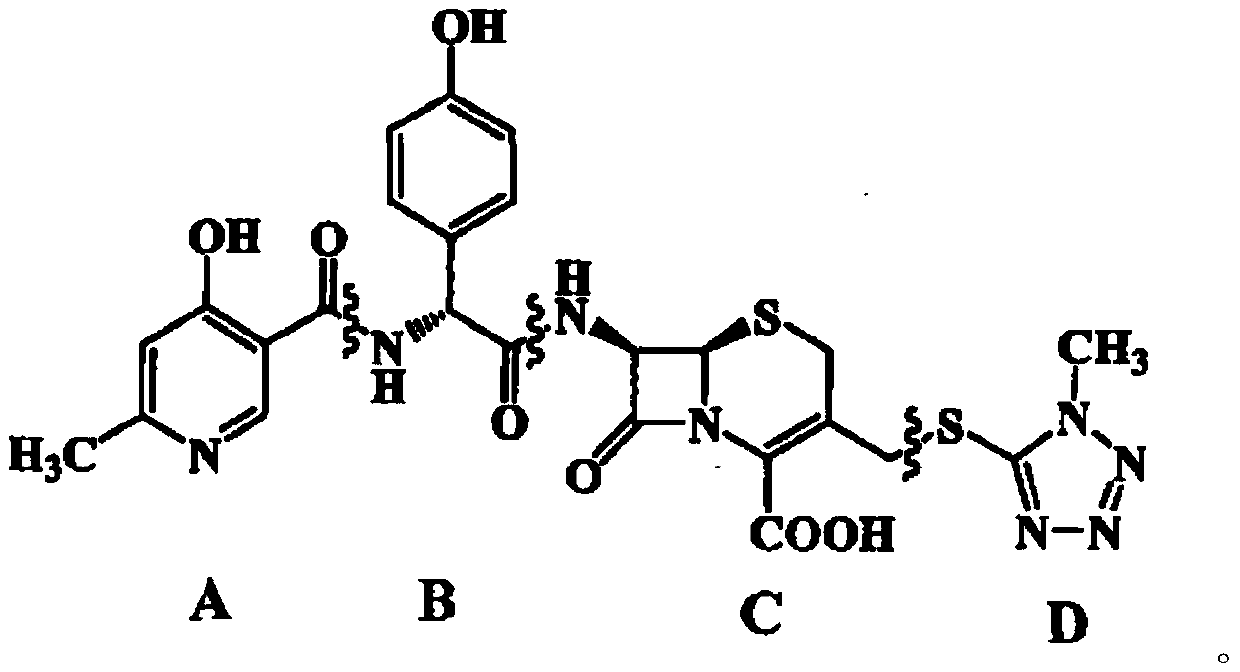
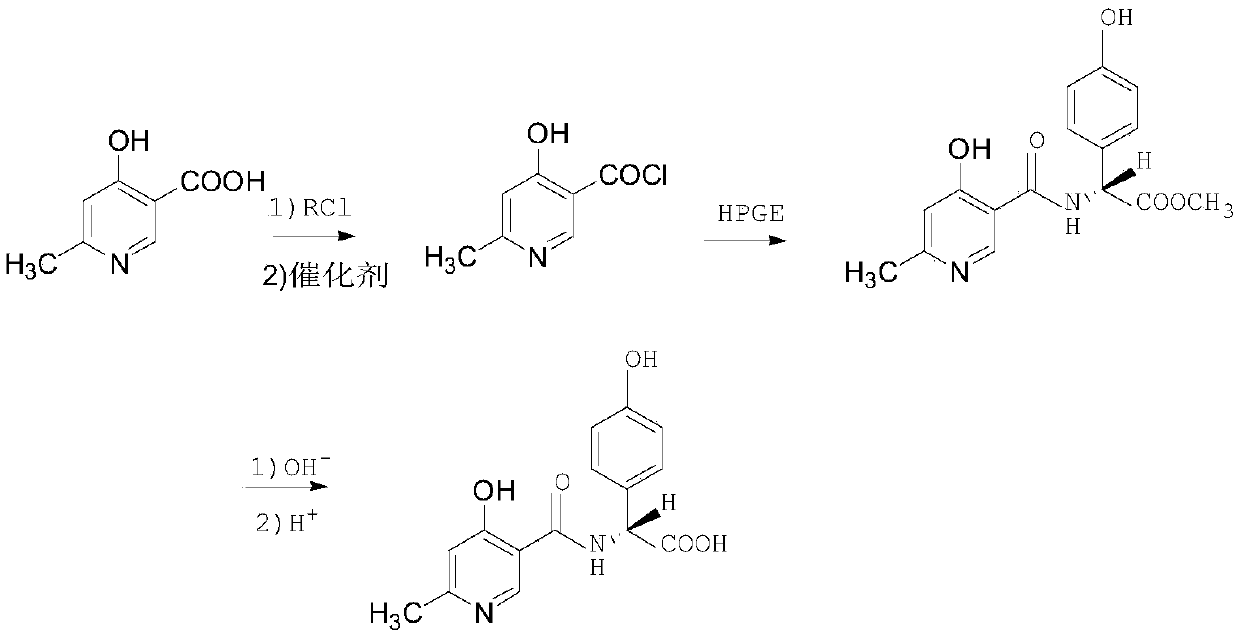
InactiveCN111116463AEasy to operateRaw materials are easy to getOrganic chemistry methodsChemical synthesisSide chain
The invention belongs to the technical field of chemical synthesis, and particularly relates to a synthesis method of cefpiramide side chain acid. Pyridine-3-formic acid and methyl p-hydroxyphenylglycinate are used as raw materials, and the cefpiramide side chain acid is obtained through chlorination, acylation, hydrolysis and crystallization. The synthesis method of the cefpiramide side chain acid is simple to operate, easily available in raw materials and low in cost, adopts a dichloromethane and N, N-dimethylacetamide mixed solvent as a solvent, greatly improves the product yield and stability, has good economic benefits, and is convenient for large-scale industrial production.
Owner:YIYUAN XINQUAN CHEM
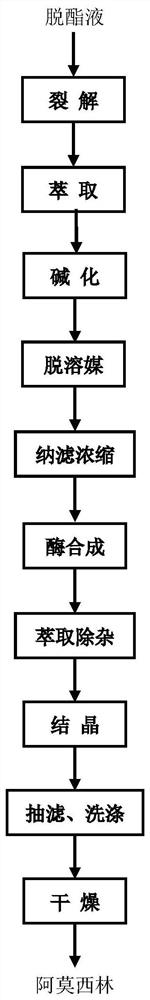
A kind of method for preparing amoxicillin by deesterification liquid
ActiveCN106399446BImprove product qualityHigh yieldOrganic chemistryFermentationPhenylacetic acidLyase
The invention relates to a method for preparing amoxicillin from a deesterification solution, belonging to the technical field of pharmacy. In the method of the present invention, the deesterification liquid is subjected to the action of lyase to obtain a lysate containing 6-APA and phenylacetic acid. The lysate is extracted to obtain an aqueous phase containing 6-APA, the aqueous phase is basified, solvent removed and nanofiltration concentrated to obtain a solution with a higher concentration of 6-APA, which is mixed with D-hydroxyphenylglycine methyl ester The crude amoxicillin was synthesized under the action of penicillin G acylase. The crude amoxicillin is extracted by acidification, crystallized, washed and dried to obtain the finished amoxicillin. The process saves steps such as crystallization, washing and drying of 6-APA, saves the treatment of 6-APA mother liquor, simplifies the production process, improves the yield of amoxicillin, and reduces the production cost.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
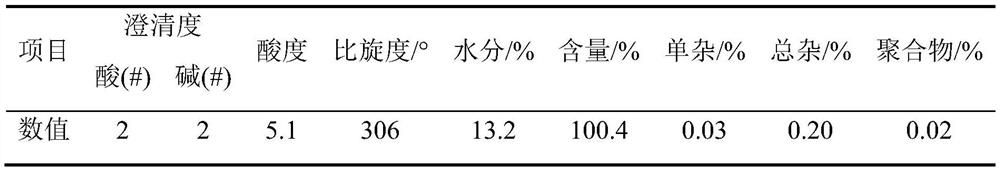
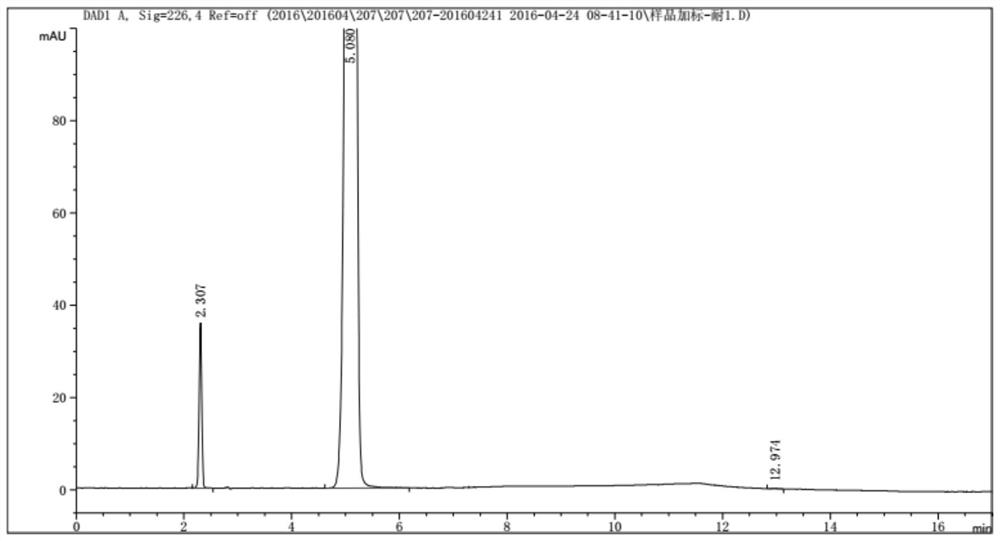
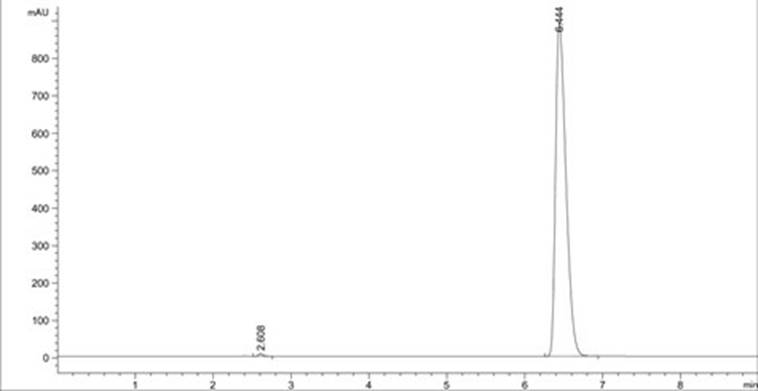
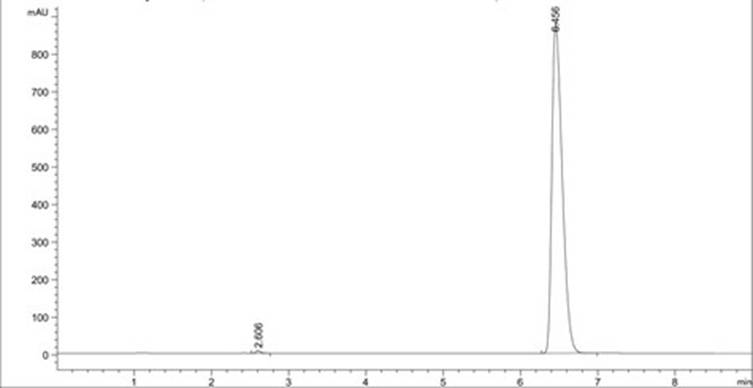
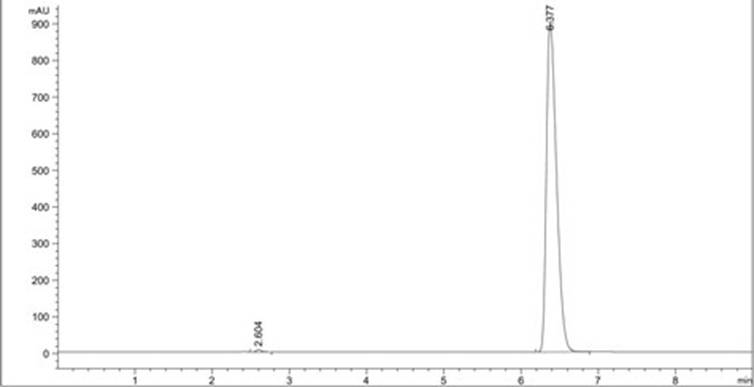
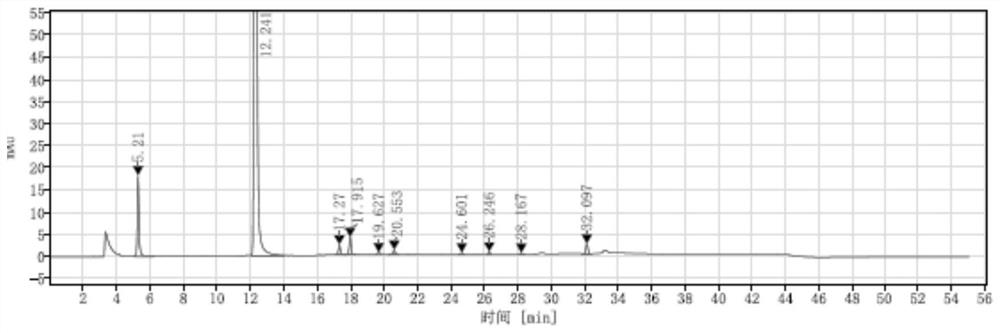
A method for analyzing d(-) p-hydroxyphenylglycine content in d(-) p-hydroxyphenylglycine methyl ester
The invention discloses an HPLC method for determining the content of D(-)p-hydroxyphenylglycine in D(-)p-hydroxyphenylglycine methyl ester. The method is to prepare a solution of D(‑) p-hydroxyphenylglycine methyl ester with dilute acid solution, adopt a chemically bonded stationary phase chromatographic column, add organic base with buffer salt, and adjust pH to 0.8-12 with acid as buffer salt solution, buffered salt solution and organic phase modifier in an appropriate ratio for gradient elution, and then detect at an appropriate detection wavelength, flow rate and column temperature, and then calculate the sample content based on the spectrum. The method of the invention can effectively separate D(-)p-hydroxyphenylglycine methyl ester and D(-)p-hydroxyphenylglycine, and accurately measure the content of D(-)p-hydroxyphenylglycine. The method is simple, reliable and rapid , high sensitivity, good reproducibility and specificity, which established the foundation for the establishment of quality standards for D(‑) p-hydroxyphenylglycine methyl ester.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
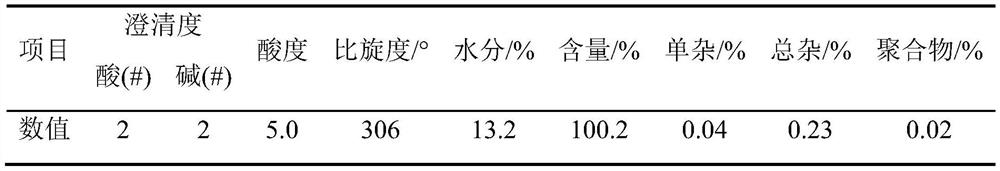
Detection method for content of D-methyl p-hydroxyphenylglycinate
PendingCN112129847AImprove accuracyEasy to operateComponent separationUltraviolet detectorsPhysical chemistry
The invention discloses a detection method for the content of D-methyl p-hydroxyphenylglycinate. According to the method, HPLC is adopted for measuring the content of D-methyl p-hydroxyphenylglycinate. D-methyl p-hydroxyphenylglycinate is dissolved with a prepared D-methyl p-hydroxyphenylglycinate diluent, the volume is fixed, D-methyl p-hydroxyphenylglycinate is eluted at different flow speeds through a C18 chromatographic column at a certain column temperature, detection is conducted through an ultraviolet detector, and the content of D-methyl p-hydroxyphenylglycinate is calculated accordingto an obtained spectrum. In order to prove that the analysis method has the advantages of being high in sensitivity, good in reproducibility, short in consumed time, high in result accuracy and the like, the analysis method is compared with a perchloric acid titration method in an experiment, and an experiment result proves that the content of D-methyl p-hydroxyphenylglycinate can be rapidly andaccurately measured by adopting the experiment method. The method plays a crucial role in measuring the content of D-methyl p-hydroxyphenylglycinate in the current market.
Owner:HENAN NEWLAND PHARMA
Preparation method of D-p-hydroxyphenylglycine methyl ester hydrochloride suitable for industrial production
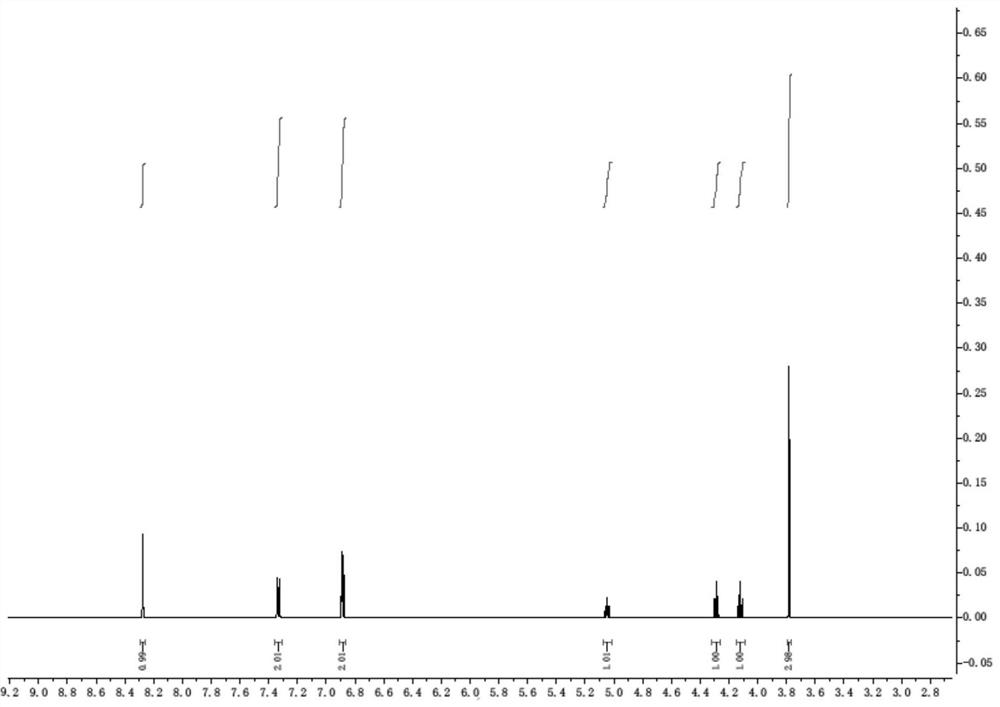
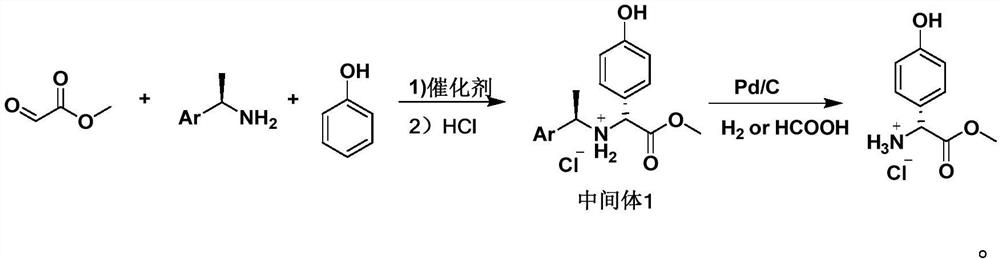
PendingCN113620826AReduce usageLow costOrganic compound preparationOrganic chemistry methodsChemical reactionEthyl group
The invention discloses a preparation method of D-p-hydroxyphenylglycine methyl ester hydrochloride suitable for industrial production, and relates to a preparation method of D-p-hydroxyphenylglycine methyl ester hydrochloride suitable for industrial production. In the first step of reaction, methyl glyoxylate and phenol are used as raw materials, cheap S-(-)-arylethylamine is used as a chiral induction reagent, and (R)-2-(4-hydroxyphenyl)-2-(((S)-1-arylethyl) amino) methyl acetate hydrochloride (intermediate 1) is obtained through reaction. In the second reaction step, the intermediate 1 is subjected to hydrogenolysis of a protecting group under the Pd / C catalytic condition. The method provided by the invention has the main characteristics that 1, the process is simple, only two-step chemical reaction is adopted, and the production efficiency is high; and 2, a cheap chiral induction reagent S-(-)-arylethylamine is used for replacing an expensive resolution reagent chiral camphorsulfonic acid or chiral phenylethanesulfonic acid, so that the raw material cost and the production cost are greatly reduced;.
Owner:HUBEI HONGYUAN PHARMA
Method of preparing antimer pure parahydroxy phenyl glycine
A process for preparing the high-purity antimer, L-p-hydroxyphenyl glycine includes such steps as sequentially adding ionic liquid BMIM.BF4, phosphoric acid buffer, methyl p-hydroxyphenylglycenate, and papain, reacting, ultrafilter to remove suspended particles and enzyme, and separating to obtain L-p-hydroxyphenyl glycine.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com