Preparation method of D-p-hydroxyphenylglycine methyl ester
A technology of p-hydroxyphenylglycine methyl ester and p-hydroxyphenylglycine, which is applied in the field of organic chemical synthesis, can solve the problems of strong volatility of hydrogen chloride, unstable intermediate process control, large fluctuation, etc., so as to avoid difficult handling and ensure yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
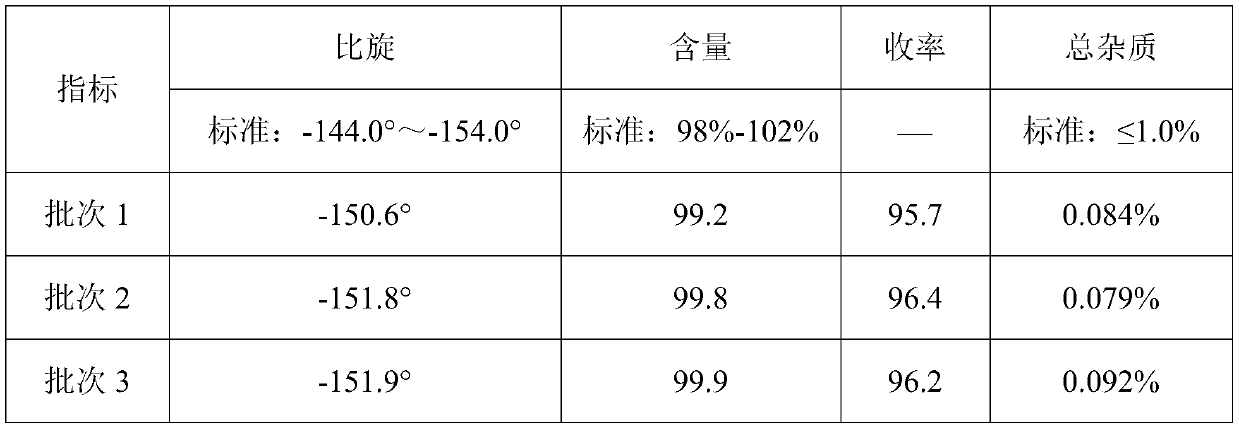
Embodiment 1
[0026] (1) Esterification step
[0027] In the reaction bottle of 500mL, add 200mL methanol, 100g D-hydroxyphenylglycine (HPG) and the 98wt% concentrated sulfuric acid of 75g, carry out esterification reaction 2 hours under reflux; Then slowly vacuumize, make the vacuum in the reaction bottle about- 0.075MPa~-0.082MPa, temperature about 80°C, collect distillate methanol and water;
[0028] Then release the vacuum, add 170mL of methanol into the reaction bottle, and carry out the esterification reaction under reflux for 2 hours; then slowly vacuumize the reaction bottle so that the vacuum in the reaction bottle is about -0.075MPa~-0.082MPa, and the temperature is about 80°C. Collect the distillate methanol and water;
[0029] Repeat the above steps of adding 170mL of methanol, esterifying for 2 hours, and distilling out methanol and water under reduced pressure;
[0030] Detected by high performance liquid chromatography (PHLC), the yield of the finally obtained D-p-hydroxyph...
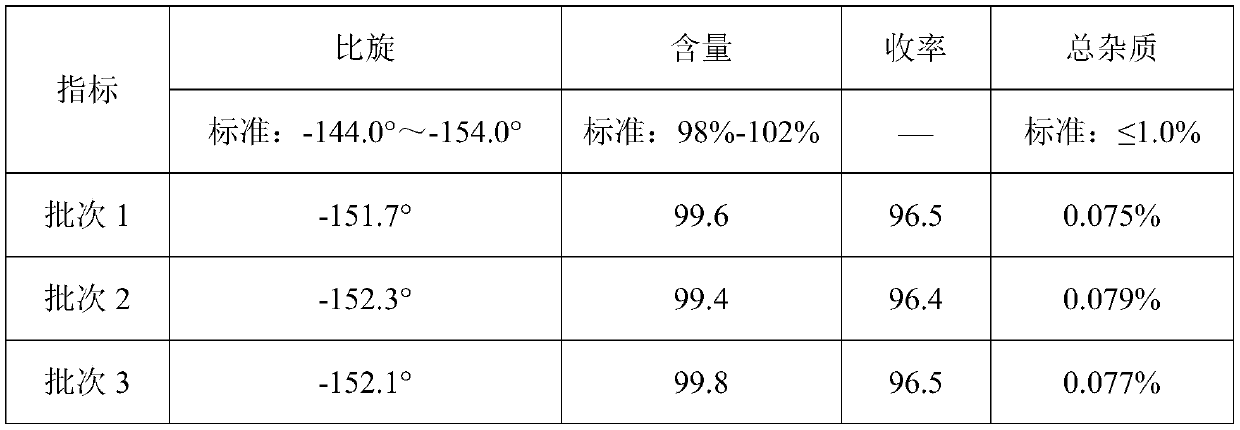
Embodiment 2
[0039] Embodiment 2 (pilot scale scale-up experiment)
[0040] (1) Esterification step
[0041] Add 12L methyl alcohol, 6kg D-p-hydroxyphenylglycine (HPG) and the 98wt% vitriol oil of 4.5kg in the reaction flask of 30L, carry out esterification reaction 2 hours under reflux; Slowly vacuumize then, make the vacuum in the reaction flask about -0.075MPa~-0.082MPa, the temperature is about 80°C, collect distillate methanol and water;
[0042]Then release the vacuum, add 10.2L methanol to the reaction bottle, and carry out the esterification reaction under reflux for 2 hours; then slowly vacuumize the reaction bottle so that the vacuum in the reaction bottle is about -0.075MPa~-0.082MPa, and the temperature is about 80°C. Methanol and water;
[0043] Repeat the steps of adding 10.2L methanol, esterifying for 2 hours, and distilling methanol and water under reduced pressure;
[0044] Detected by high performance liquid chromatography (PHLC), the yield of the finally obtained D-p-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com