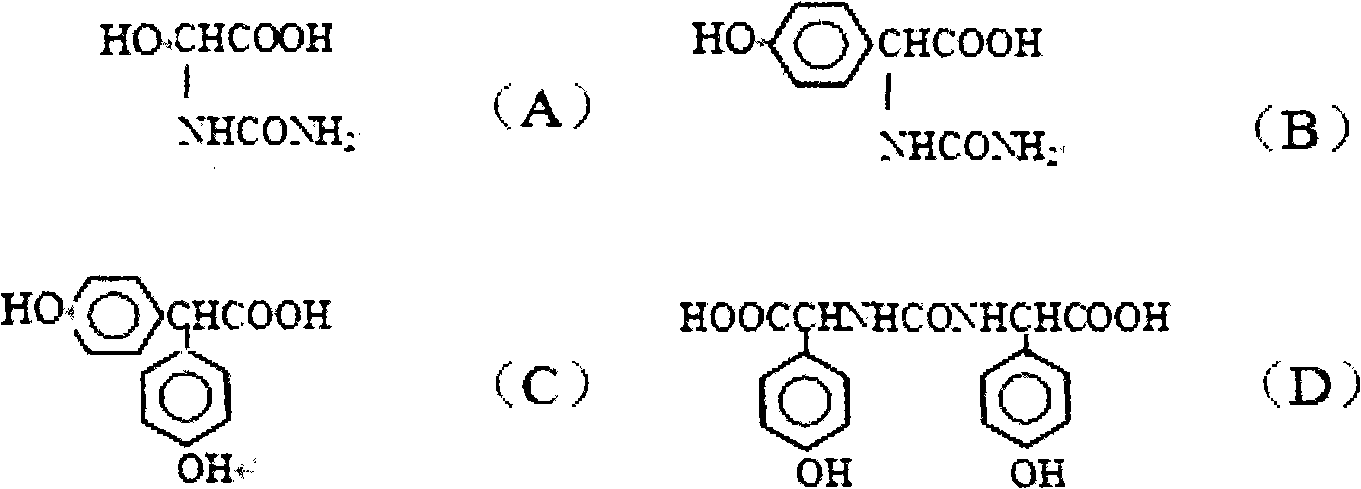
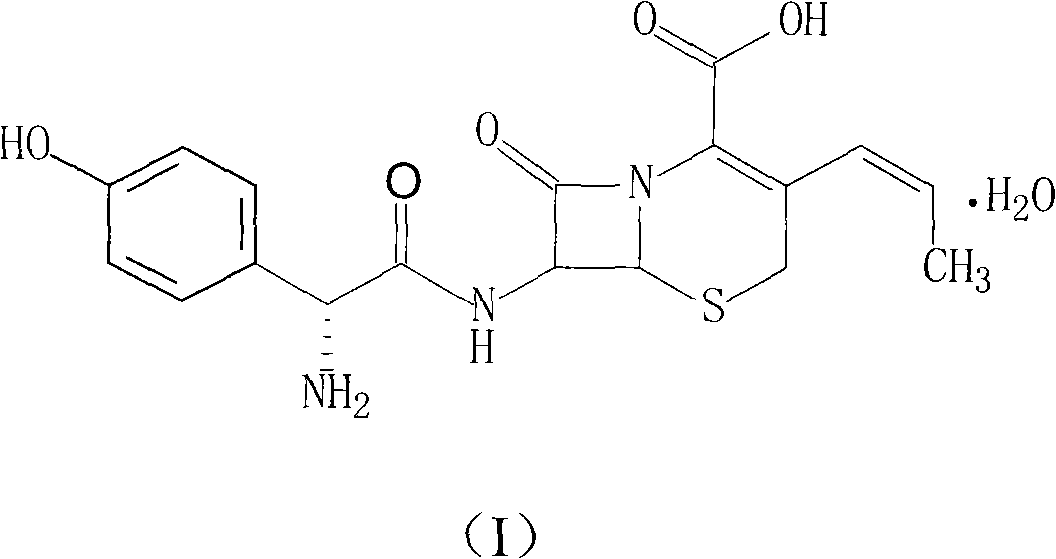
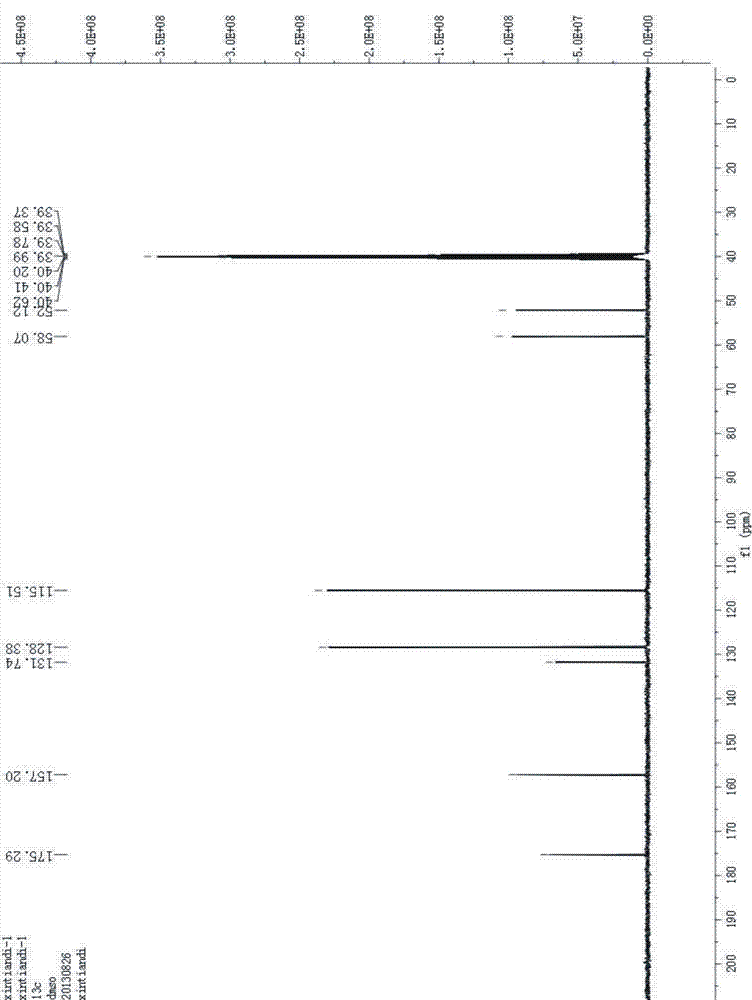
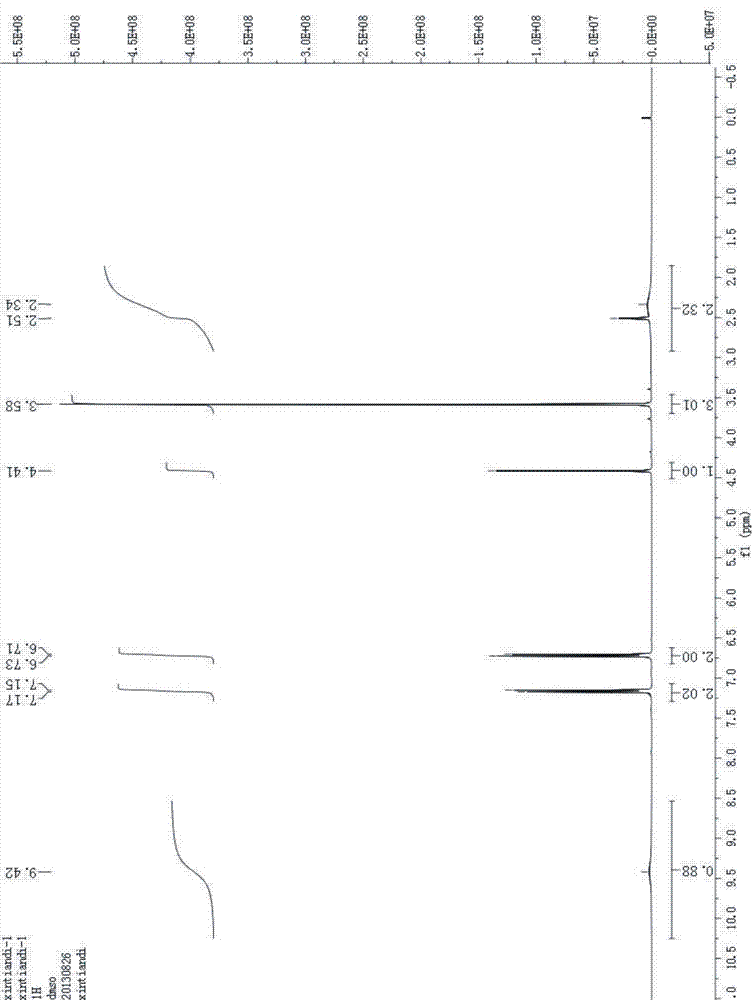
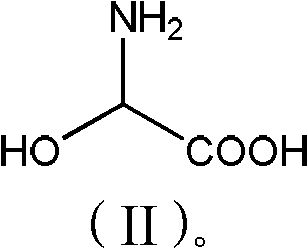
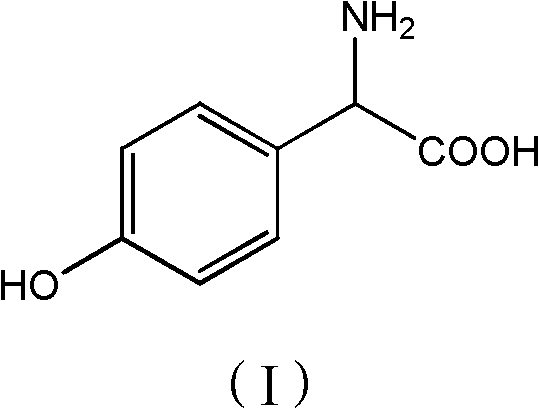
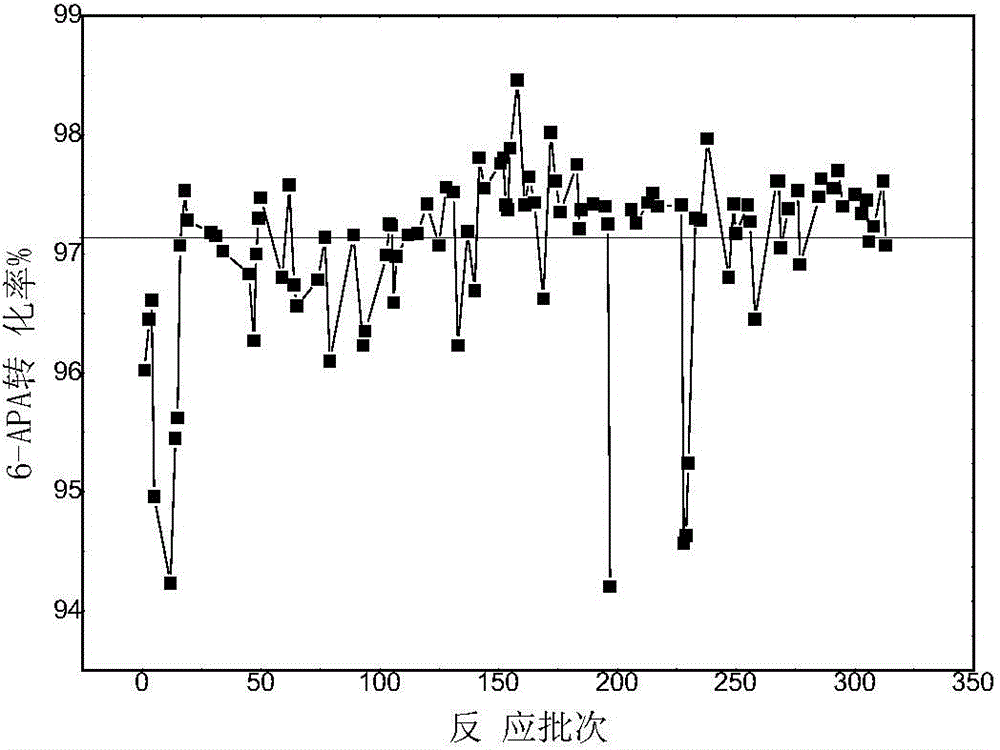
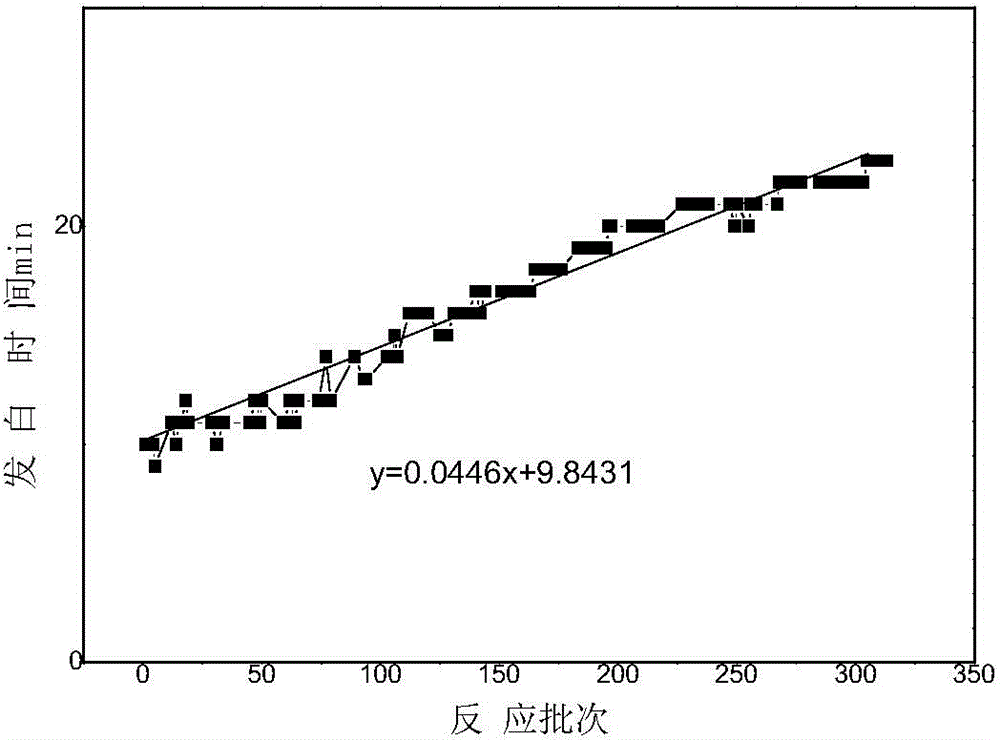
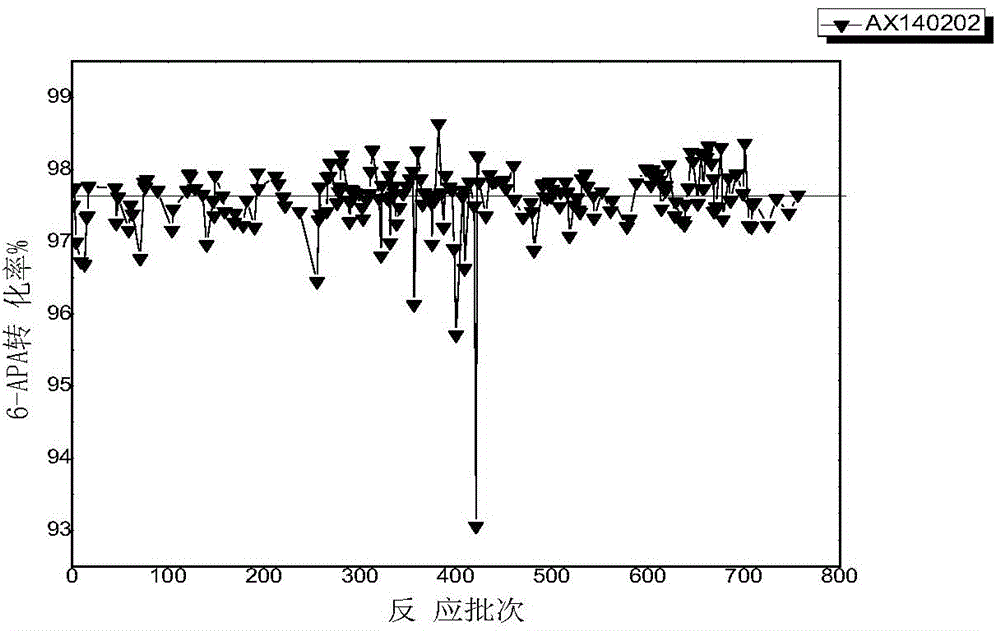
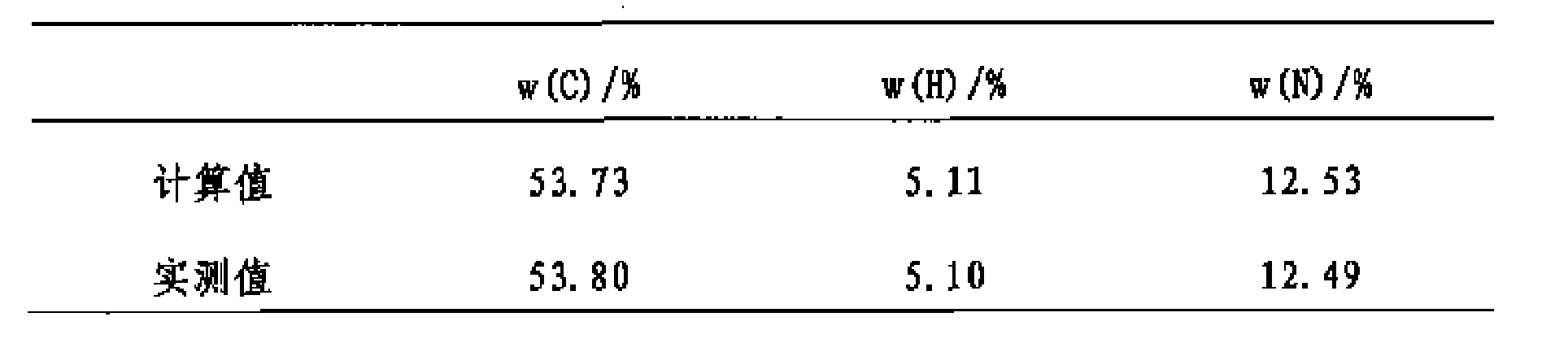
Patents
Literature
148 results about "P-hydroxyphenylglycine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
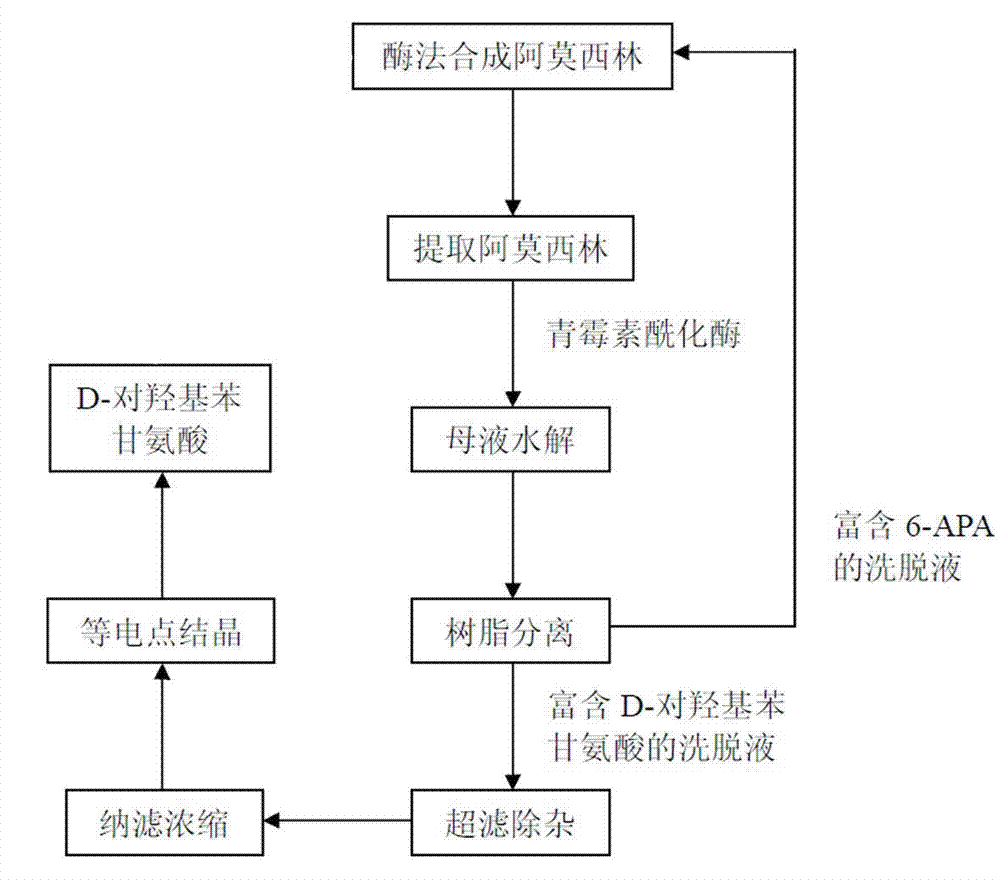
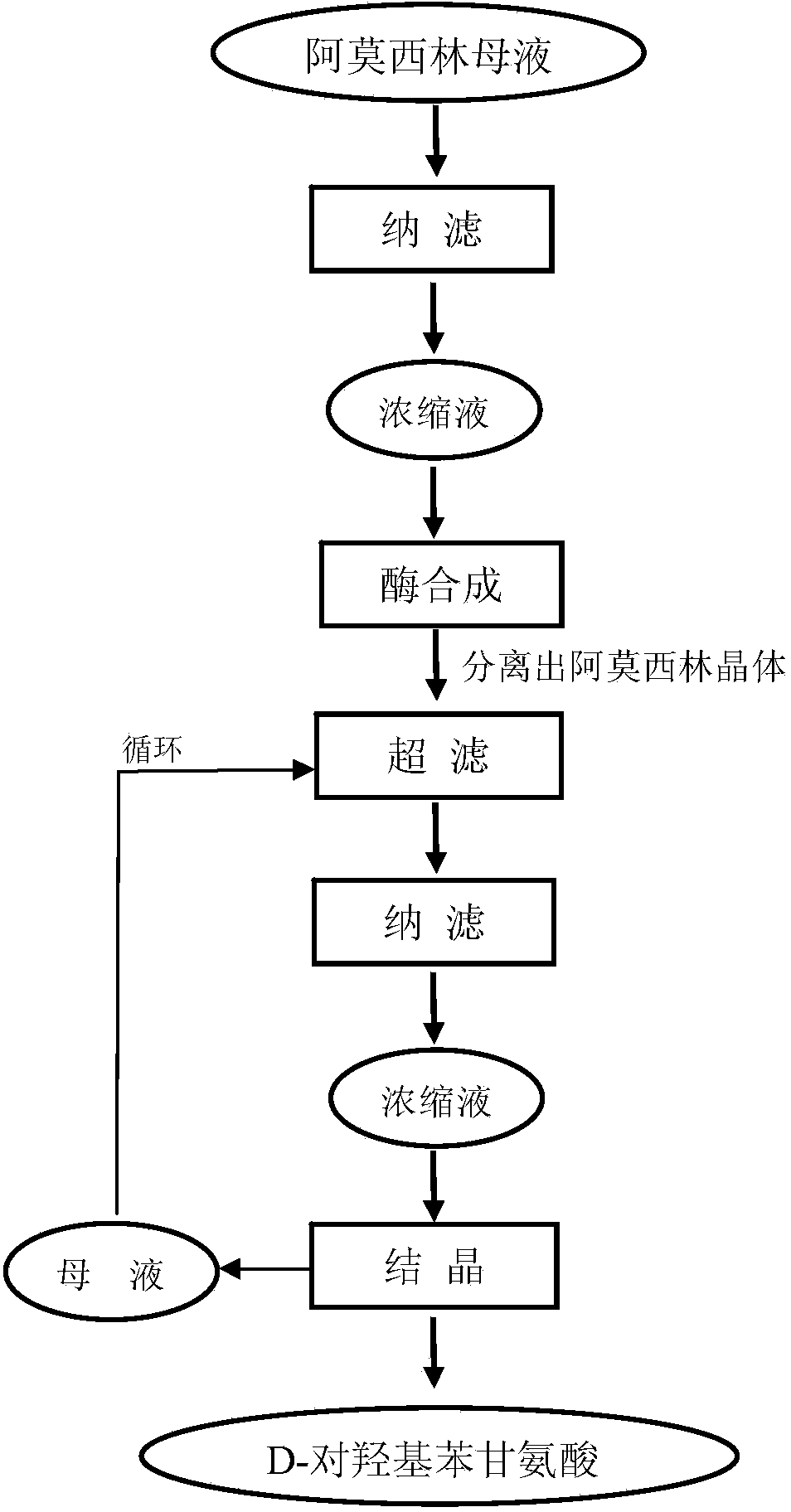
Method for recycling active ingredients in amoxicillin mother liquor synthesized by enzymatic method
ActiveCN102816803AAvoid adverse reactionsRelieve pressureOrganic compound preparationChemical industryBULK ACTIVE INGREDIENTP-hydroxyphenylglycine
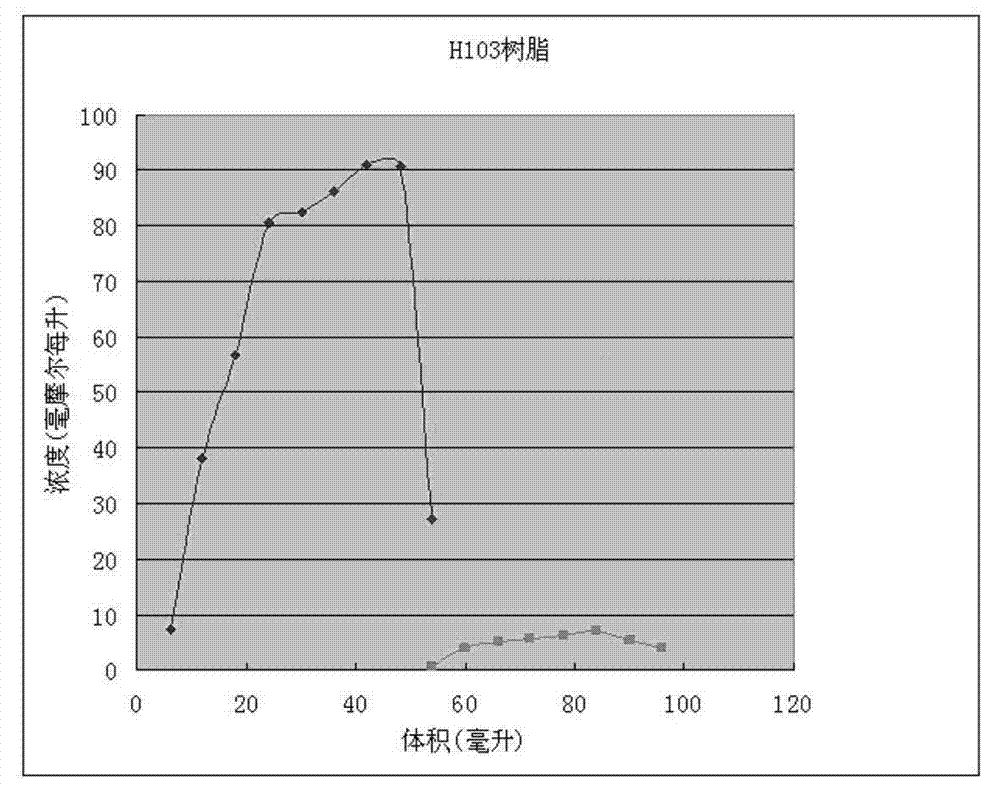
The invention discloses a method for recycling active ingredients in the amoxicillin mother liquor synthesized by an enzymatic method. The method includes synthesizing the amoxicillin mother liquor by the enzymatic method; separating the amoxicillin mother liquor through a macro-porous resin column, eluting the separated amoxicillin mother liquor by deionized water, and collecting an eluant rich in D-4-Hydroxyphenylglycine and an eluant rich in 6-amino penicillanic acid (APA) respectively; filtering the eluant rich in D-4-Hydroxyphenylglycine with Daltonian ultrafiltration membranes with a cutoff molecular weight of 150 to 200; performing nanofiltration concentration on the Daltonian ultrafiltration membranes for the filtered liquor with the cutoff molecular weight of 150 to 200, and standing, crystallizing and filtering the concentrated liquor to obtain solids, and drying the solids to obtain the D-4-Hydroxyphenylglycine. According to the method, the technological design is reasonable, the operation is convenient, the recycling effect is good, and energy is saved and the environment is protected.
Owner:NORTH CHINA PHARM GRP SEMISYNTECH CO LTD
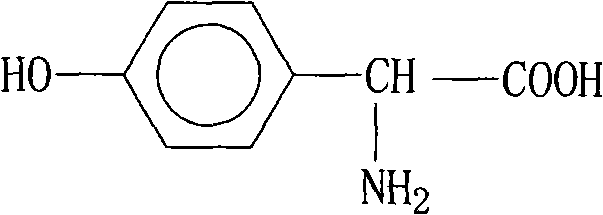
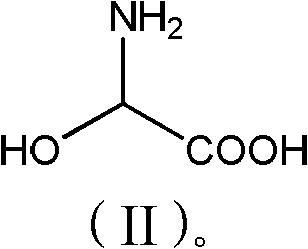
P-hydroxybenzene glycine synthesis technology
InactiveCN101362703AOrganic compound preparationAmino-carboxyl compound preparationSulfite saltHydroxylamine Hydrochloride
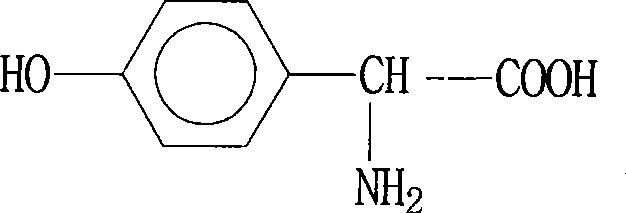
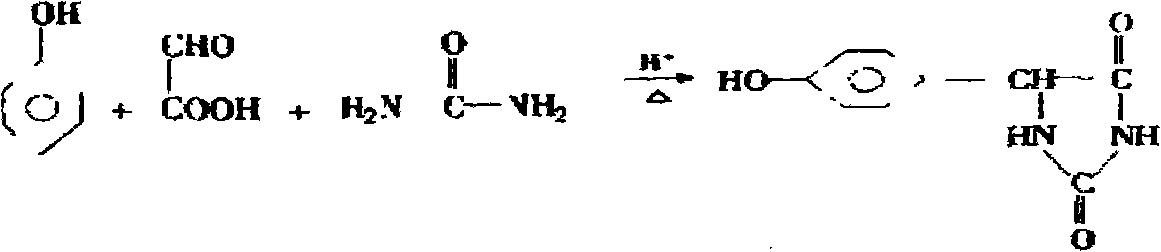
The invention belongs to the field of pharmaceutical chemical engineering intermediate production, which relates to a synthesis technology of p-hydroxyphenylglycine (HPG). The purpose of the invention is achieved by the following steps: phenol, glyoxylic acid, water and sulfamic acid carry out the one-pot braise reaction under the action of catalysts such as benzene sulfonic acid, p-toluenesulfonic acid, o-toluenesulfonic acid, and the like; after the reaction is finished, a small amount of reducing substances such as sodium sulfite, sodium bisulfite, hydroxylamine hydrochloride, and the like, are added; finally, the PH value is adjusted by alkali, the mother liquor separation is washed by large amount of water and then washed by organic solvents such as methanol, ethanol, acetone, glacial acetic acid, and the like; the obtained product HPG is white powder, wherein, the content of NPLC is equal to or more than 98.5, which can meet the requirement of splitting. The method has the advantages of low production cost, simple operation, stable quality, etc.
Owner:谢建中
Method for preparing 4-hydroxyphenyl hydantoin
InactiveCN101973941AQuality improvementHigh synthetic yieldOrganic compound preparationAmino-carboxyl compound preparationPhenylacetic acidPhenol
The invention discloses a method for preparing 4-hydroxyphenyl hydantoin from a glyoxylic acid, phenol and urea by condensation under acid condition, which is characterized in that: the 4-hydroxyphenyl hydantoin is prepared in the presence of a sulfamic acid, the production of polymerization impurities is inhibited and simultaneously a phenylglycine byproduct is produced. The mol ratio of the reaction raw materials of the glyoxylic acid to the phenol to the urea to the sulfamic acid is 1:1.0-1.1:1-1.5:0.2-0.5. The method comprises the following steps of: adding dropwise glyoxylic acid solution at the relatively lower temperature of 50 to 60 DEG C to form a 2-ureidobenzeneacetic acid intermediate, preparing the 4-hydroxyphenyl hydantoin at the relatively higher temperature of 80 or 105 DEG C by ring formation and simultaneously producing the p-hydroxyphenylglycine byproduct, wherein reaction solution is subjected to post-treatment to obtain white 4-hydroxyphenyl hydantoin crystals with the purity of 99.6 percent and the yield of 62.7 percent; and mother solution is further treated to obtain white p-hydroxyphenylglycine crystals with the purity of 99.2 percent ad the yield of 10.1 percent. In the method, the main byproduct is p-hydroxyphenylglycine, the utilization rate of the synthesis raw materials is increased and the problems of unstable quality of product and high treatment cost of the mother solution in the prior art are solved.
Owner:TIANJIN VOCATIONAL INST
Preparation method of para-hydroxybenzol gylcine
InactiveCN101045693AHigh yieldReduce generationOrganic compound preparationAmino-carboxyl compound preparationGlycineBenzaldehyde
Owner:BAOSHAN IRON & STEEL CO LTD
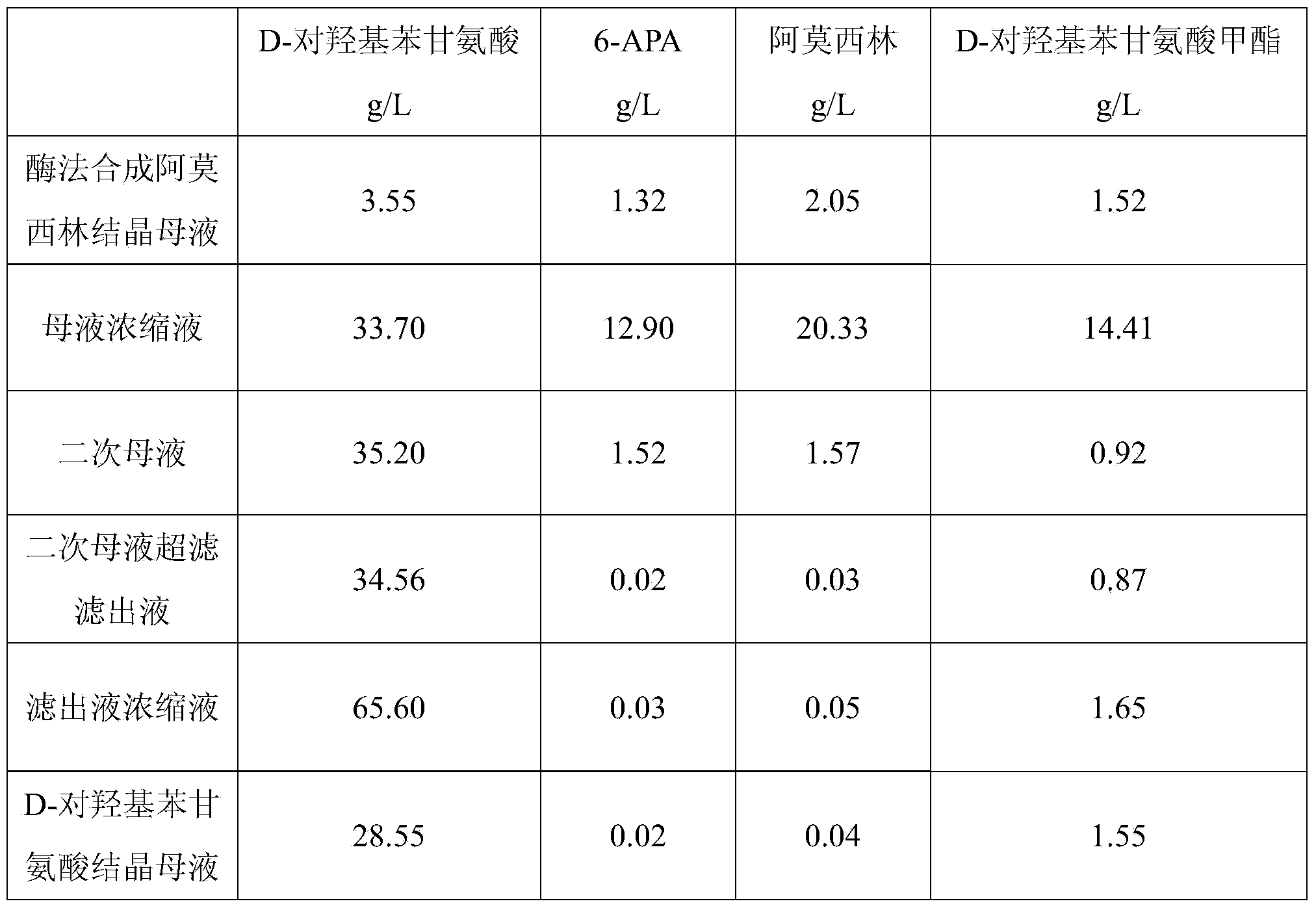
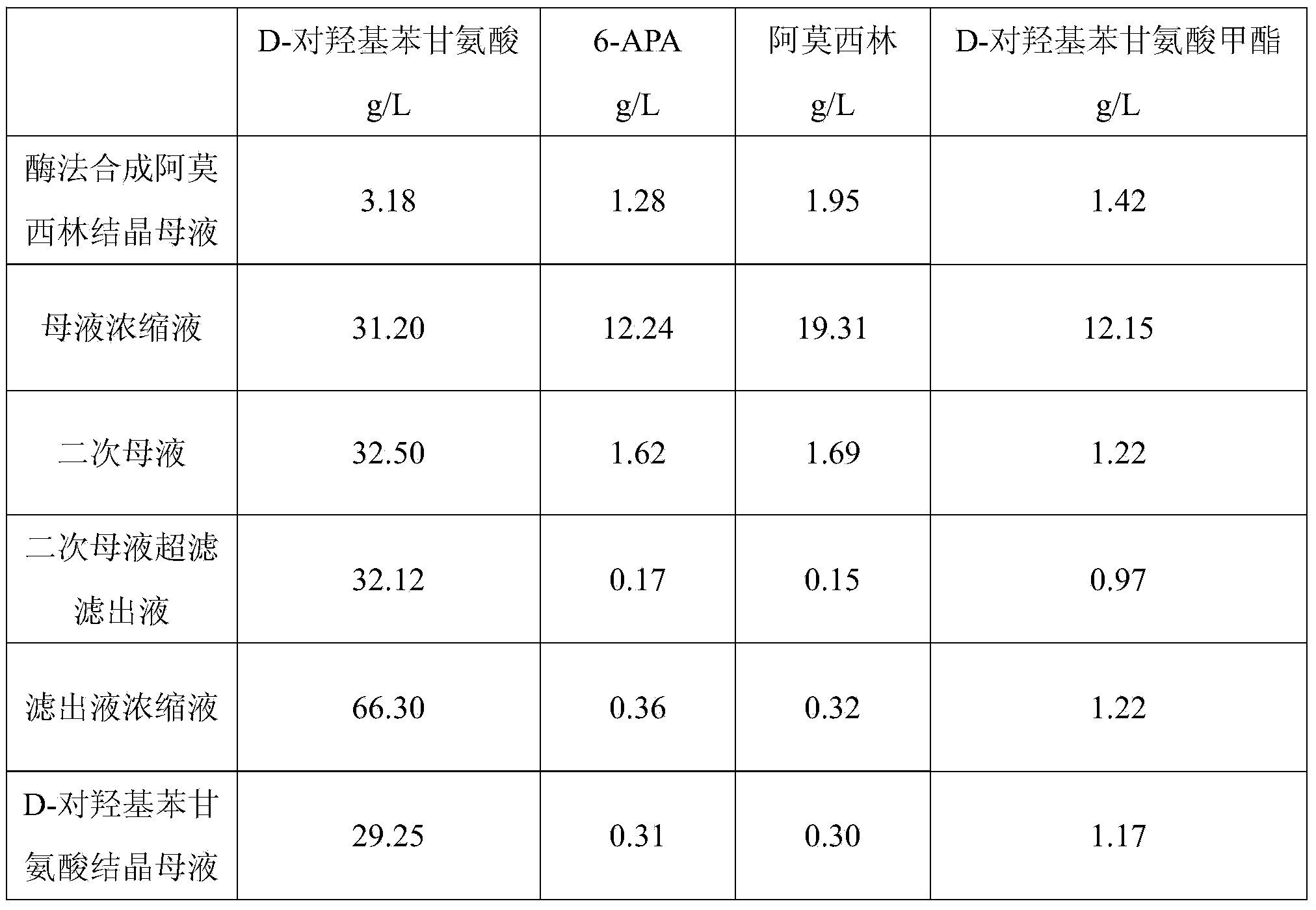
Method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by enzyme process
ActiveCN104357528AImprove product qualityCreate economic growthOrganic compound preparationAmino-carboxyl compound preparationUltrafiltrationChemistry
The invention relates to a method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by an enzyme process. The method comprises the following steps: (1) concentrating the amoxicillin mother liquid, namely adjusting the pH value of the amoxicillin mother liquid prepared by the enzyme process to be 8.0-9.5, and performing nanofiltration and concentration to obtain concentrated mother liquid; (2) synthesizing amoxicillin under enzyme catalysis, namely adjusting the pH value of the concentrated mother liquid to be 5.8-7.0, and converting 6-APA (6-amino penicillanic acid) and D-methyl hydroxyphenyl glycinate into amoxicillin in the presence of immobilized penicillin acylase for synthesis; (3) preparing D-hydroxyphenyl glycine concentrated liquid by ultrafiltration and nanofiltration, namely separating after the enzyme catalysis reaction is ended to obtain amoxicillin crystals and secondary amoxicillin mother liquid, and performing ultrafiltration and nanofiltration on the secondary mother liquid to obtain the D-hydroxyphenyl glycine concentrated liquid; (4) crystallizing D-hydroxyphenyl glycine. According to the method, 6-APA and D-methyl hydroxyphenyl glycinate remained in the mother liquid are consumed through an indirect process of synthesizing amoxicillin under enzyme catalysis, the product quality of D-hydroxyphenyl glycine is improved, and the yield of D-hydroxyphenyl glycine is increased.
Owner:SHANXI WEIQIDA PHARMA IND
Method for treating L-p-hydroxyphenylglycine desalting mother liquor by adopting bipolar membrane electrodialysis technology
InactiveCN101967106ANo pollutionReduce pollutionGeneral water supply conservationOrganic compound preparationPhysical chemistryImpurity ions
The invention discloses a method for treating an L-p-hydroxyphenylglycine desalting mother liquor by adopting a bipolar membrane electrodialysis technology, comprising the following steps of diluting the desalting mother liquor, regulating a pH value thereof to be alkaline, heating, filtering, and using cation exchange resin to remove inpurity ions in the mother liquor; separating the mother liquor after impurity ion removal by adopting bipolar membrane electrodialysis to respectively obtain liquid alkali and a resolution agent; secondly using the obtained liquid alkali in alkali regulation or an activation treatment process of cation exchange resin in the last step, and using the obtained resolution agent for production of L-p-hydroxyphenylglycine again. Therefore, various components reach closed cycle without causing material loss. The method is beneficial to recovering valuable substances in the mother liquor, lowers the environmental pollution and also avoids introduction of new impurities, thereby improving the quality of a product.
Owner:石药集团中诺药业(石家庄)有限公司
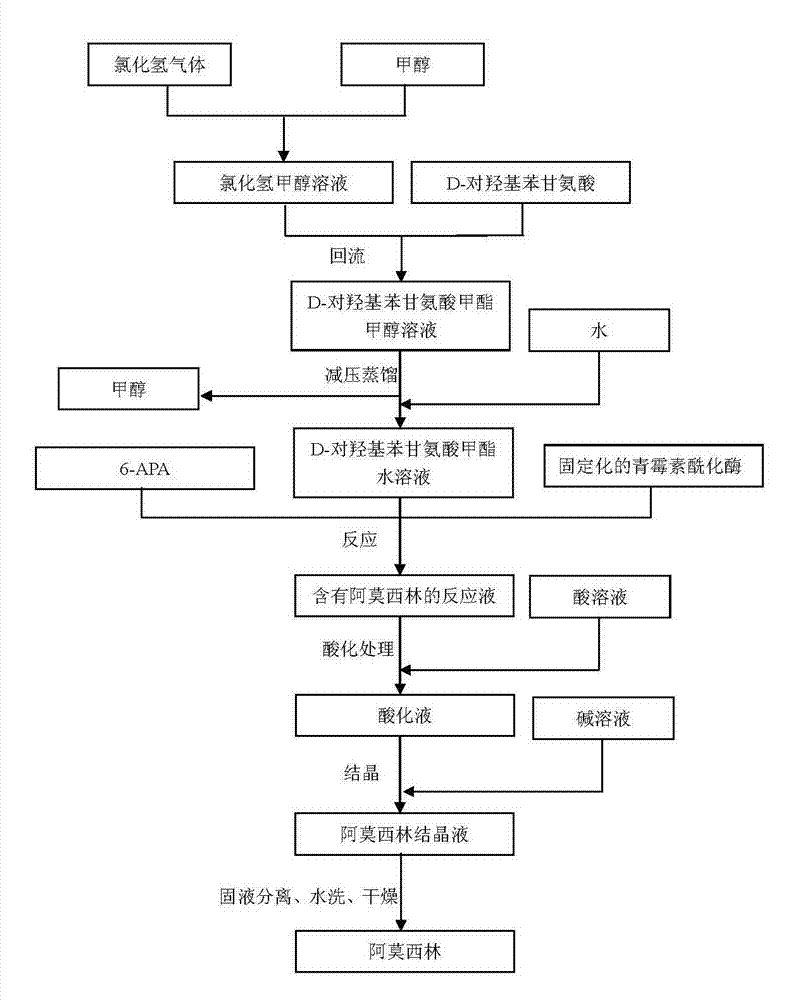
Preparation method of D-para hydroxybenzene glycine methyl ester
InactiveCN103113250AHigh yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsDistillation
The invention provides a preparation method of D-para hydroxybenzene glycine methyl ester. The method comprises the following steps of: firstly, preparing a hydrochloric acid methanol solution; adding D-para hydroxybenzene glycine into the hydrochloric acid methanol solution to perform reflux reaction for 2-4 hours at 65-80 DEG C; performing pressure reduction distillation and removing methanol; and adding water to obtain a D-para hydroxybenzene glycine methyl ester aqueous solution. Based on that, the invention also provides anenzymatic synthesis method of amoxicillin. The method comprises the following steps of: adding 6-APA and immobilized penicillin acylase into the D-para hydroxybenzene glycine methyl ester aqueous solution to react for 1-8 hours at 10-30 DEG C; regulating the pH value of a reaction liquid to 0.8-1.0 by using hydrochloric acid or sulfuric acid aqueous solution; regulating the pH value to 4.5-6.0 by using ammonia water or sodium hydroxide aqueous solution to crystallize for 1-5 hours at 0-30 DEG C; separating solid from liquid; collecting a solid; and washing and drying to obtain amoxicillin. The method is simple in steps, and low in cost; and the obtained -para hydroxybenzene glycine methyl ester is high in yield and less in impurities and can be directly applied to anenzymatic synthesis of amoxicillin.
Owner:NORTH CHINA PHARM GRP SEMISYNTECH CO LTD
Method for synthesizing p-hydroxyphenylglycine
InactiveCN102050752ASimple processLow costOrganic compound preparationAmino-carboxyl compound preparationGlyoxylic acidOrganic solvent
The invention belongs to the technical field of medicinal and chemical intermediate production, and relates to a method for synthesizing p-hydroxyphenylglycine. The method comprises the following steps of: adding catalyst in a ratio of phenol to glyoxylic acid of 0.9 to 2 at one time at the temperature of between 35 and 75 DEG C, and reacting for 5 to 20 hours; adding a small amount of reductive substance for decolorization, adjusting the pH value by using alkali, and crystallizing for 1 to 10 hours at the temperature of between 0 and 40 DEG C; and washing the crystal by using a large amount of water, and then flushing the crystal by using an organic solvent. The method has simple process and low cost, the yield can reach 60 percent, and the color and the purity cannot meet the resolution requirements.
Owner:HENAN NEWLAND PHARMA
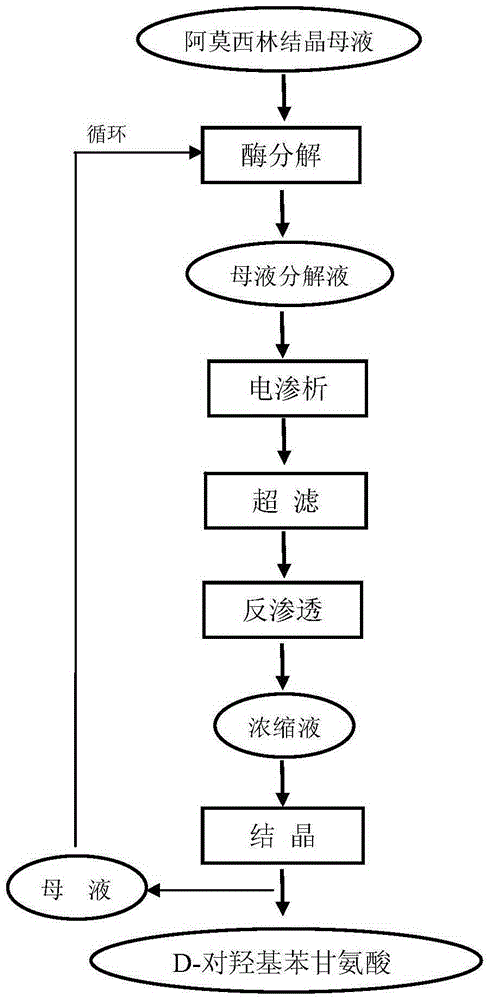
Recovery method for D-hydroxyphenylglycine in amoxicillin crystallization mother liquor of enzymatic synthesis
ActiveCN105254520AImprove product qualityEfficient ConcentrationOrganic compound preparationAmino-carboxyl compound preparationEnzymatic synthesisRecovery method
The invention belongs to the technical field of pharmacy and relates to a recovery method for D-hydroxyphenylglycine in amoxicillin crystallization mother liquor of enzymatic synthesis. The recovery method comprises the following steps that 1, amoxicillin crystallization mother liquor is decomposed; 2, electrodialysis, ultra-filtration and reverse osmosis are adopted for preparing a D-hydroxyphenylglycine concentrated solution; 3, D-hydroxyphenylglycine is crystallized. According to the method, the product quality of recovered D-hydroxyphenylglycine is promoted, efficient concentration of D-hydroxyphenylglycine in the mother liquor is achieved, and therefore the yield of recovered D-hydroxyphenylglycine products is greatly increased; a brand-new technology is used for recovering D-hydroxyphenylglycine in the mother liquor successfully, new economic benefits can be increased, and the recovery method plays an important role in environmental protection.
Owner:SHANXI WEIQIDA PHARMA IND
Method for preparing optical pure p-hydroxy phenyl glycine by separation method
InactiveCN101792398AReduce manufacturing costOrganic compound preparationAmino-carboxyl compound preparationGlycineOrganic solvent
The invention discloses a method for preparing optical pure p-hydroxy phenyl glycine by a separation method, and belongs to the field of separating racemic compounds. In the method, laevo-rotation cycle phosphoric acid serving a main separating agent and cycle phosphoric acid serving as an auxiliary separating agent are used together to separate DL-p-Hydroxyphenylglycine, and particularly, the DL-p-Hydroxyphenylglycine and the combined cycle phosphoric acid separating agent are reacted in a water-containing organic solvent, the reaction solution is heated and refluxed until the reaction solution is clear, the reaction solution is stirred and cooled to precipitate crystals, the reaction solution is filterer and washed, the obtained filter cakes are treated to form the D-(-)-p-hydroxy phenyl glycine, and the filtrate and the water-washing solution are treated to obtain the L-(+)-p-hydroxy phenyl glycine. The method has the advantages of high yield, high optical purity, high separation efficiency, easy and convenient operation, and the like.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method of synthesizing cefadroxil by enzyme process
The invention relates to a method of synthesizing cefadroxil by an enzyme process. The method comprises the following steps of: by taking 7-ADCA as an initial raw material, performing a reaction on D-tyrosine methyl ester or D-p-hydroxyl phenylglycine ethyl ester and 7-ADCA in water by directly inputting the solid in the presence of penicillin acylase at 10-25 DEG C; after reaction, separating a cefadroxil coarse product and enzyme reaction mother liquor; further purifying the cefadroxil coarse product to obtain a white cefadroxil product; and adding beta-naphthol or 2,7-dioxynaphthalene into the enzyme reaction mother liquor to obtain a cefadroxil compound. Cefadroxil can be further treated and recovered from the cefadroxil compound, so that the recovery rate of cefadroxil is increased. The product obtained by the method is high in yield and purity. The product is white in appearance, multi-step reaction of a chemical process and various solvents and auxiliary materials are not needed, and green synthesis of cefadroxil is realized.
Owner:苏州盛达药业有限公司 +1
Novel route cefprozil compound
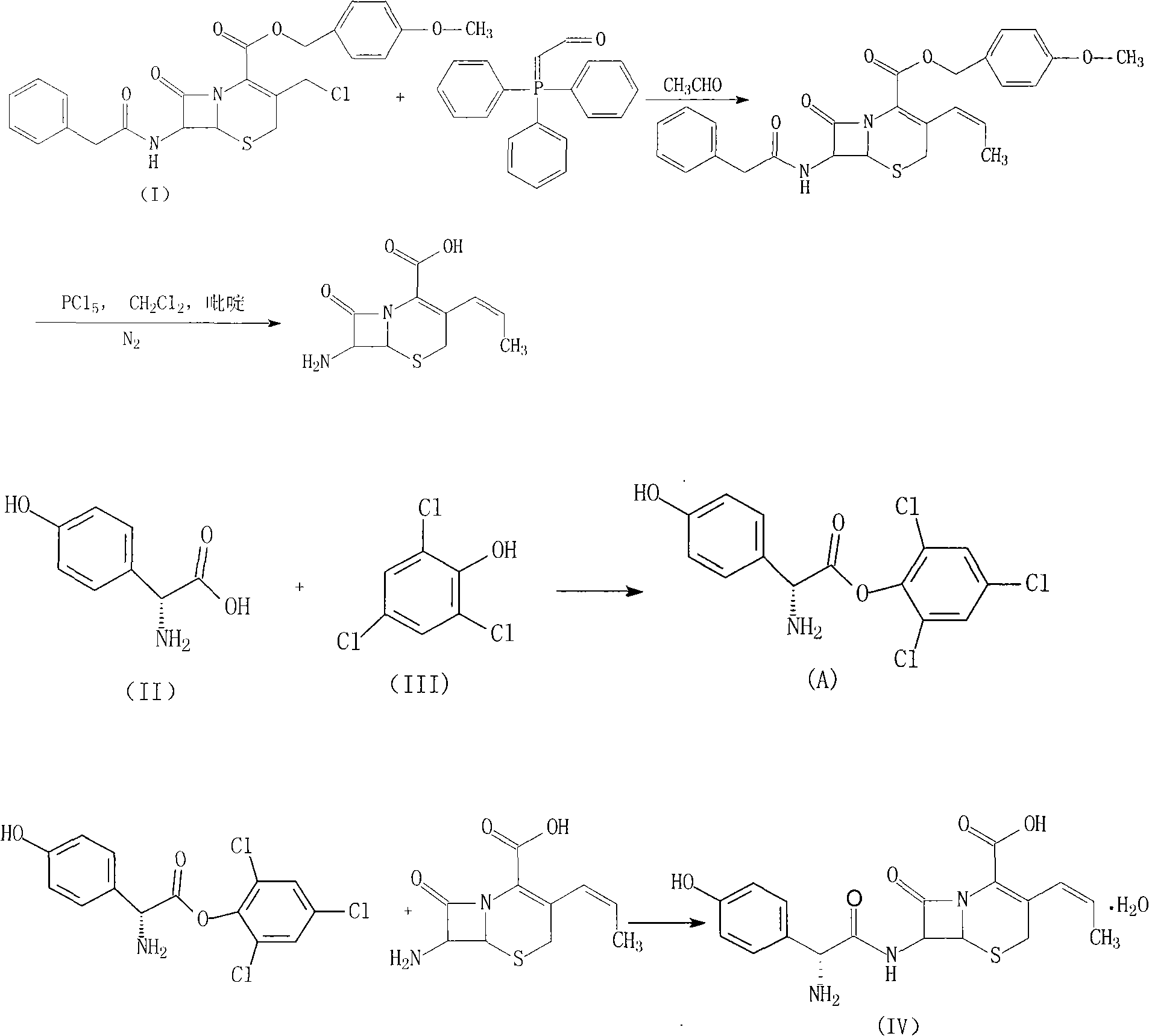
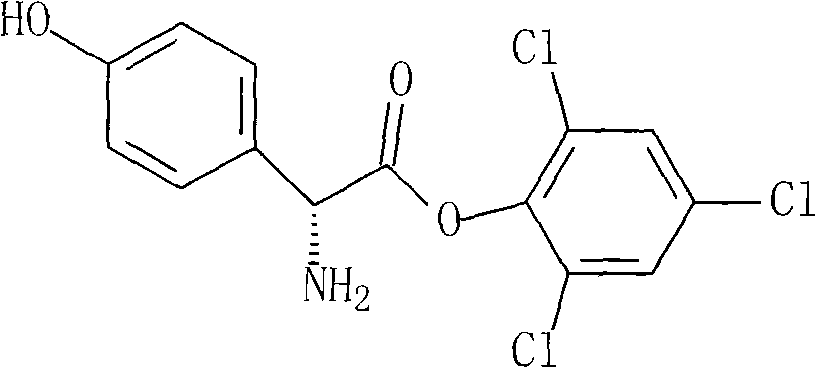
InactiveCN101798312ASimple processMild stabilityOrganic compound preparationAmino-carboxyl compound preparationP-hydroxyphenylglycineAnisyl acetate
The invention provides a novel route cefprozil compound, which simplifies the processes of 7-phenylacetamide-3-chloromethyl cephalosporanic acid anisyl acetate and triphenylphosphine phyllocaline Benedict's reagents, and activating reagents such as sodium iodide and the like do not need to be added, so the post treatment is simple and convenient, the production amplification can be realized more easily, and at the same time, para hydroxybenzene glycine is adopted to react with 2, 4, 6-trichlorophenol to generate a novel intermediate product of para hydroxybenzene glycine 2, 4, 6- phenyl ester trichlorine. The stability and the activity of the compound are mild, by-products produced in the reaction are fewer, the reaction yield is improved, and in addition, the obtained products have high purity and low isomeride content.
Owner:HAINAN MEIDA PHARMA
Synthesis method of p-hydroxyphenylglycine methyl ester
ActiveCN102718672AHigh purityReduce pollutionOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsSolid acid
The invention discloses a synthesis method of p-hydroxyphenylglycine methyl ester. The method comprises the steps of: reacting p-hydroxyphenylglycine or salt thereof which is used as an initial raw material with methanol in the presence of solid acid; and treating so as to obtain p-hydroxyphenylglycine methyl ester. The preparation method of p-hydroxyphenylglycine methyl ester is available in raw material, simple and convenient to operate, low in cost, high in yield, high in product purity, and is suitable for commercial production.
Owner:SHANDONG HANXING PHARM TECH CO LTD +2
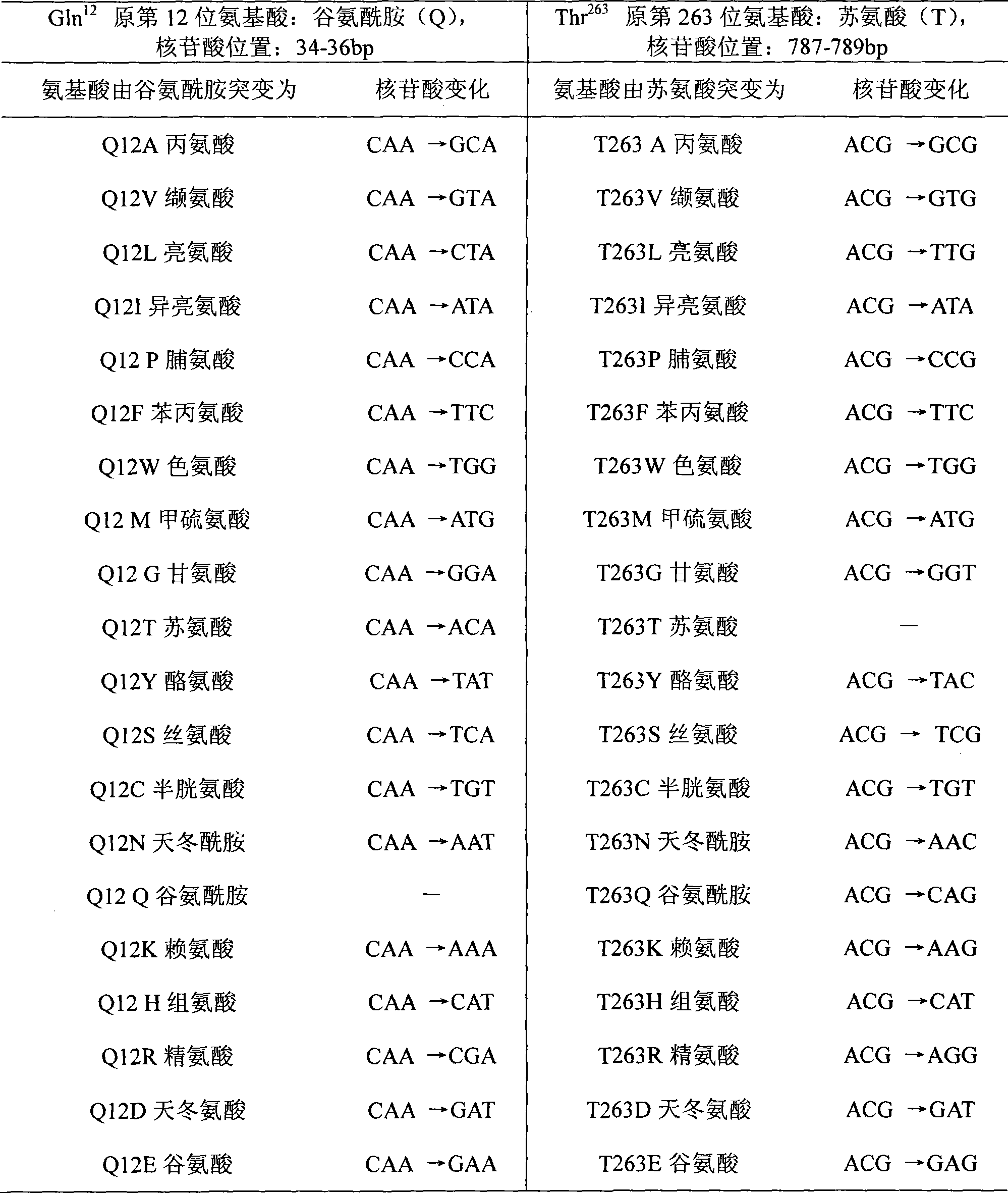
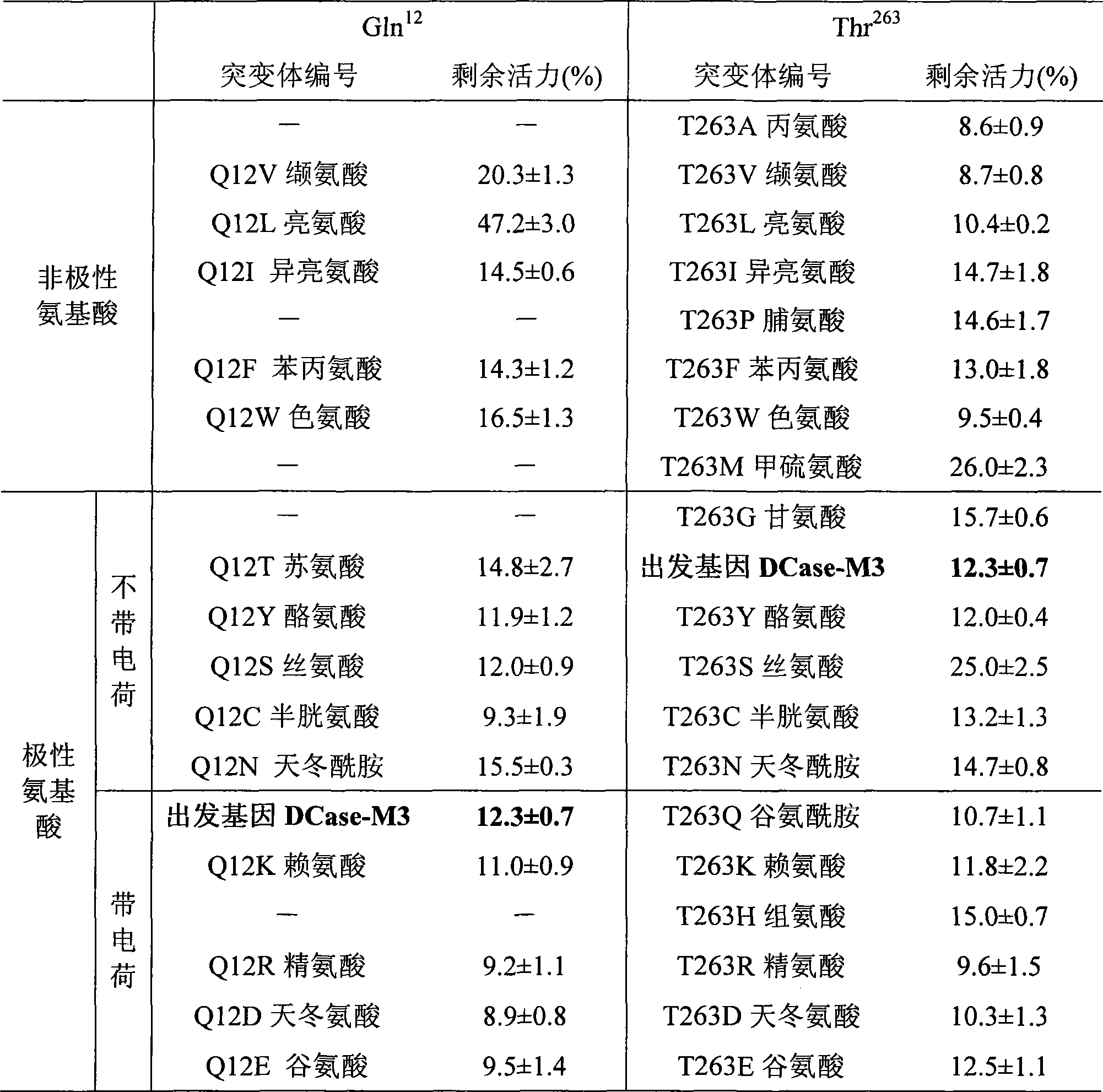
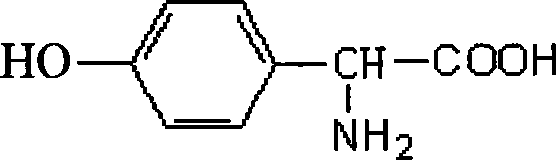
Mutant of D-carbamyl hydrolysis enzyme and application thereof
The invention discloses a mutant of D-carbamyl hydrolysis enzyme and application thereof in producing D-p-hydroxyphenylglycine. The method comprises the following steps that: a mutant is obtained by the directed evolution technology and mutates into nonpolar amino acid in 12-glutamine of the D-carbamyl hydrolysis enzyme; another mutant mutates into methionine in 263-threonine of the D-carbamyl hydrolysis enzyme; a third mutant mutates into serine in 263-threonine of the D-carbamyl hydrolysis enzyme; a fourth mutant mutates into the threonine in 12-glutamine of the D-carbamyl hydrolysis enzyme; and the 263-threonine mutates into the serine. The mutant enzyme shows higher thermal stability and enzyme activity in the production of the D-p-hydroxyphenylglycine, and indicates that using the directed evolution technology to reconstruct industrial enzyme is a quite effective method.
Owner:洛阳华荣生物技术有限公司
Method of preparing D-p-hydroxyphenylglycine
ActiveCN101239926AGood split effectLow costOrganic compound preparationAmino-carboxyl compound preparationRoom temperatureP-hydroxyphenylglycine
The invention provides a preparing method of D-p-hydroxyphenylglycine, which comprises adding DL-p-hydroxyphenylglycine and resolving agent beta-naphthalenesulfonic acid in water to prepare solution, rising temperature and reacting under agitating, adding induction seeds, adjusting the specific totation of the solution, performing heat-preservation reaction, cooling, sufficiently crystallizing, reducing pressure, filtering and drying, thereby obatining solid D-p-hydroxyphenylglycine beta-naphthalenesulfonic acid complex salts or L-p-hydroxyphenylglycine beta naphthalenesulfonic acid complex salts; dissolving D-p-hydroxyphenylglycine beta-naphthalenesulfonic acid complex salts in water to prepare aqueous solution, rising temperature, adding active carbon to perform decoloring treatment, adjusting the PH value by alkali liquid, cooling the sollution to room temperature, filtering, washing, and drying, thereby obatining D-p-hydroxyphenylglycine. The D-p-hydroxyphenylglycine produced by the method of the invention has good quality, short reaction time and low cost.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Penicillin G acylase mutant, and coding gene and application thereof
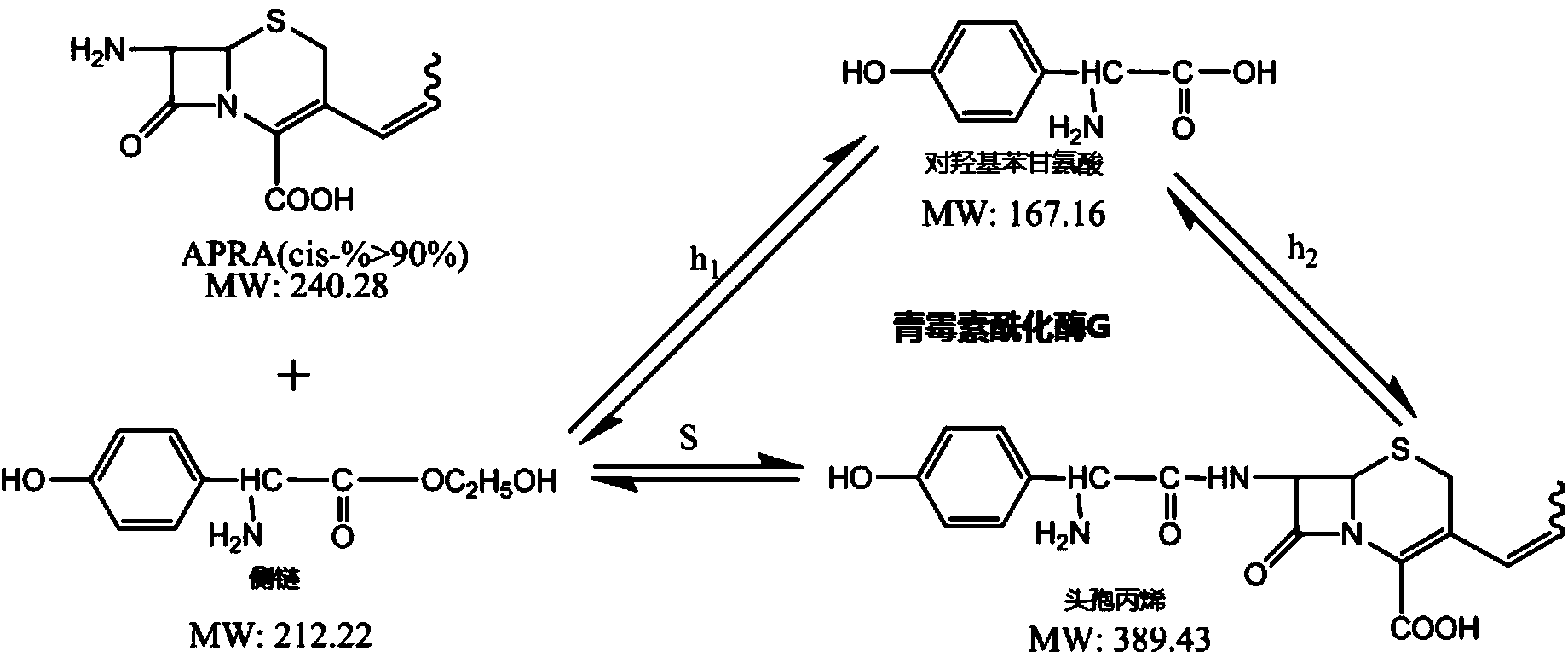
The invention discloses a penicillin G acylase mutant, and coding gene and application thereof; the amino acid sequence of the penicillin G acylase mutant is displayed by SE Q ID NO.1; the nucleotide sequence of the coding gene is presented by the SEQ ID NO.2; the invention also provides the application of the penicillin G acylase mutant in synthesis of cephalo-type antibiotic. Compared with wild type, the novel penicillin G acylase mutant has higher activity in synthesis of the cephalo-type antibiotic like cephalosporin propylene, cofactor or cephalosporin amoxicillin, and has highly improved enzyme vitality when catalyzing 7-APRA and side chain 2-hydroxy ethyl to react with hydroxy benzenes glycine ester so as to synthesis the cephalosporin propylene; specific vitality is improved from 1.5U / mg to 35U / mg, and synthesis hydrolysis vitality ratio is not reduced, and the synthesis hydrolysis vitality ratio can reach 1.8.
Owner:ZHEJIANG APELOA TOSPO PHARMA +1
Splitting process of racemic para hydroxybenzene glycine
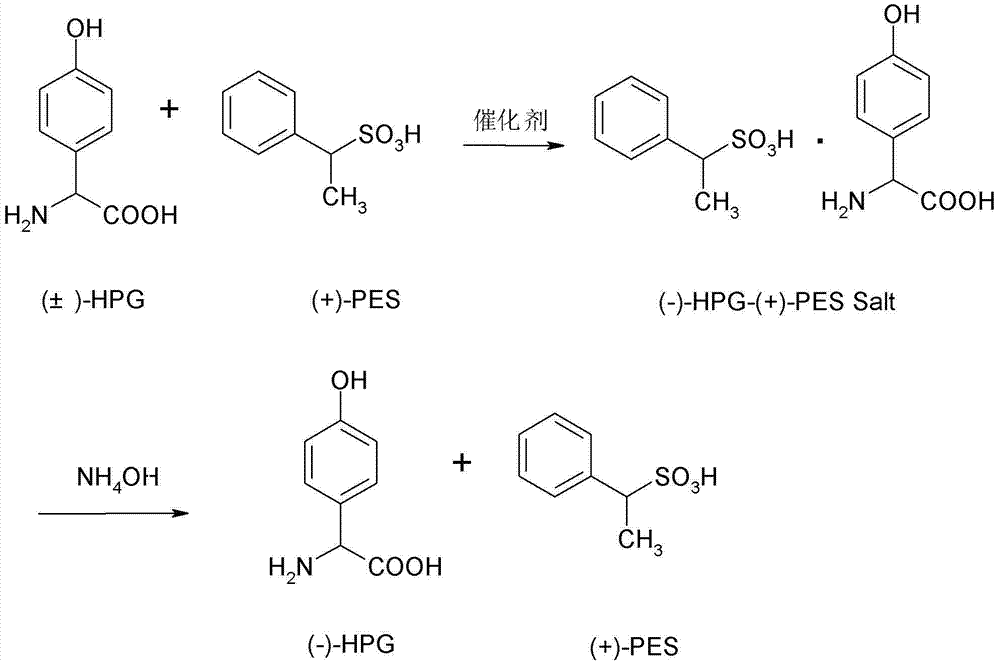
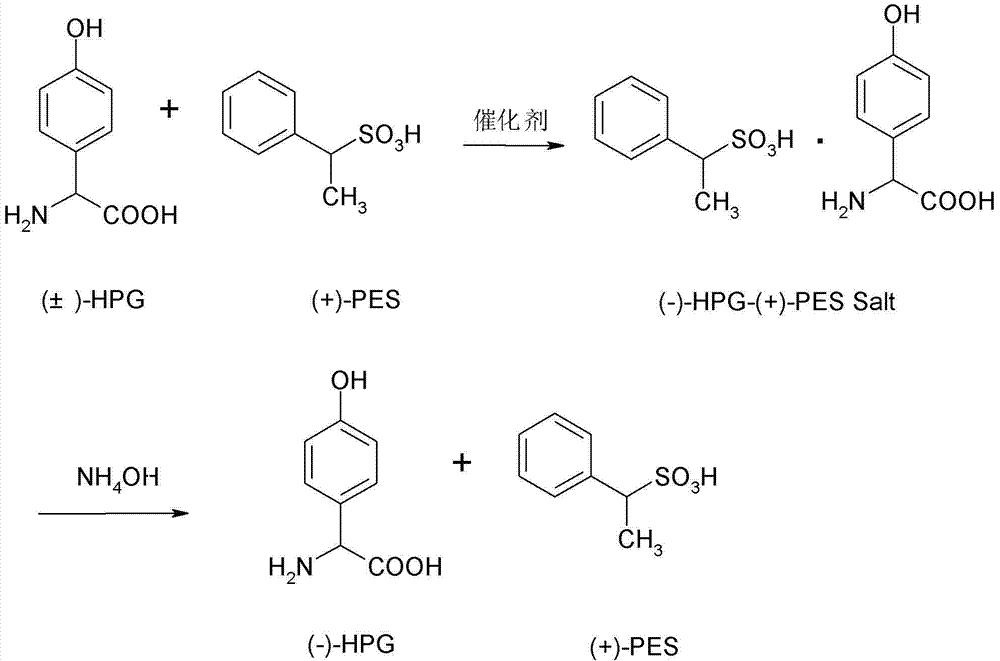
ActiveCN102757356AIncrease profitEmission reductionOrganic compound preparationAmino-carboxyl compound preparationHigh concentrationInorganic salts
The invention relates to a splitting process of racemic para hydroxybenzene glycine. As to the problems that the splitting process of the racemic para hydroxybenzene glycine is high in price, much high-concentration waste water is generated in a production process, and pollution on the environment is easily generated at present, the splitting process of the racemic para hydroxybenzene glycine is characterized by comprising the steps of: dispersing 1mol of complex salt prepared from L-p-hydroxyphenylglycine glycine and D-ethyl benzene sulfonic acid into 20mol of water by a hydrolysis process, and slowly dropping inorganic base solution to neutralize until the pH is 4-8; devitrifying, separating, washing and drying the solution to obtain the L-p-hydroxyphenylglycine glycine, storing the mother liquid for use at low temperature; dissolving 1mol of 98% concentrated sulfuric acid into 20mol of water by the splitting process, adding racemic para hydroxybenzene glycine and the mother liquor, then adding 0.05mol of catalyst to reflux for 10-20 hours; devitrifying, separating, washing and drying the solution to obtain the complex salt of the L-p-hydroxyphenylglycine glycine and D-ethyl benzene sulfonic acid, and applying the hydrolysis process and splitting process in circulation until the concentration of inorganic salt of the mother liquid is saturated. According to the invention, racemization and splitting integration is achieved; and the conversion per pass is kept over 80%, and the reaction efficiency is high.
Owner:大丰云涛生物技术有限公司
Synthetic method of D-p-hydroxyphenylglycine methyl ester
InactiveCN104744281AAvoid the risk of hydrolysisReduce processOrganic compound preparationAmino-carboxyl compound preparationGlycineBiochemical engineering
The invention relates to the medicine field, and especially relates to a synthetic method of D-p-hydroxyphenylglycine methyl ester. The invention provides a synthetic method by directly using d-p-hydroxyphenylglycine as an initial raw material without splitting and separation, a target compound D-p-hydroxyphenylglycine methyl ester can be obtained through one-pot synthesis, a technology process is shortened, and hydrolysis risk of ester in a long production process can be avoided. According to the invention, yield can reach more than 96%, the ee value of D-p-hydroxyphenylglycine methyl ester can reach more than 99%, and the quality index is excellent.
Owner:HENAN NEWLAND PHARMA
P-hydroxy phenylglycine synthesis method
ActiveCN102816076AHigh purityReduce pollutionOrganic compound preparationAmino-carboxyl compound preparationGlycineSynthesis methods
The invention discloses a p-hydroxy phenylglycine synthesis method. P-hydroxy phenylglycine is obtained by carrying out reaction of phenol and 2-hydroxy glycine in the presence of catalysts. The p-hydroxy phenylglycine synthesis method is available in raw materials, simple and convenient to operate, low in cost, high in yield and product purity, environment-friendly and suitable for commercialized production.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
Method for recovering D-p-hydroxyphenylglycine in amoxicillin production waste liquid
ActiveCN104628587AReduce energy consumptionHigh purityOrganic compound preparationAmino-carboxyl compound preparationLiquid wasteDesorption
The invention discloses a method for recovering D-p-hydroxyphenylglycine in amoxicillin production waste liquid by use of ion exchange resin. The method comprises the following steps: firstly, exchanging D-p-hydroxyphenylglycine onto the resin by use of the ion exchange resin, dissolving D-p-hydroxyphenylglycin by use of a 0.5mol / L aqueous hydrochloric acid solution, and meanwhile, concentrating the solution; secondly, purifying the desorption solution by use of macroporous adsorption resin, and drying the dripping liquid to obtain a finished product. The method is characterized in that D-p-hydroxyphenylglycine is concentrated and purified by use of the ion exchange resin, the process is greatly simplified, the recovery effect is good, the energy is saved, the environment can be protected, and the subsequent production process of synthesizing amoxicillin by use of an enzyme method is further perfected.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
D-carboxamide hydrolase mutant and its uses
InactiveCN1995336AIncrease enzyme activityShow potential application valueBacteriaHydrolasesTyrosineThreonine
The invention discloses four mutants of D-carbamoyl hydrolase and application to manufacture D-p-hydroxybenzene glycine in the biological engineering domain, which is characterized by the following: mutating the amino acid at 18th position of mutation enzyme 1 from alanine into threonine and tyrosine at 30th position of mutation enzyme 2 to asparagine; switching the lysine at 30th of mutation enzyme 2 into glutacid; overlapping three mutation positions; obtaining the mutation enzyme 4.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
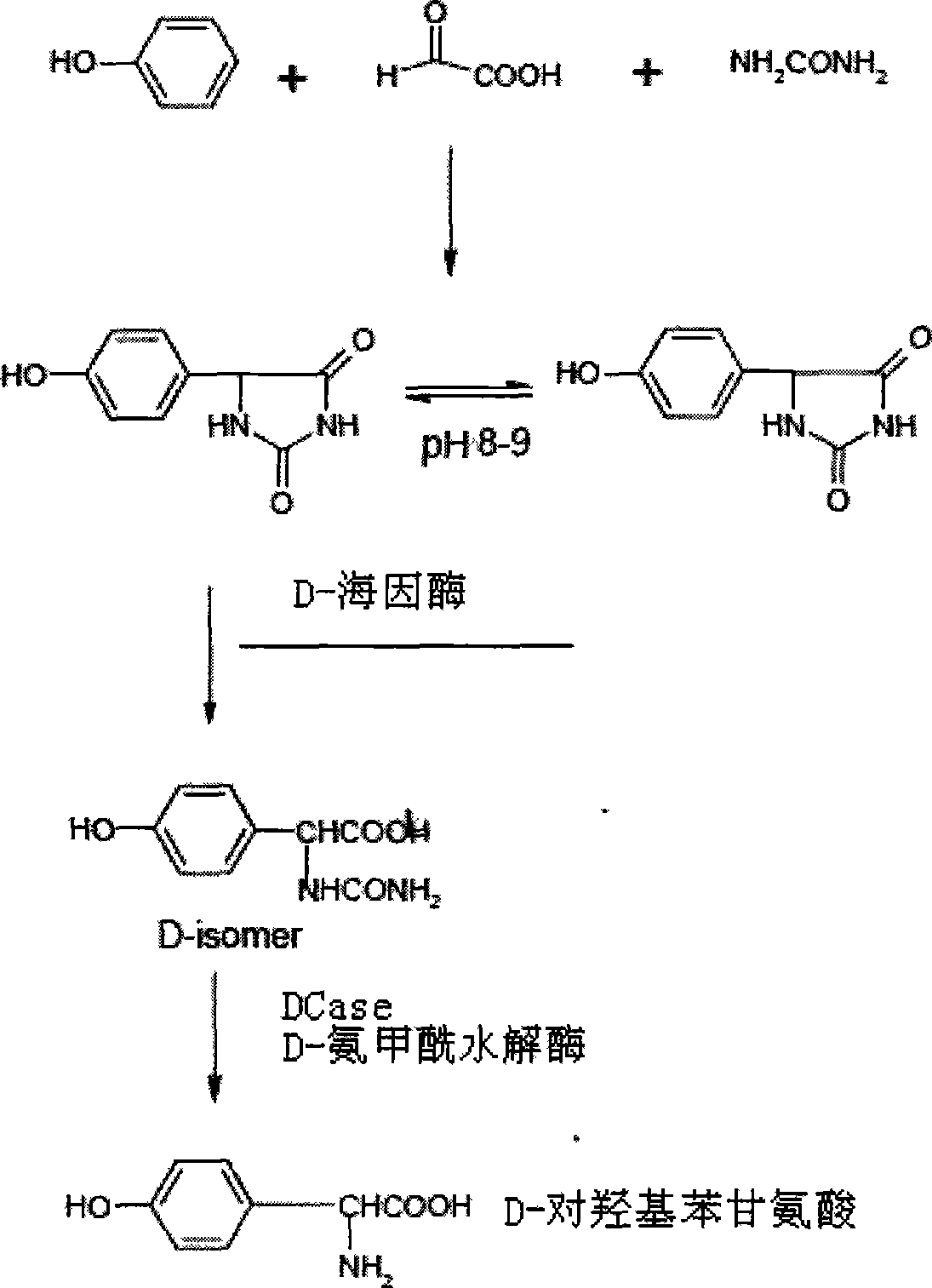
Method of producing D-p-hydroxy-phenyl glycine by heterogeneous enzyme catalysis method
The present invention relates to medicine material production, and is especially inhomogeneous enzyme catalytic process for producing D-p-hydroxyphenyl glycine. After solvent and DL-p-hydroxyphenyl hydantoin in the amount of 4-28 vol% of the solvent are added into enzyme catalyzed reactor, enzyme preparation of activity higher than 0.3 U / ml in the amount of 1-12 % total reactant liquid is added to react at pH 6.2-8.6 and 30-44 deg.c through stirring for 6-12 hr to separate out D-p-hydroxyphenyl glycine crystal until the concentration of N-carbamino-p-hydroxyphenyl glycine inside the reaction system is lowered to below 0.25 wt%. During the reaction, the reaction system is sampled and detected by means of high pressure liquid chromatography. The present invention has the advantages of high yield, low power consumption, high product quality, etc.
Owner:SHIJIAZHUANG ZHONGTIAN BIOTECH
Method for synthesizing cefprozil through green enzymatic method
The invention relates to a method for synthesizing cefprozil through a green enzymatic method. The method includes following steps: S1, adding parent nucleus 7-APRA or hydrochloride thereof into a buffer solution, and adding D-para hydroxybenzene glycinate derivative and / or D-para hydroxybenzene glycine amide under the condition that pH is 5-8; S2, adding cefprozil synthetase into the S1, reacting at temperature of 15-30 DEG C and pH of 6.5-7.8 for 1-3h, separating out separation liquid after reaction finishes, and immobilizing cefprozil synthetase to obtain a cefprozil crude product; S3, subjecting the crude product obtained in the S2 to acidolysis dissolved clarification, filtering and re-crystallizing to obtain cefprozil. Raw materials are cheap and easy to obtain, reaction is simple, hydrolysis activity of penicillin acylase can be effectively inhibited, and production cost is low; compared with conventional chemical synthesis methods, the method is simple and convenient to operate and low in cost, synthesis period is shortened, production efficiency is improved, total yield is high, controllability is high, and needs on industrial production are met.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Preparation method of D-p-hydroxyphenylglycine methyl ester
PendingCN111153821AGuaranteed yieldGuaranteed quality indicatorsOrganic compound preparationOrganic chemistry methodsMethanolEster sulfate
Owner:SHANXI WEIQIDA PHARMA IND
An enzymatic synthesis process of Amoxicillin
InactiveCN104830940AShort reaction timeHigh yieldOn/in organic carrierFermentationD-p-hydroxyphenylglycineP-hydroxyphenylglycine
The invention relates to a synthesis method of medicines, and particularly relates to screening of an immobilized Amoxicillin synthase and an enzymatic synthesis process of Amoxicillin. The process includes immobilizing by adoption of an amino epoxy type carrier to obtain an immobilized Amoxicillin enzyme LK218, adding the immobilized Amoxicillin enzyme LK218, 6-aminopenicilanic acid and a D-p-hydroxyphenylglycine derivative into water, stirring, mixing to obtain a mixture, adjusting the pH value of the mixture by utilization of a hydrochloric acid solution and a sodium hydroxide solution, controlling the temperature and reaction time of the mixture, and finishing the reaction until the residual concentration of the 6-APA is 0-2 mg / mL. Aiming at problems, namely difficult screening and evaluation of immobilized enzymes, tedious production steps, poor reference points, long reaction time, low conversion ratios, and the like, the immobilized Amoxicillin enzyme and the novel synthesis process of the Amoxicillin are provided.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Synthesis of D(-)-alpha-(4-ethyl-2,3-dioxygen ethylene imine-1-formamido) p-hydroxybenzene acetic acid
The invention provides a synthetic method of D(-)-alpha-(4-ethyl-2,3-dioxypiperazine-1-formamido) p-hydroxyphenylacetic acid. In the synthetic method, p-hydroxyphenylglycine is taken as a starting material, water is taken as a solvent, the p-hydroxyphenylglycine react with 4-ethyl-(2,3-dioxopiperazinyl)formyl chloride-1 in the presence of alkali to directly obtain a target product D(-)-alpha-(4-ethyl-2,3-dioxypiperazine-1-formamido)p-hydroxyphenylacetic acid after acidification. Hydroxyl protection and carboxyl protection of the p-hydroxyphenylglycine are not needed in the synthetic method, and condensation reaction can be directly carried out in aqueous phase, which simplifies the production process and reduces the consumption, and the prepared target product has stable quality.
Owner:山西新天源药业有限公司
P-hydroxybenzene glycine synthesis technology
InactiveCN101362703BOrganic compound preparationAmino-carboxyl compound preparationSulfite saltHydroxylamine Hydrochloride
The invention belongs to the field of pharmaceutical chemical engineering intermediate production, which relates to a synthesis technology of p-hydroxyphenylglycine (HPG). The purpose of the invention is achieved by the following steps: phenol, glyoxylic acid, water and sulfamic acid carry out the one-pot braise reaction under the action of catalysts such as benzene sulfonic acid, p-toluenesulfonic acid, o-toluenesulfonic acid, and the like; after the reaction is finished, a small amount of reducing substances such as sodium sulfite, sodium bisulfite, hydroxylamine hydrochloride, and the like, are added; finally, the PH value is adjusted by alkali, the mother liquor separation is washed by large amount of water and then washed by organic solvents such as methanol, ethanol, acetone, glacial acetic acid, and the like; the obtained product HPG is white powder, wherein, the content of NPLC is equal to or more than 98.5, which can meet the requirement of splitting. The method has the advantages of low production cost, simple operation, stable quality, etc.
Owner:谢建中
Green method of enzymatic synthesis of cefaclor
InactiveCN106222230AHigh selectivityImprove catalytic performanceImmobilised enzymesHydrolasesChemical synthesisEnzymatic synthesis
The invention relates to a green method of enzymatic synthesis of cefaclor. The method includes the steps of: (S1) adding a parent nucleus 7-ACCA into a buffer liquid; (S2) under the pH of 5-8, adding a D-p-hydroxyphenylglycinate derivative or a salt thereof and / or D-p-hydroxyphenylglycine amide, and immobilized cefaclor synthesis enzyme, and performing a reaction for 1-3 h at 5-30 DEG C under the pH value of 6.2-7.8; after a certain reaction time, adding a seed crystal to perform crystallization, and when the reaction is finished, separating a reaction liquid and the immobilized cefaclor synthesis enzyme to obtain a cefaclor coarse product; and (S3) acid-hydrolyzing and dissolve-clarifying the coarse product, filtering and re-crystallizing the product to prepare the cefaclor. The enzymatic synthesis method, compared with a conventional chemical synthesis method, is simple in operation, is low in cost, reduces synthetic period, improves production efficiency and total yield, has strong controllability and satisfies industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
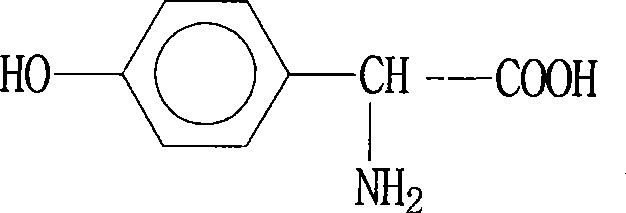
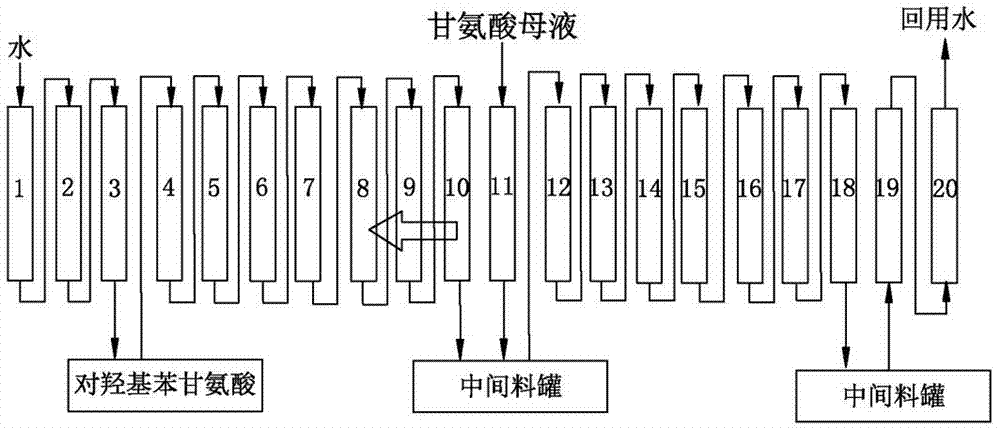
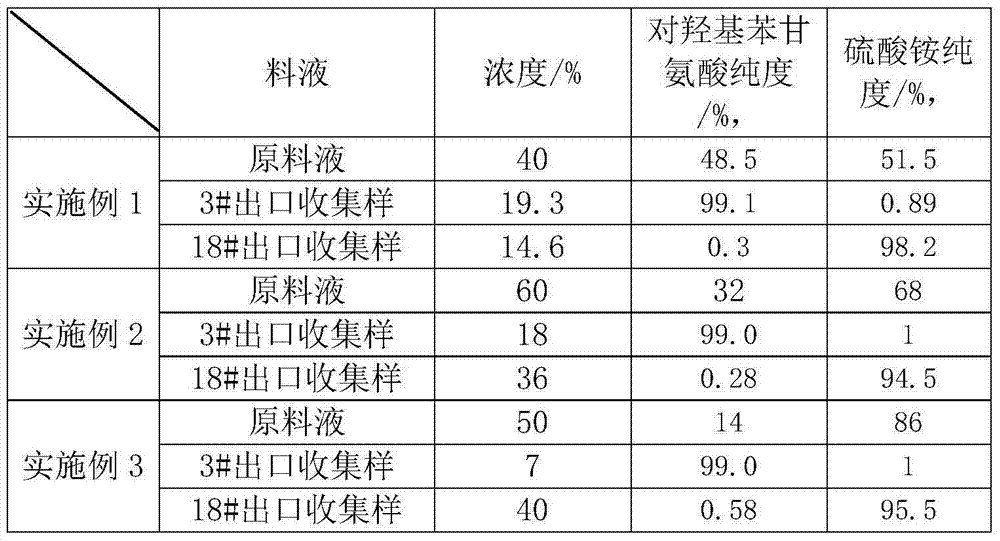
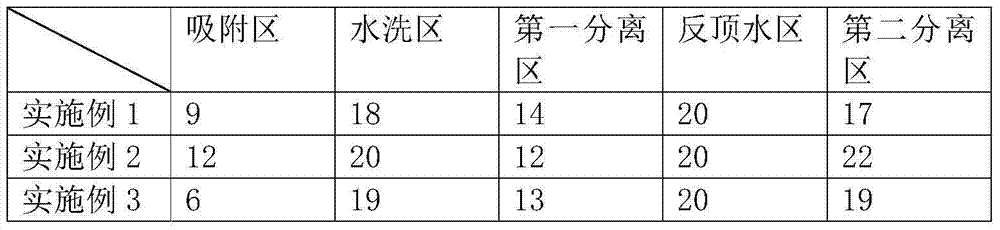
Method for separating p-hydroxyphenylglycine and ammonium sulfate from glycine mother solution
ActiveCN103483213ASmall footprintComposition is stableOrganic compound preparationAmmonium sulfatesChromatographic separationGlycine
The invention discloses a method for separating p-hydroxyphenylglycine and ammonium sulfate from a glycine mother solution, which is characterized by comprising the following steps: regulating the pH value of a glycine mother solution containing p-hydroxyphenylglycine and ammonium sulfate to 3.0-8.0, sending into a continuous chromatographic separation system filled with a strong acid cation exchange resin at 10-80 DEG C, eluting by using water as an eluting agent, and respectively collecting an eluate and a residue solution to obtain the two products p-hydroxyphenylglycine and ammonium sulfate, thereby implementing efficient separation of the p-hydroxyphenylglycine and ammonium sulfate. The method has the advantages of compact equipment, simplified system, reduced pipelines and smaller occupied area; the method is continuously operated under continuous operation, the composition and concentration of the product are basically kept stable; the method has favorable operating flexibility, and can automatically regulate the rotation speed according to the variance in production load; the method lowers the operating cost and equipment investment; and the separating effect is good, and the purity of the separated p-hydroxyphenylglycine is high.
Owner:XIAMEN STARMEM TECH
Technology for treating phenol-contained wastewater in synthetic process of L-(+)-D-p-hydroxyphenylglycine
InactiveCN101759268AWater contaminantsMultistage water/sewage treatmentDistillationTherapeutic effect
The invention relates to a technology for treating phenol-contained wastewater in the synthetic process of L-(+)-D-p-hydroxyphenylglycine, which belongs to the technical field of the treatment of organic chemical wastewater. In the method, after the distillation pretreatment is carried out on synthesized wastewater, the macroporous resin adsorption treatment is carried out on the collected phenol-contained wastewater, wherein the pretreatment comprises the step of carrying out acidized pretreatment on the synthesized wastewater. In the method, because a phenol-contained substance in the wastewater is removed in advance, the recycling and processing difficulty of the subsequent inorganic salt can be greatly improved. The recycled sodium phenolate can be charged back to the synthesizing section of p-hydroxyphenylglycine. The method has stable and reliable treatment effect, low treatment cost and simple, convenient and easily-applied operation and is easy to realize industrialized application.
Owner:HENAN NEWLAND PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com