Synthetic method of D-p-hydroxyphenylglycine methyl ester
A technology of p-hydroxyphenylglycine methyl ester and p-hydroxyphenylglycine, which is applied in the field of synthesis of D-p-hydroxyphenylglycine methyl ester, can solve the problem that D-p-hydroxyphenylglycine methyl ester cannot meet the raw material index of β-lactam antibiotics Requirements and other issues to achieve the effect of avoiding hydrolysis risk, excellent quality indicators, and shortening the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
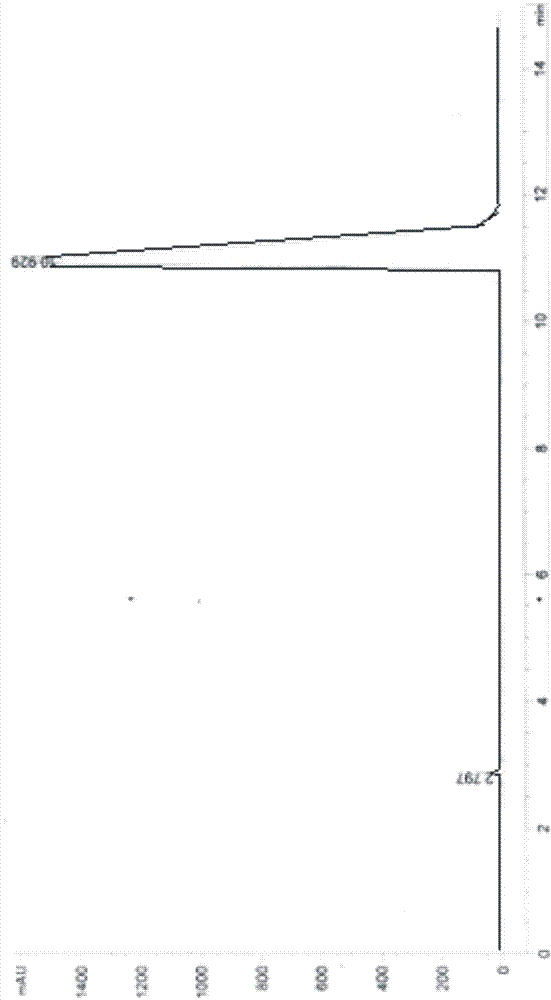
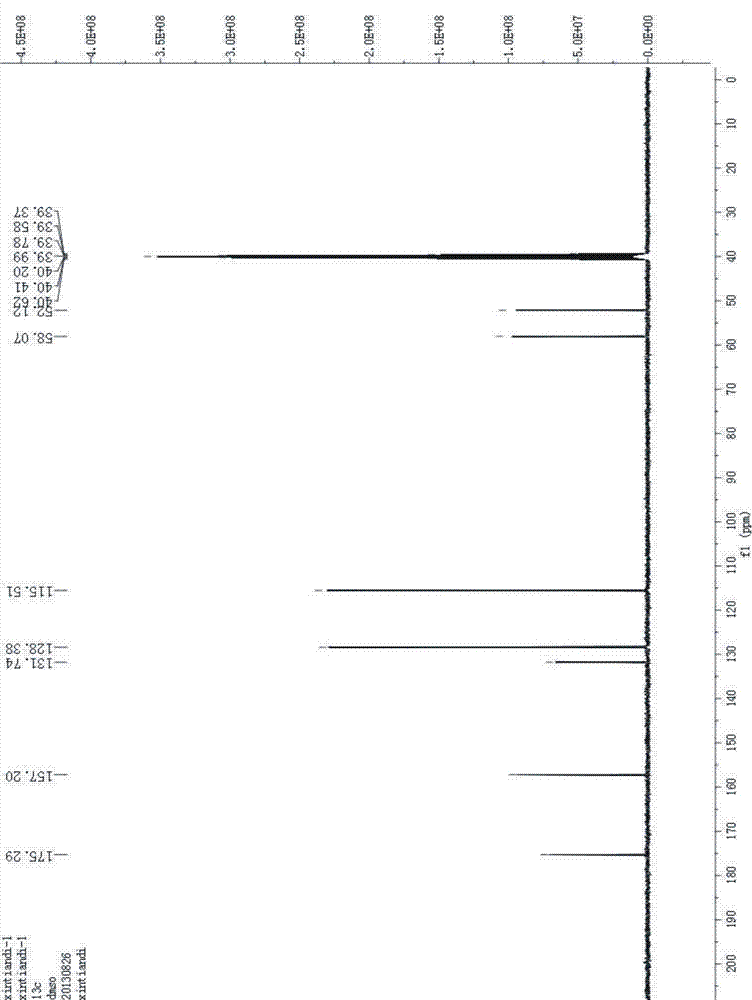
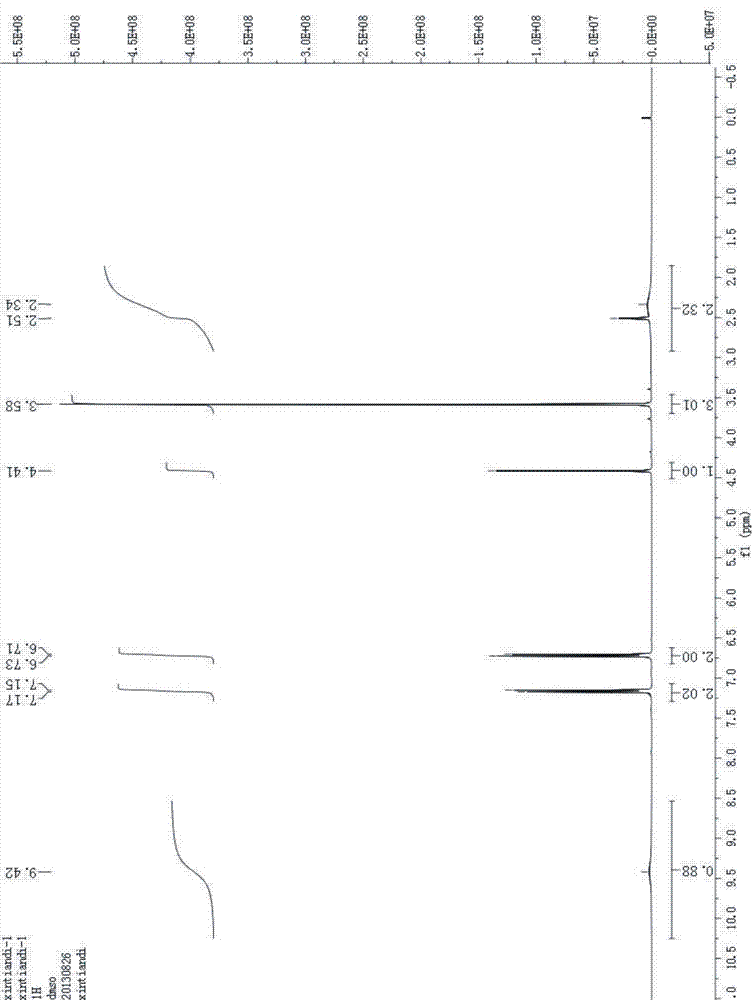
Image
Examples
Embodiment 1
[0040] The synthesis of embodiment 1 D-p-hydroxyphenylglycine methyl ester hydrochloride
[0041] Add 800 g of D-p-hydroxyphenylglycine and 800-8000 g of methanol into a 3000 mL three-necked flask, and stir. Weigh 569.4-683.2 g of thionyl chloride and add it dropwise slowly. During the dropping process, control the temperature to ≤65°C. After the dropwise addition of thionyl chloride is completed, control the reaction temperature to -10-65°C and keep the temperature for 10 hours. After the heat preservation reaction was completed, the temperature was lowered to 10°C, stirred for 1 h, filtered with suction, and the filter cake was rinsed with ethyl acetate to obtain 867.7 g of solid, with a yield of 83.3%.
[0042] After testing, the obtained solid was D-p-hydroxyphenylglycine methyl ester hydrochloride.
[0043]
Embodiment 2
[0044] The synthesis of embodiment 2 D-p-hydroxyphenylglycine methyl ester
[0045] Add 100 g of D-p-hydroxyphenylglycine methyl ester hydrochloride prepared in Example 1 to a 2L beaker, add methanol to the beaker, stir and dissolve, then add ammonia water dropwise to the above solution for neutralization, and the neutralization process controls the reaction The temperature is ≤80°C, and when the pH value of the reaction solution is higher than 7, the neutralization is completed, and the dropwise addition is stopped. After suction filtration, 83.25 g of solids were obtained, with a yield of 97.5%.
[0046] After testing, the obtained solid is D-p-hydroxyphenylglycine methyl ester, and the ee value of D-p-hydroxyphenylglycine methyl ester can reach more than 99%.
[0047]
Embodiment 3
[0048] The synthesis of embodiment 3 D-p-hydroxyphenylglycine methyl ester
[0049]Add 800g of D-p-hydroxyphenylglycine and 800-2400mL of methanol into a 3000mL three-necked flask, stir, and cool down in an ice-water bath. Weigh 569.4-683.2 g of thionyl chloride and add it dropwise slowly. During the dropping process, control the temperature to ≤65°C. After the dropwise addition of thionyl chloride is completed, control the reaction temperature to -10-65°C and keep the temperature for 10 hours. After the heat preservation reaction is completed, transfer the material to a 50L reactor, add 8-15L of distilled water, stir and dissolve, then add ammonia water dropwise to the above solution for neutralization, and control the reaction temperature ≤ 80°C during the neutralization process until the pH value of the reaction solution When it is higher than 7, the neutralization is completed and the dropwise addition is stopped. After centrifugation, 814.3 g of solids were obtained, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com