Preparation method of D-para hydroxybenzene glycine methyl ester
A technology for p-hydroxyphenylglycine methyl ester and p-hydroxyphenylglycine, which is applied in the field of enzymatic synthesis of D-p-hydroxyphenylglycine methyl ester of amoxicillin, can solve the problems of cumbersome steps, unsatisfactory, difficult to control, and the like, To achieve the effect of simple process steps, simplification of operation steps, and reduction of workshops
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of hydrogen chloride gas: Take 49g of ammonium chloride and add it to the gas generating device, add 90g of concentrated sulfuric acid dropwise, and collect 13.9g of hydrogen chloride gas for future use.
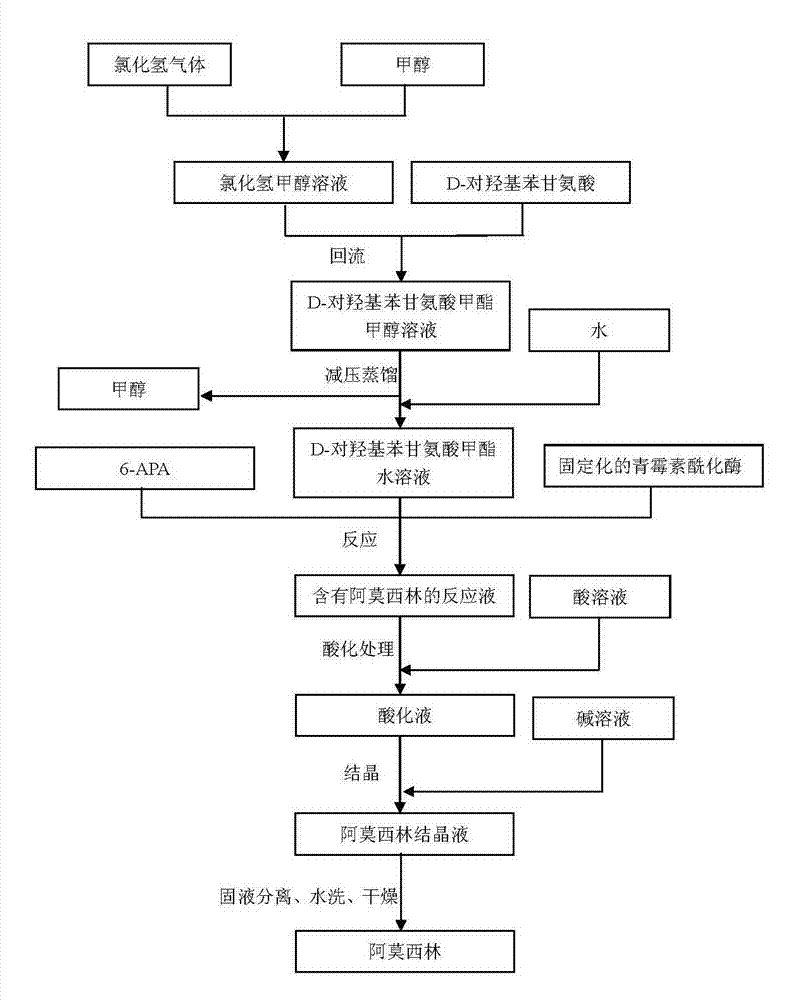
[0019] Preparation of D-p-hydroxyphenylglycine methyl ester (as figure 1 shown):
[0020] a. Passing pre-prepared hydrogen chloride gas into methanol to obtain a methanolic hydrogen chloride solution, wherein the mass-volume concentration of hydrogen chloride is 7%.
[0021] b. Add D-p-hydroxyphenylglycine to the hydrogen chloride methanol solution, wherein the molar ratio of D-p-hydroxyphenylglycine to hydrogen chloride is 2:1, then heat it in a water bath to 65°C, and reflux for 2 hours to obtain D-p-hydroxyphenylglycine Phenylglycine methyl ester methanol solution, the reaction conversion rate is 96.8%.
[0022] c. Place the methanol solution of D-p-hydroxyphenylglycine methyl ester above under the conditions of 40°C and vacuum degree of -0.08MPa, dis...
Embodiment 2
[0024] Prepare hydrogen chloride gas according to a conventional method: take 40 g of ammonium chloride and add it to the gas generator, add 73 g of concentrated sulfuric acid dropwise, and collect 15.27 g of hydrogen chloride gas for future use.
[0025] Preparation of D-p-hydroxyphenylglycine methyl ester:
[0026] a. Passing pre-prepared hydrogen chloride gas into methanol to obtain a methanolic hydrogen chloride solution, wherein the mass-volume concentration of hydrogen chloride is 14%.
[0027] b. Add D-p-hydroxyphenylglycine to the above hydrogen chloride methanol solution, wherein the molar ratio of D-p-hydroxyphenylglycine to hydrogen chloride is 3.5:1, then heat it in a water bath to 80°C, and reflux for 4 hours to obtain D-p-hydroxyphenylglycine Phenylglycine methyl ester methanol solution, the reaction conversion rate is 97.6%.
[0028] c. Place the above D-hydroxyphenylglycine methyl ester methanol solution at 70°C and a vacuum of -0.09MPa, distill under reduced ...
Embodiment 3
[0030] Preparation of hydrogen chloride gas: Measure 500 mL of concentrated hydrochloric acid, heat, collect and obtain 8.2 g of hydrogen chloride gas for future use.
[0031] Preparation of D-p-hydroxyphenylglycine methyl ester:
[0032] a. Passing pre-prepared hydrogen chloride gas into methanol to obtain a methanolic hydrogen chloride solution, wherein the mass-volume concentration of hydrogen chloride is 10%.
[0033] b. Add D-p-hydroxyphenylglycine to the methanolic hydrogen chloride solution, wherein the molar ratio of D-p-hydroxyphenylglycine to hydrogen chloride is 2.5:1, then heat it in a water bath to 75°C, and reflux for 3 hours to obtain D-p-hydroxyl Phenylglycine methyl ester methanol solution, the reaction conversion rate is 98.5%.
[0034] c. Place the methanol solution of D-p-hydroxyphenylglycine methyl ester above under the conditions of 50°C and vacuum degree of -0.085MPa, distill under reduced pressure until no distillate is distilled, and add water until t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com