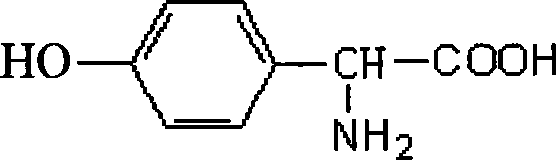
Method of preparing D-p-hydroxyphenylglycine
A technology of p-hydroxyphenylglycine and naphthalene sulfonic acid is applied in the field of preparing high-purity D-p-hydroxyphenylglycine, can solve the problems of long reaction time, low yield and the like, achieves short reaction time, good quality, and avoids Effects of Environmental Pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the preparation method of the present invention comprises the following steps: (1), in 3000ml water, add 1030g DL-p-hydroxyphenylglycine (i.e. dexterous p-hydroxyphenylglycine) and 1350g resolution agent β-naphthalenesulfonic acid, be mixed with A solution with a concentration of about 44%. Under stirring conditions, the temperature was raised to 60°C for reaction, and a small amount of induced seed crystal (D-p-hydroxyphenylglycine β-naphthalenesulfonic acid double salt, 20g) was added to adjust the specific rotation value of the solution to 1.5°. Fully crystallized at 25°C, filtered under reduced pressure, and dried (in a vacuum oven) to obtain 1730 g of solid (white crystal) D-p-hydroxyphenylglycine β-naphthalenesulfonic acid double salt. The whole split time is 2.5 hours. The 1730g D-p-hydroxyphenylglycine β-naphthalenesulfonic acid double salt prepared in step (1) was dissolved in 1730g water, recrystallized and cooled to room temperature, and filter...
Embodiment 2
[0017] Embodiment 2: the preparation method of the present invention comprises the following steps: (1), in 3000ml water, add 1030g DL-p-hydroxyphenylglycine and 1350g resolving agent β-naphthalenesulfonic acid, be mixed with the solution that concentration is about 44%, in Under stirring conditions, heat up to 65°C for reaction, add a small amount of induced seed crystal (L-p-hydroxyphenylglycine β-naphthalenesulfonic acid double salt), adjust the specific rotation value of the solution to 1.7°, keep the reaction for 1.5 hours, and cool to 30°C Fully crystallized, filtered under reduced pressure and vacuum dried to obtain 1610 g of solid L-p-hydroxyphenylglycine β-naphthalenesulfonic acid double salt.
[0018] (2-1), the 1610g L-p-hydroxyphenylglycine β-naphthalenesulfonic acid double salt prepared in step (1) is dissolved in 2000g water, adds 5g benzaldehyde, is warming up to 120 ℃, racemization reaction (insulation 7 hours) until the optical rotation of L-p-hydroxyphenylgly...
Embodiment 3
[0020]Embodiment 3: the preparation method of the present invention takes β-naphthalenesulfonic acid as the resolving agent, resolves racemic DL-p-hydroxyphenylglycine, obtains D-p-hydroxyphenylglycine, and it comprises the steps: (1), in Add the DL-p-hydroxyphenylglycine and the resolving agent β-naphthalenesulfonic acid with a weight ratio of 1: 1.2 (or 1: 1.4 or 1: 1.6) in water, and prepare a concentration of 25% (or 35% or 45%) solution, heated up to 60°C (or 70°C or 80°C) under stirring conditions for reaction, adding induced seed crystals (D-p-hydroxyphenylglycine β-naphthalenesulfonic acid double salt) to adjust the specific rotation of the solution When the value reaches 1.5° (or 2.0°), heat preservation reaction for 1.4 hours, cool to 20°C (or 30°C or 40°C) to fully crystallize, filter and dry under reduced pressure to obtain solid D-p-hydroxyphenylglycine β-naphthalenesulfonic acid Double salt. (2), the D-p-hydroxyphenylglycine β-naphthalenesulfonic acid double sal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com