Beta-cyclodextrin derivative and preparation method thereof, and polyurea-bond cyclodextrin chiral stationary phase prepared from beta-cyclodextrin derivative
A chiral stationary phase and cyclodextrin technology, applied in chemical instruments and methods, other chemical processes, bulk chemical production, etc., can solve the problems of low chemical stability, poor water resistance, short service life, etc., and achieve chemical stability Good performance, good batch reproducibility and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
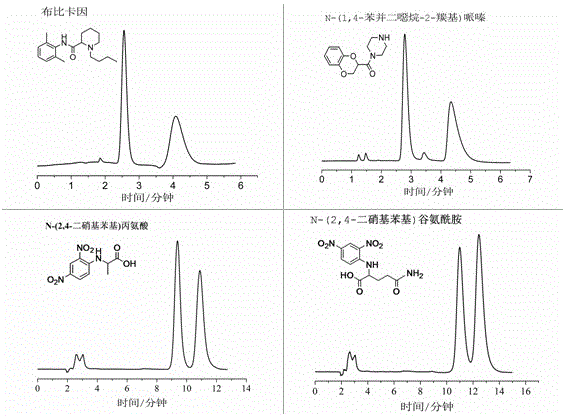
Examples
Embodiment 1
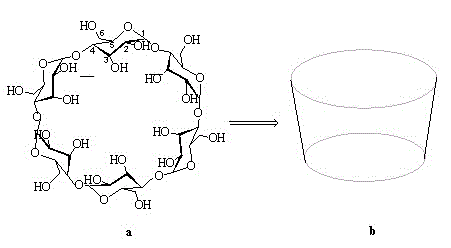
[0051] (1) Preparation of β-cyclodextrin derivatives
[0052] S1. Preparation of seven substituted 6-iodo-6-deoxy-β-cyclodextrins
[0053] Add 1 g β-cyclodextrin, 2 g iodine and 2 g triphenylphosphine in sequence to 50 mL DMF, stir and react at 90 °C for 8 h under nitrogen protection; after the reaction is stopped, add acetone to the reaction liquid to precipitate precipitate, filter The solid was collected and dried to give a light brown product.
[0054] S2. Preparation of seven-substituted 6-azido-6-deoxy-β-cyclodextrin:
[0055] Weigh 1 g of the obtained solid of S1 and 1 g of sodium azide, dissolve it in 50 mL of DMF, and react with stirring at 60 °C for 8 h under the protection of nitrogen. After the reaction was completed, 200 mL of ice water was added to precipitate a white precipitate, which was filtered, washed with water 2 to 3 times, and dried to obtain the product.
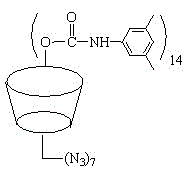
[0056] S3. Preparation of hepta-substituted 6-azido-6-deoxy-3, 5-dimethylphenylcarbamoylated β-...
Embodiment 2
[0063] (1) Preparation of β-cyclodextrin derivatives
[0064] S1. Preparation of seven substituted 6-iodo-6-deoxy-β-cyclodextrins
[0065] Add 1 g β-cyclodextrin, 4 g iodine and 4 g triphenylphosphine in sequence to 60 mL DMF, stir and react at 100 °C for 6 h under nitrogen protection; after the reaction stops, add acetone to the reaction solution to precipitate a precipitate, filter The solid was collected and dried to give a light brown product.
[0066] S2. Preparation of seven-substituted 6-azido-6-deoxy-β-cyclodextrin:
[0067] Weigh 1 g of the obtained solid of S1 and 2 g of sodium azide, dissolve it in 50 mL of DMF, and react with stirring at 70 °C for 7 h under the protection of nitrogen. After the reaction was completed, 200 mL of ice water was added to precipitate a white precipitate, which was filtered, washed with water 2-3 times, and dried in vacuo to obtain the product.
[0068] S3. Preparation of hepta-substituted 6-azido-6-deoxy-3, 5-dimethylphenylcarbamoyla...
Embodiment 3
[0075] (1) Preparation of β-cyclodextrin derivatives
[0076] S1. Preparation of seven-substituted 6-iodo-6-deoxy-β-cyclodextrin:
[0077] Add 1 g β-cyclodextrin, 5 g iodine and 5 g triphenylphosphine in sequence to 70 mL DMF, and stir the reaction at 110 °C for 6 h under the protection of nitrogen; Solid, which yielded a light brown product after drying in vacuo.
[0078] S2. Preparation of seven-substituted 6-azido-6-deoxy-β-cyclodextrin:
[0079] Weigh 1 g of the obtained solid of S1 and 2.5 g of sodium azide, dissolve it in 50 mL of DMF, and stir the reaction at 80 °C for 6 h under the protection of nitrogen. After the reaction, add 200 mL of ice water to precipitate a white precipitate, filter, wash with water 2-3 times, and dry in vacuo.
[0080] S3. Preparation of hepta-substituted 6-azido-6-deoxy-3, 5-dimethylphenylcarbamoylated β-cyclodextrin:
[0081] 1 g of the product obtained from S2 was dissolved in 70 mL of pyridine, 8 g of 3,5-dimethylphenylisocyanate was a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com